Abstract
Background: Although BiPAP has been used as an adjunct to exercise, little is know about its effect on exercise in COPD. We aimed to evaluate the acute effect of BiPAP delivered with a standard valve (Vision, Respironics), compared to no assist, on exercise capacity in individuals with COPD. Methods: Peak exercise workload (WLpeak), dyspnea (Borg), end-expiratory lung volume (EELV), tidal volume (VT), minute ventilation (VE), O2 uptake (VO2), and CO2 production (VCO2) were assessed in 10 COPD patients (FEV1 53 ± 22% pred) during three symptom-limited bicycle exercise tests while breathing i) without a ventilator (noPS), ii) with a pressure support (PS) of 0 cm H2O (PS0; IPAP & EPAP 4 cm H2O) and iii) PS of 10 cm H2O (PS10; IPAP 14 & EPAP 4 cm H2O) on separate days using a randomized crossover design. Results: WLpeak was significantly lower with PS10 (33 ± 16) and PS0 (30.5 ± 13) than noPS (43 ± 19) (p < 0.001). Dyspnea at peak exercise was similar with noPS, PS0 and PS10; at isoload it was lower with noPS compared to PS10 and PS0 (p < 0.01). VT and VE were highest with PS10 and lowest with noPS both at peak exercise and isoload (p < 0.001). EELV was similar at peak exercise with all three conditions. VO2 and VCO2 were greater with PS10 and PS0 than noPS (p < 0.001), both at peak exercise and isoload. Conclusion: Use of BiPAP with a standard exhalation valve during exercise increases VT and VE at the expense of augmenting VCO2 and dyspnea, which in turns reduces WLpeak in COPD patients.
| Abbreviations | ||
| BiPAP | = | Bi-level positive airway pressure |
| CPET | = | Cardiopulmonary exercise test |
| CPAP | = | Continuous positive airway pressure |
| CO2 | = | Carbon dioxide |
| COPD | = | Chronic Obstructive Pulmonary Disease |
| EELV | = | The end-expiratory lung volume |
| EPAP | = | Expiratory positive airway pressure |
| FEV1 | = | Forced expiratory volume in one second |
| FRC | = | Functional residual capacity |
| FVC | = | Forced vital capacity |
| IPAP | = | Inspiratory positive airway pressure |
| NIVS | = | Noninvasive ventilatory support |
| NRV | = | Non-rebreathing valve |
| noPS | = | No pressure support = without ventilator |
| PEEPi | = | Intrinsic positive end-expiratory pressure |
| PETCO2 | = | End-tidal carbon dioxide pressure |
| PS | = | Pressure Support |
| PS0 | = | Pressure support of 0 cm H2O |
| PS10 | = | Pressure support of 10 cm H2O of pressure support |
| Pmo | = | Mouth pressure |
| RR | = | Respiratory Rate |
| SaO2 | = | Oxygen saturation |
| TLC | = | Total Lung Capacity |
| VE | = | Minute Ventilation |
| VO2 | = | Oxygen consumption |
| VCO2 | = | Carbon dioxide production |
| VT | = | Tidal Volume |
| WLmax | = | Maximum work load / exercise capacity |
| WLpeak | = | Peak work load / exercise capacity |
| WOB | = | Work of breathing |
Introduction
Chronic obstructive pulmonary disease (COPD), principally defined by a progressive irreversible airflow limitation, is a major cause of morbidity and mortality and a source of substantial economic burden worldwide (Citation1). Although exercise training as part of pulmonary rehabilitation has been shown to improve exercise capacity and health related quality of life (Citation2-5), patients with COPD typically have a limited ability to perform high intensity exercise due to a heightened sense of dyspnea and/or leg fatigue secondary to reduced ventilatory capacity and skeletal muscle dysfunction (Citation6), respectively.
Noninvasive ventilatory support (NIVS) was proposed as an adjunct to exercise (Citation3,Citation7–12) in order to assist patients with COPD to reach higher exercise intensities and thereby to enhance the physiological benefits of exercise (Citation13,14).
Bi-level positive airway pressure (BiPAP) is a form of NIVS often used for providing ventilation as an adjunct to exercise training (Citation9,Citation12,Citation15-20). The standard BiPAP circuit consists of single-limb tubing for both inspiration and expiration, and includes an intentional standard disposable exhalation port located at the distal end of the circuit, which serves to eliminate exhaled gas. There is some evidence that use of a standard exhalation device with BiPAP may result in CO2 rebreathing in individuals with chronic respiratory failure (Citation21). Although some authors have recommended a non-rebreathing valve (NRV) for reduction of the CO2 rebreathing with BIPAP (Citation21,22), such a valve has also been shown to contribute to greater expiratory resistance and an increased work of breathing (WOB) (Citation22). Moreover, use of a NRV with BIPAP during exercise has not been found to necessarily provide immediate effects of improved exercise endurance or maximum exercise capacity (Citation9). In one study, exercising with a mouthpiece alone was reported to result in a shorter distance walked compared to unencumbered breathing. The same study also reported no difference between NIVS delivered using a standard exhalation valve and exercise with a mouthpiece alone (Citation23), putting to question the role of CO2 rebreathing during exercise with BiPAP delivered using a standard exhalation valve.
The aim of the current study was to evaluate the acute effect of BiPAP, in the form of pressure support of 0 and 10 cm H2O, on maximum exercise capacity and related physiological parameters, compared to no assist, in individuals with COPD.
Methods
Study participants
Ten clinically stable patients between 45 and 80 years of age, with a diagnosis of moderate-to-severe COPD (FEV1/FVC ≤ 0.7) (Citation24) were included in the study. Stable disease was operationally defined as unchanged respiratory symptoms, no change in medications and no exacerbation in the previous 4 weeks. Exclusion criteria: individuals with known cardiovascular, musculoskeletal or locomotor disease, alpha1-antitrypsin deficiency, and those requiring supplemental oxygen. Ethical approval was obtained from the hospital's ethics committee (IRB committee name and project approval number, HÙpital du SacrÈ-Coeur de MontrÈal, 2009_08_57), and all participants gave their written informed consent according to the Helsinki Declaration.
Procedure
This was a randomized, cross-over study comprising three separate experimental visits (Figure ).
Figure 1. Schematic representation of the experimental protocol. Inspiratory pressure support PS of 0 cmH2O and PS 10 cmH2O were administrated in a random order.

Visit 1 included anthropometric measurements, pulmonary function testing (25), a resting 12-lead electrocardiogram, and a cycling symptom limited-incremental cardiopulmonary exercise test (CPET) without any ventilator (noPS).
Visits 2 and 3 included a CPET with the participant assisted via BiPAP (Vision, Respironics) receiving 0 cm H2O and 10 cm H2O of pressure support (PS) in a randomized order. The expiratory positive airway pressure (EPAP) was set to 4 cm H2O, since the EPAP on the BiPAP Vision cannot be reduced below 4 cm H2O, and the inspiratory positive airway pressure (IPAP) was set to 14 cm H2O and 4 cm H2O to deliver 10 cm H2O and 0 cm H2O of PS, respectively. Each visit was separated by at least 48 hours and patients were asked to abstain from caffeine, smoking, heavy meals, alcohol, and major physical exertion on the day undergoing testing.
Pulmonary function tests
Spirometry, lung volumes, and respiratory muscle pressures were determined using a body plethysmograph (Medical Graphics, USA) (Citation25). Maximum inspiratory and expiratory muscle pressures were measured at FRC and TLC, respectively. Spirometry, lung volumes, maximum pressures, and single-breath diffusion capacity for carbon monoxide were compared with reported norms (Citation26,27).
Cardiopulmonary Exercise Testing
Exercise testing was performed on an electronically braked cycle ergometer (Lode, Groningen, Netherlands). CPET consisted of 6-minutes of steady-state resting breathing, followed by 1-minute of unloaded exercise, after which work rate was increased by 5 W each minute until a symptom limited end-point (Citation28). Pedaling rate was maintained between 50 and 70 rpm. At the end of the rest period, at each exercise workload, and at peak exercise participants performed an inspiratory capacity (IC) manoeuvre for determination of end-expiratory lung volume (EELV), and were asked to rate their dyspnea using the Borg scale (Citation29).
Instrumentation and measurement
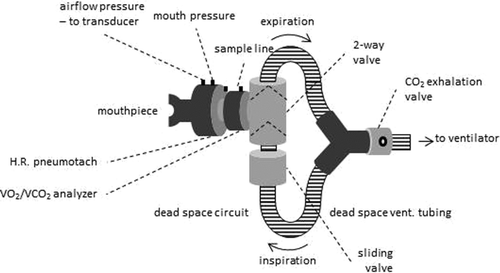
At rest and during exercise, participants wore nose clips and breathed through a mouthpiece connected in series with a pneumotachograph (Hans Rudolph, USA), a pulmonary gas exchange analyzer (Cosmed K4b2, Rome, Italy) and a 2-way valve (Hans Rudolph, USA). During visits 2 and 3, an additional sliding valve (Hans Rudolph), ventilator tubing with disposable exhalation valve and BiPAP ventilator, were added to the breathing circuit (Figure ).
Airflow was continuously measured using the pneumotachograph and mouth pressure (Pmo) via tubing connected to a differential pressure transducer (Biopac Systems, Santa Barbara, CA, USA). The airflow and Pmo signals were digitized using a 16-bit A/D converter at 100 Hz and stored for offline analysis. Oxygen consumption (VO2), carbon dioxide production (VCO2), heart rate (HR) and oxygen saturation (SpO2) were continuously measured using the portable gas analysis system (Cosmed K4b2, Rome, Italy).
Off-line data analysis
Tidal volume (VT) was obtained by digital integration of airflow, and was used to determined inspiratory and expiratory time, breathing cycle time, respiratory rate (RR), and minute ventilation (VE). Measured parameters from each breath were averaged over 30-s intervals. EELV was obtained by subtracting the IC volume from measures of total lung capacity previously obtained during pulmonary function testing (Citation30,31).
Maximum exercise capacity was operationally defined as the highest workload (WLmax) reached and maintained for at least 30 seconds. Isoload was defined as the lowest maximum exercise work load reached from any of the three exercise test conditions.
Statistical analysis
Differences in WLmax between the three CPETs and the other measured parameters at rest and peak exercise were examined using one-way repeated measures ANOVA and the Student-Newman-Keuls test for post hoc comparisons. The level of significance for all statistical tests was α ≤ 0.05. Results are expressed as means ± SD unless otherwise specified.
Results
Participant characteristics
Table provides the main characteristics of the ten study participants. Patients had moderate-to-severe airflow obstruction, exhibited static hyperinflation and a reduced diffusion capacity.
Table 1. Descriptive characteristics of patients
Ventilator pressures
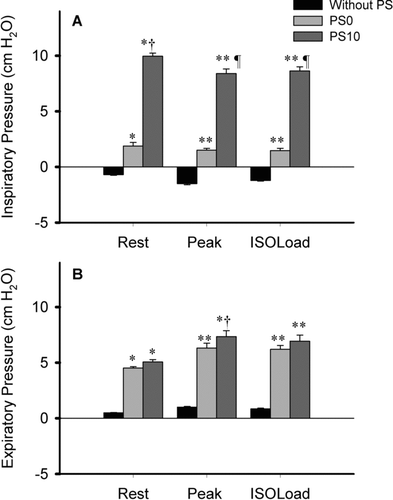
Mean inspiratory and expiratory Pmo during exercise without the ventilator (without/no PS) as well as with PS0 and PS10 are shown in Figure .
Figure 3. Mean inspiratory (panel A) and expiratory mouth pressure (panel B) at rest, peak exercise and isoload. Plots are average values ±SE obtained in the 10 patients. Without assist, mean Pmo was negative during inspiration, whereas it averaged +3 cm H2O and +10cm H2O during inspiration with PS0 and PS10, respectively. The expiratory Pmo was similar during PS0 and PS10, and was significantly higher than without assist. For post-hoc contrasts: *p ≤ 0.05 and **p ≤ 0.001, relative to without PS; † p ≤ 0.05, and ¶ p ≤ 0.001, relative to PS0.
The effect of NIVS on maximum exercise
WLmax was significantly lower during exercise performed with PS0 and PS10 compared to without PS (Table ). WLmax was lower with PS10 than without PS in 7 individuals, whereas it was unaltered in 3 (Figure ). Compared to PS0, 6 subjects reached a higher WLmax with PS10; however, the group difference did not achieve statistical significance.
Figure 4. Maximum exercise capacity reached during the three experimental conditions. Group mean values of maximum workload reached during symptom-limited incremental bicycle exercise without PS (black bar), with PS0 (light grey bar) and with PS10 (dark grey bar) are presented in the panel A. Individual values are shown in panel B. For post-hoc contrasts: **p ≤ 0.001, relative to without PS.
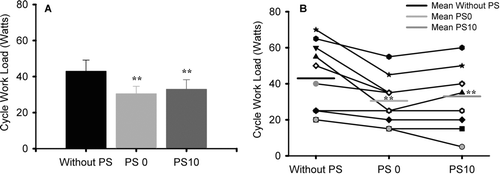
Table 2. Physiological parameters at rest (N = 10)
Table 3. Physiological parameters at peak exercise (N = 10)
Breathing pattern during rest and exercise
Average values for breathing pattern variables at rest and during exercise are shown in Tables and . Compared to without PS, VT was larger with PS10 at rest and peak exercise, whereas it was not different with PS0 in either condition. At rest, RR was similar with PS0 and without PS, whereas it was significantly higher with PS10, due to a shortened inspiratory and expiratory time. In contrast, at peak exercise, RR and other breath timing components were unaltered with the addition of the ventilator. The VE was significantly increased with PS10 compared to without PS or PS0, at rest and at peak exercise. At isoload, VE was higher with both PS0 and PS10 compared to without PS (Figure ).
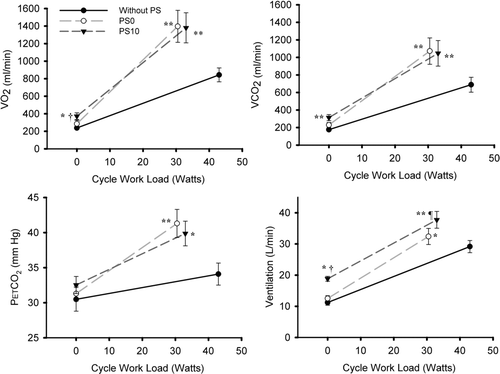
Figure 5. Metabolic parameters comparisons at rest and peak exercise were performed between the three experimental conditions of breathing without PS, with PS0 and with PS10. Plots are average values ±SE obtained in the 10 patients. For post-hoc contrasts: *p ≤ 0.05, relative to without PS; **p ≤ 0.001, relative to without PS; †p ≤ 0.05, relative to PS0.
Operational lung volumes
Resting EELV was marginally increased with PS10 compared to noPS (∼170 ml); in contrast, no difference in the EELV was found between PS0 and PS10. Whereas EELV increased by ∼500 ml during exercise without PS, there was no difference at peak exercise between the three conditions.
Gas exchange parameters
As illustrated in Figure , both VO2 and VCO2 were higher with the application of PS10 compared to without PS and PS0 at rest, whereas they were significantly higher at peak exercise and at isoload with both PS10 and PS0 compared to without PS. No difference in SaO2 or HR was observed between runs.
Exertional symptoms
Despite differences in maximum exercise capacity, the levels of dyspnea at peak exercise were similar for PS0 and PS10 (Figure ). Compared to noPS, dyspnea was significantly higher at isoload with both PS10 and PS0 (p = 0.009 and p = 0.003, respectively).
Figure 6. Dyspnea Borg score comparison at rest and peak exercise was made between the three conditions. *p ≤ 0.05, relative to without PS.
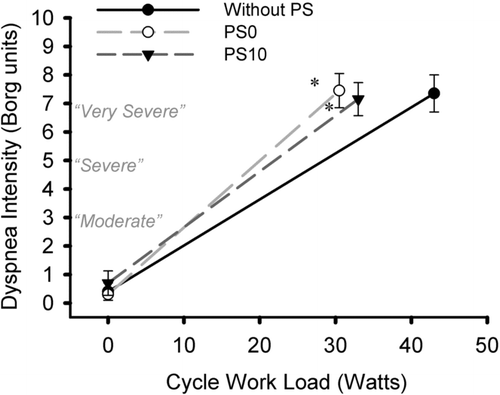
Maximum exercise capacity without ventilator was limited primarily by intolerable dyspnea in nine patients and by a combination of shortness of breath and leg discomfort in one patient. With PS10 and PS0, dyspnea was reported as the exercise limiting symptom by all patients.
Discussion
This is the first study to evaluate the effect of BiPAP, delivered using a standard exhalation valve during bicycle CPET, on maximum exercise capacity and related physiological parameters in patients with COPD. The main finding is that BiPAP (PS0 and PS10) resulted in a reduced maximum exercise capacity compared to exercise without the ventilator. Although PS10 resulted in a significant increase in VT and VE during exercise compared to exercise without the ventilator and PS0, both PS0 and PS10 resulted in larger increases in VO2, VCO2, and dyspnea compared to exercise without PS.
In the current study, the mode of NIVS and the PS setting of 10 cm H2O were selected based on the fact that these have been used as adjuncts to exercise in previous studies (Citation14,Citation32). BiPAP delivers two levels of positive pressure, which can be adjusted independently. However, the EPAP on the Vision cannot be lowered below 4 cm H2O, therefore the PS0 level which was used as the sham condition in the current study was essentially equivalent to a continuous positive airway pressure (CPAP) of 4 cm H2O. The BiPAP ventilator delivers ventilatory assist via single-limb tubing which can increase the risk of CO2 rebreathing (Citation21). Although the purpose of the standard exhalation port is to help eliminate exhaled CO2 gas and avoid rebreathing, Ferguson et al. (Citation21) reported an increased dead space ventilation and greater CO2 rebreathing in individuals breathing at rest. In the current study, compared to the response observed during exercise without PS, 0 and 10 cm H2O of PS delivered using BIPAP and a standard exhalation port resulted in a significantly lower WLmax (p ≤ 0.001).
Our findings are consistent with those of Highcock et al., (Citation23) whereby exercise endurance was lower during treadmill exercise with BiPAP delivered using a mouthpiece and standard exhalation valve compared to unencumbered breathing. Although the purpose of NIVS as an adjunct to exercise training is to increase exercise capacity by decreasing respiratory muscle work, evidence from our study and others (Citation23) suggests that this may not be accomplished with BiPAP delivered using a standard exhalation valve. Other non-rebreathing valves, such as the plateau valve, have been shown to be more effective in eliminating CO2 rebreathing in patients with respiratory failure (Citation21,22); however, such valves may also increase expiratory resistance and the work of breathing (Citation22). Moreover, incorporating a CO2-NRV within the BiPAP circuit was not found to provided immediate improvements in maximum walking capacity (Citation9). Whether or not a CO2-NRV can help increase WLmax has yet to be determined.
To standardize the intervention, it was decided a priori not to individualize IPAP and EPAP levels. However, higher IPAP levels tend to increase airflow and the amount of air that is exhaled into the ventilator hose, thereby increasing the CO2 rebreathing (Citation21,22). Ferguson and Gilmartin (Citation21) reported a reduced WOB when EPAP levels of 8 cm H2O or greater were administered to patients with respiratory failure. Moreover, the same study found that increasing the EPAP resulted in less CO2 rebreathing. However, setting EPAP above the PEEPi can also contribute to a rise in EELV and the WOB (Citation33,34). Although we did not measure PEEPi in our subjects, EELV at end-exercise was not increased by the addition of either PS0 or PS10 (Citation35).
Other studies have reported a marked increase in expiratory muscle activation with CPAP during exercise in patients with COPD (Citation13,Citation36), which could also have occurred in the current study with PS0. Thus, the lack of tailoring of both IPAP and EPAP could have contributed to ineffective ventilatory muscle unloading and to why PS10 and PS0 did not improve maximum exercise capacity in our patients.
In the present study, compared to exercise without PS, PS10 increased VE and VT both at rest and during exercise. These findings are consistent with studies which used a separate inspiratory and expiratory breathing circuit for assist delivery in patients with stable COPD (Citation14,Citation37,Citation38) and respiratory failure (Citation39), suggesting that ventilatory muscle unloading could have contributed to the breathing pattern changes observed in our study, independent of any potential effects from CO2 rebreathing (Citation40). Nevertheless, we cannot dismiss that rebreathing CO2 gas from the BiPAP single limb tubing may additionally have contributed to the observed breathing pattern changes, given that breathing CO2 is known to stimulate respiratory drive and increase both VT and VE (Citation40).
In a previous study investigating the effects of added external dead space on ventilatory and metabolic responses during exercise, healthy individuals were likewise reported to have an increased VE during moderate exercise as a result of an increased CO2 load. (Citation41). Whereas the RR tends to decrease when ventilator assist is increased (Citation14,Citation38), in our study it was increased only with PS10 at rest and was similar with or without assist during exercise. Similar breathing pattern changes have been reported in healthy older individuals breathing 3% CO2 while performing graded cycle ergometry to exhaustion (Citation42), indicating that some degree of CO2 rebreathing may have occurred in our subjects.
Metabolic parameters (VCO2, VO2, and PETCO2) increased progressively more for a given change in exercise workload with both PS0 and PS10, compared to exercise without a ventilator (Figure ). The increased VE and associated increased respiratory drive likely contributed to the higher metabolic demand, the development of dyspnea, and exercise termination in our participants. Although dyspnea was the same during all three sessions, it was reached earlier during exercise with PS10 and PS0 (Figure ).
The slope of the VE response for a given VCO2 was lower with both PS0 and PS10 compared to exercise without PS (Figure ). In line with this, Poon (37) showed that CO2 stimulation results in a less steep VE-VCO2 relationship compared to eupneic breathing during exercise in older individuals. Mechanical ventilatory limitations during exercise, which are more pronounced in individuals with severe COPD and in older individuals (Citation42-44) have been implicated in this phenomena. Reduced chemoreceptor responsiveness in hypercapnic patients can also suppress the VE response (Citation45). Thus despite an increased CO2 stimulation during exercise with the ventilator, our subjects appear to have been unable to adequately increase ventilation to meet the increased demands of breathing.
Methodological considerations
A number of limitations must be considered when interpreting the results. First, the possibility of type II error cannot be dismissed; the small sample size likely limited our ability to observe an effect of PS10 compared to PS0. Second, our exclusion criteria, especially the exclusion of individuals who receive supplemental O2, limit our ability to generalize the findings. Our findings might have been different had patients with more severe COPD been recruited; it is possible that such individuals may have had a greater need for ventilator assistance and thus benefitted more from its use as an adjunct during exercise. Third, exercise without the ventilator (visit 1) always preceded exercise with NIVS.
Although this could have affected the findings, it is more likely that a learning effect related to exercise performance would have contributed to improving WLmax rather than reducing it as was observed in the current study. Whether a lack of familiarity with NIVS might have contributed to the decreased exercise capacity is unknown. There is no consensus in the literature regarding this; some studies have suggested having at least one preliminary visit to familiarize the patient with the equipment (46,Citation47), whereas others have felt this to be unnecessary (Citation48).
Clinical implications
The most relevant practical implication is that using a Vision BiPAP ventilator to deliver PS via a mouthpiece and a standard valve incorporated into the ventilatory circuit does not increase exercise capacity in COPD patients. It is conceivable that if early mobilization of a patient following acute exacerbation is performed with the patient receiving assist from the BiPAP system using a standard exhalation valve, such a system might not respond to the patient's ventilatory needs and could potentially limit their walking and exercise capacity. Several studies investigating the effect of PS on exercise performance found that BiPAP administration during exercise training as part of pulmonary rehabilitation program increased exercise capacity post training. Most of those studies did not report the use of a CO2 NRV. In view of our findings, we wonder if instead of unloading the respiratory muscles, such BiPAP administration might have additionally worked the respiratory muscles during the exercise training, which ultimately helped to increase post-training maximum exercise. Investigating the potential advantages and disadvantages of BiPAP versus other modes of NIVS would also be of importance prior to their inclusion as an adjunct to exercise.
Conclusion
In conclusion, the current study found that in patients with COPD, maximum exercise capacity was not increased with the administration of 10 cm H2O of pressure support delivered using BiPAP with a standard exhalation valve, compared to no ventilator. Although there was a tendency to increase maximum exercise capacity with PS10, compared to PS0, we were unable to demonstrate a significant difference probably due to the lack of power. Our findings may be related to a combination of a lack of individual tailoring of both the IPAP and EPAP, the remaining 4 cm H2O EPAP support, and CO2 rebreathing occurring with use of the standard disposable exhalation port and breathing circuit. Whether incorporating a CO2 plateau valve can alter the findings remains to be elucidated.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
A. Moga (guarantor) participated in the study conception, design, data acquisition and analysis, interpretation of the results, wrote the first draft of the manuscript, reviewed and approved the manuscript. Dr. M. de Marchie contributed to the clinical supervision of the studies, interpretation of the results, and approving the manuscript. Dr. D. Saey contributed to review and final approval of the manuscript. Dr. J. Spahija (guarantor) contributed to the study conception, design, data acquisition, interpretation of the results, writing the manuscript and approving the manuscript.
Acknowledgment
The authors would like to thank V. Rose from the Jewish Rehabilitation Hospital, Laval and the Pneumology Department at HÙpital du SacrÈ-Cúur de MontrÈal for their assistance with patient recruitment and PFT assessment.
References
- Saetta M, Finkelstein R, Cosio MG. Morphological and cellular basis for airflow limitation in smokers. Eur Respir J 1994; 7:1505–1515.
- Troosters T, Casaburi R, Gosselink R, et al. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172:19–38.
- Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173:1390–1413.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007; 131:4S–42S.
- O'Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease - 2007 update. Can Respir J 2007; 14 Suppl B:5B–32B.
- Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 1998; 30:1467–1474.
- O'Donnell DE, Sanii R, Younes M. Improvement in exercise endurance in patients with chronic airflow limitation using continuous positive airway pressure. Am Rev Respir Dis 1988; 138:1510–1514.
- Ambrosino N, Strambi S. New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J 2004; 24:313–322.
- Johnson JE, Gavin DJ, Adams-Dramiga S. Effects of training with heliox and noninvasive positive pressure ventilation on exercise ability in patients with severe COPD. Chest 2002; 122:464–472.
- Costes F, Agresti A, Court-Fortune I, et al. Noninvasive ventilation during exercise training improves exercise tolerance in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2003; 23:307–313.
- van ‘t Hul A, Gosselink R, Hollander P, et al. Training with inspiratory pressure support in patients with severe COPD. Eur Respir J 2006; 27:65–72.
- Reuveny R, Ben-Dov I, Gaides M, et al. Ventilatory support during training improves training benefit in severe chronic airway obstruction. Isr Med Assoc J 2005; 7:151–155.
- Maltais F, Reissmann H, Gottfried SB. Pressure support reduces inspiratory effort and dyspnea during exercise in chronic airflow obstruction. Am J Respir Crit Care Med 1995; 151:1027–1033
- van ‘t Hul A, Gosselink R, Hollander P, et al. Acute effects of inspiratory pressure support during exercise in patients with COPD. Eur Respir J 2004; 23:34–40.
- Costa D, Toledo A, Silva AB, et al. Influence of noninvasive ventilation by BiPAP on exercise tolerance and respiratory muscle strength in chronic obstructive pulmonary disease patients (COPD). Rev Lat Am Enfermagem 2006; 14:378–382.
- Borghi-Silva A, Reis MS, Mendes RG, et al. Noninvasive ventilation acutely modifies heart rate variability in chronic obstructive pulmonary disease patients. Respir Med 2008; 102:1117–1123.
- Borghi-Silva A, Di Thommazo L, Pantoni CB, et al. Non-invasive ventilation improves peripheral oxygen saturation and reduces fatigability of quadriceps in patients with COPD. Respirology 2009; 14:537–544.
- Borghi-Silva A, Mendes RG, Toledo AC, et al. Adjuncts to physical training of patients with severe COPD: oxygen or noninvasive ventilation? Respir Care 2010; 55:885–894.
- Toledo A, Borghi-Silva A, Sampaio LM, et al. The impact of noninvasive ventilation during the physical training in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). Clinics (Sao Paulo) 2007; 62:113–120.
- Kohnlein T, Schonheit-Kenn U, Winterkamp S, et al. Noninvasive ventilation in pulmonary rehabilitation of COPD patients. Respir Med 2009; 103:1329–1336.
- Ferguson GT, Gilmartin M. CO2 rebreathing during BiPAP ventilatory assistance. Am J Respir Crit Care Med 1995; 151:1126–1135.
- Lofaso F, Brochard L, Touchard D, et al. Evaluation of carbon dioxide rebreathing during pressure support ventilation with airway management system (BiPAP) devices. Chest 1995; 108:772–778.
- Highcock MP, Shneerson JM, Smith IE. Increased ventilation with NiIPPV does not necessarily improve exercise capacity in COPD. Eur Respir J 2003; 22:100–105.
- Disease GIfCOL. Global Strategy for the Diagnosis, Management and Prevention of COPD (Revised 2011), 2011; http://www.goldcopd.org.
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Knudson RJ, Slatin RC, Lebowitz MD, et al. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis 1976; 113:587–600.
- Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 1969; 99:696–702.
- ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167:211-277.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14:377–381.
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164:770–777.
- Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997; 156:55–59.
- Keilty SE, Ponte J, Fleming TA, et al. Effect of inspiratory pressure support on exercise tolerance and breathlessness in patients with severe stable chronic obstructive pulmonary disease. Thorax 1994; 49:990–994.
- Bianchi L, Foglio K, Porta R, et al. Lack of additional effect of adjunct of assisted ventilation to pulmonary rehabilitation in mild COPD patients. Respir Med 2002; 96:359–367.
- Ranieri VM, Giuliani R, Cinnella G, et al. Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilation. Am Rev Respir Dis 1993; 147:5–13.
- Moga AM, de Marchie M, Delisle S, et al. The effect of BiPAP on symptom-limited maximum exercise in COPD patients. Euro Respir J 2011; 38:4809.
- Petrof BJ, Calderini E, Gottfried SB. Effect of CPAP on respiratory effort and dyspnea during exercise in severe COPD. J Appl Physiol 1990; 69:179–188.
- Poon CS. Potentiation of exercise ventilatory response by airway CO2 and dead space loading. J Appl Physiol 1992; 73:591–595.
- Vanpee D, El Khawand C, Rousseau L, et al. Effects of nasal pressure support on ventilation and inspiratory work in normocapnic and hypercapnic patients with stable COPD. Chest 2002; 122:75–83.
- Passam F, Hoing S, Prinianakis G, et al. Effect of different levels of pressure support and proportional assist ventilation on breathing pattern, work of breathing and gas exchange in mechanically ventilated hypercapnic COPD patients with acute respiratory failure. Respiration 2003; 70:355–361.
- Read DJ. A clinical method for assessing the ventilatory response to carbon dioxide. Australas Ann Med 1967; 16:20–32.
- Ward S, and Whipp B. Ventilatory control during exercise with increased external dead space. J Appl Physiol Respir Environ Exerc Physiol 1980; 48:225–231.
- Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol 1997; 82:746–754.
- Mead J, Milic-Emili J, Turner JM. Factors limiting depth of a maximal inspiration in human subjects. J Appl Physiol 1963; 18:295–296.
- Clark JM, Sinclair RD, Lenox JB. Chemical and nonchemical components of ventilation during hypercapnic exercise in man. J Appl Physiol 1980; 48:1065–1076.
- Scano G, Spinelli A, Duranti R, et al. Carbon dioxide responsiveness in COPD patients with and without chronic hypercapnia. Eur Respir J 1995; 8:78–85.
- Dolmage TE, Goldstein RS. Proportional assist ventilation and exercise tolerance in subjects with COPD. Chest 1997; 111:948–954.
- Bianchi L, Foglio K, Pagani M, et al. Effects of proportional assist ventilation on exercise tolerance in COPD patients with chronic hypercapnia. Eur Respir J 1998; 11:422–427.
- Hernandez P, Maltais F, Gursahaney A, et al. Proportional assist ventilation may improve exercise performance in severe chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2001; 21:135–142.