Abstract
It is well-established that COPD patients have a burden of vascular disease that cannot be fully-explained by smoking history but the mechanistic links between atherosclerosis and pulmonary disease in COPD patients are not well-understood. Moreover, in ex-smokers without symptoms or other evidence of COPD, subclinical pulmonary and vascular disease, although potentially present, has not been described or evaluated. Hence our aim was to use sensitive three-dimensional (3D) pulmonary and carotid imaging to quantify pulmonary airway/parenchyma abnormalities and atherosclerosis in ex-smokers without airflow limitation or symptoms consistent with COPD. We evaluated 61 subjects without airflow limitation including 34 never- (72 ± 6 years) and 27 ex-smokers (73 ± 9 years), who provided written informed consent to spirometry, plethysmography, 3He magnetic resonance imaging (MRI) and carotid ultrasound (US) and, for ex-smokers alone, thoracic X-ray computed tomography (CT). Ex-smokers had significantly greater 3He ventilation defect percent (VDP = 7%, p = 0.001) and carotid total plaque volume (TPV = 250 mm3, p = 0.002) than never-smokers, although there were no significant differences for spirometry or plethysmography, and CT airway and emphysema measurements were normal. There were univariate relationships for 3He VDP with carotid intima media thickness (IMT, r = 0.42, p = 0.004), TPV (r = 0.41, p = 0.006) and vessel wall volume (VWV, r = 0.40, p = 0.007). Multivariate models that included age, BMI, FEV1, DLCO and VDP showed that only VDP significantly predicted IMT (β = 0.41, p = 0.001), VWV (β = 0.45, p = 0.003) and TPV (β = 0.38, p = 0.005). In summary, there was imaging evidence of mild airways disease and carotid plaque burden that were related and significantly greater in ex-smokers without airflow limitation than in never-smokers.
Introduction
Cardiovascular disease is the single largest cause of hospitalization in patients with mild and moderate chronic obstructive pulmonary disease (COPD), and after lung cancer, the leading cause of death (Citation1, 2). In addition, in COPD patients, there is a dose-response relationship for pulmonary structure-function abnormalities with carotid atherosclerosis (Citation3–8), coronary artery calcification (Citation9–12) and vascular dysfunction (Citation13–16). Although these studies have shown the presence of cardiovascular disease in patients with COPD that cannot be explained by smoking history alone (Citation3, 4, Citation6–8, Citation17), the mechanisms by which cardiovascular disease may be accelerated in COPD are not clear, nor is our understanding of these relationships in early or milder subclinical stages.
Although the diagnosis and monitoring of COPD is mainly based on spirometry measurements of airflow obstruction (Citation18, 19), such measurements cannot fully characterize the pathophysiological changes that occur in obstructive lung disease (Citation20). These include regional small-airways disease and microstructural changes in the terminal bronchi and parenchyma. In this regard, regional imaging measurements provided by hyperpolarized 3He magnetic resonance imaging (MRI) are highly sensitive to pulmonary abnormalities in asymptomatic ex-smokers (Citation21, 22) and never-smokers with second-hand smoke exposure (Citation23). Similarly, airway morphology and parenchyma density X-ray computed tomography (CT) measurements from the ECLIPSE (Citation24) and COPDGene (Citation25) studies showed the utility of CT phenotypes (Citation26) for stratifying COPD patients (Citation27–29).
Cardiovascular disease, predominated by large vessel atherosclerosis, is associated with obstructive lung disease (Citation30, 31) that can be regionally and quantitatively evaluated using carotid ultrasound (US). The burden of atherosclerosis can be quantified (Citation32) using carotid intima-media thickness (IMT) measurements and this is believed to reflect medial hypertrophy and intima abnormalities, and importantly, IMT correlates with cardiovascular outcomes (Citation32, 33). In a similar manner, three-dimensional ultrasound (3DUS) carotid atherosclerosis measurements (Citation34) of carotid wall and plaque abnormalities are sensitive predictors of cardiovascular risk (Citation35–37) and in some patients, these provide a better estimate of plaque burden and risk than IMT alone (Citation37).
There is a paucity of direct evidence relating subclinical lung disease and atherosclerosis in otherwise healthy ex-smokers. To provide a better understanding of the relationship between carotid atherosclerosis and pulmonary abnormalities common to ex-smokers with obstructive lung disease, our objective was to acquire highly sensitive pulmonary and carotid 3D imaging measurements in never- and ex-smokers with normal pulmonary function. We hypothesized that quantitative 3D imaging phenotypes would provide a way to tease out potential relationships for early or mild sub-clinical emphysema or airways disease with atherosclerosis in subjects at risk.
Methods
Study subjects
Ex-smokers (310 pack-year smoking history) without symptoms or a physician-diagnosis of COPD and normal spirometry (FEV1/FVC ≥ 70%) as well as never-smokers (≤1 pack-year smoking history) with no history of chronic respiratory or significant or uncontrolled cardiovascular disease between 50–90 years of age were recruited. These subjects enrolled in response to advertisements placed within the community and at local healthy ageing exercise and atherosclerosis prevention clinics. All subjects provided written informed consent to a protocol approved by a local research ethics board and Health Canada.
Spirometry and plethysmography
Spirometry, plethysmography and diffusing capacity of carbon monoxide (DLCO) measurements were performed according to the American Thoracic Society guidelines (Citation19). An ndd EasyOne spirometer (ndd Medizintchnik AG, Zurich, Switzerland) was used to measure FEV1 and FVC. Whole body plethysmography was performed for lung volumes and DLCO measurements were recorded using a gas analyzer (MedGraphics Corporation, St. Paul, Minnesota, USA).
Image acquisition
High-resolution B-mode ultrasound (US) images were acquired (ATL HDI 5000; Philips, Bothel, Washington, USA) as previously described (Citation38) using a 50 mm L12-5 MHz transducer (frequency = 8.5 MHz, Philips). Gain, focal points and time-depth compensation were optimized by the sonographer taking into consideration neck size, carotid anatomy and tissue depth. Two dimensional images were reconstructed into a 3D volume as previously described (Citation39, 40). MRI was performed on a whole body 3.0 Tesla Discovery 750MR (General Electric Health Care, Milwaukee, Wisconsin, USA) MRI system. 1H and 3He MRI were performed as previously described (Citation41) with the subject in an inspiration breath-hold (FRC+1L). For ex-smokers only, computed tomography (CT) was acquired within 10 minutes of MRI and at the same lung volume (FRC+1L) to ensure similar parenchymal distension in a method previously described (Citation41).
Image analysis
Carotid IMT was measured from the longitudinal plane of the 3DUS volume using Prowin 24.0 software (Medical Technologies International Inc., Palm Desert, California, USA) as previously described (Citation42). Carotid total plaque volume (TPV) and vessel wall volume (VWV) were measured as previously described (Citation43).
3He MRI ventilation defect percent (VDP) and apparent diffusion coefficients (ADC) were measured using semi-automated segmentation generated using custom-built software in MATLAB R2007b (The Math-works Inc., Natick, Massachusetts, USA) as previously described (Citation44, 45). CT volumes were analyzed using Pulmonary Workstation 2.0 (PW2, VIDA Diagnostics Inc., Coralville, Iowa, USA). Pulmonary CT images were analyzed for airway dimensions including wall area percent (WA%), lumen area (LA) and standardized wall thickness of airways with an inner perimeter of 10 mm (Pi10) (Citation46). In addition, the relative area of the lung with CT attenuation values less than -950HU (RA950) and total airway count were also measured using PW2.
Statistics
Normality of data was tested using the Shapiro–Wilk test and when significant, the Mann-Whitney U-test for nonparametric data was performed using SPSS Statistics V20.0 (SPSS Inc, Chicago, Illinois, USA). Unpaired two tailed t-test comparisons were performed using GraphPad Prism V4.0 (GraphPad Software Inc, California, USA) and Welch's correction used when the F-test for equal variances was significant. The Holm-Bonferroni correction (Citation47) was performed for multiple unpaired t-test comparisons. Multiple regression analyses were performed in SPSS to determine the relationship between carotid US measurements with 3He MRI VDP and pulmonary function parameters. Partial correlations were computed using SPSS. Multiple regression and correlation models were adjusted for age, BMI and DLCO since these parameters are established risk factors for cardiovascular and pulmonary diseases (Citation8, Citation48). Results were considered significant when the probability of two-tailed type I error was less than 5% (p ≤ 0.05) and summary data are presented as mean ± SD unless otherwise indicated.
Results
As shown in Table , 61 subjects including 27 ex-smokers (73 ± 9 years, 18 male) and 34 never-smokers (72 ± 6 years, 18 male) were evaluated. Except for BMI, (p = 0.001) there were no significant differences between subject groups for demographic characteristics.
Table 1. Demographic, pulmonary function, thoracic CT and carotid ultrasound data for all study subjects
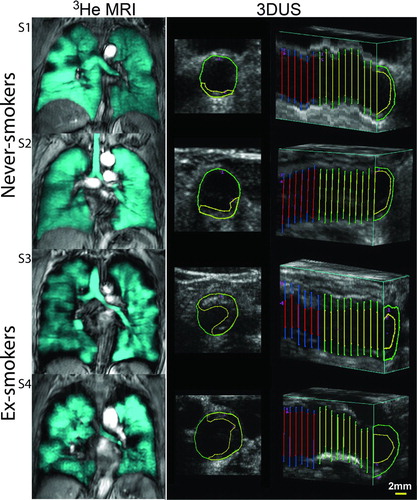
Figure shows 3He MRI center coronal slices and 3DUS axial and longitudinal images for representative ex- and never-smokers. 3He MRI ventilation images show homogeneous ventilation in never-smokers, whereas there is heterogeneous signal intensity with visually obvious ventilation defects in ex-smokers. 3DUS axial images show carotid plaque that is qualitatively greater in the two ex-smokers.
Figure 1. Representative 3He MRI and 3DUS images for two never-smokers (S1 and S2) and two ex-smokers (S3 and S4). Never-smoker S1 is a 67-year-old female with FEV1 = 120%, FEV1/FVC = 0.77, 3He MRI VDP = 3%, TPV = 20 mm3 and IMT = 0.79 mm. Never-smoker S2 is a 68-year-old male with FEV1= 93%, FEV1/FVC = 0.79, 3He MRI VDP = 2%, TPV = 30 mm3 and IMT = 0.73 mm. Ex-smoker S3 is a 79-year-old female with FEV1 = 88%, FEV1/FVC = 0.71, VDP = 8%, TPV = 500 mm3 and IMT = 0.94 mm. Ex-smoker S4 is a 85-year-old male with FEV1 = 139%, FEV1/FVC = 0.79, VDP = 8%, TPV = 340 mm3 and IMT = 0.96 mm. The axial 3DUS image of the common carotid artery shows the intima-lumen boundary in green and carotid plaque-lumen boundary in yellow. The longitudinal 3DUS carotid image shows IMT segmentation of the common carotid artery.
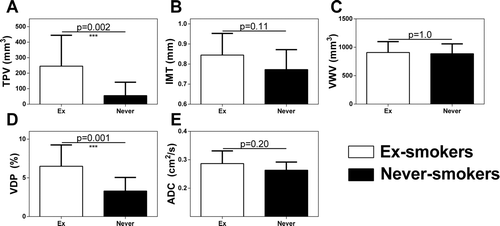
Quantitative results are provided in Figure and Table that show that the ex-smokers subgroup had significantly greater TPV, (250 ± 200 mm3, p = 0.002) and 3He MRI VDP, (7 ± 3%, p = 0.001) than never-smokers. No significant differences were observed for VWV (p = 1.0), IMT (p = 0.11), ADC (p = 0.20) or FEV1 (p = 0.89). For ex-smokers, CT mean RA950 (1.5 ± 1.4%), airway wall thickness at an internal perimeter of 10 mm (Pi10, 4.1 ± 0.17 mm), WA% (61 ± 2%), and LA (17 ± 11 mm2) were within ≠normal range as previously published (Citation49, 50).
Figure 2. Imaging phenotypes in asymptomatic ex-smokers and never-smokers. Ex-smokers have significantly greater: A) TPV (p = 0.002) and D) VDP (p = 0.001) than never-smokers. No significant differences were observed for: B) IMT (p = 0.11), C) VWV (p = 1.0), or E) ADC (p = 0.20). Holm-Bonferroni corrected p values are shown.
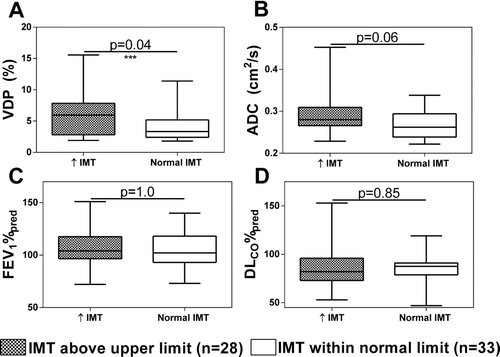
Using previously established age-normalized values for IMT (Citation51), 28 subjects (15 ex-smokers and 13 never-smokers, 28/61 = 46%) exceeded the upper limit of normal for IMT and 33 subjects (12 ex-smokers and 21 never-smokers, 33/61 = 54%) had normal IMT. As shown in Figure , subjects with abnormally elevated IMT had significantly greater VDP (p = 0.04) than subjects with normal IMT, but the two subgroups were not significantly different with respect to ADC (p = 0.06), FEV1%pred (p = 1.0) or DLCO%pred (p = 0.85). CT measurements for the 15 ex-smokers with abnormal IMT were not significantly different than ex-smokers with normal IMT for RA950 (p = 0.96), WA% (p = 0.66) or LA (p = 0.63).
Figure 3. Comparisons between never- and ex-smokers with IMT 3 age-related upper limit of normal (IMT >97.5% confidence interval (CI)) and subjects with IMT ≤ age-related normal limit (IMT ≤ 97.5 CI). A) Subjects with abnormally elevated IMT have statistically significantly different VDP (p = 0.04) than subjects with normal IMT. No significant differences were observed for: B) ADC (p = 0.06), C) FEV1 (p = 1.0) or D) DLCO (p = 0.85). Comparisons displayed are Holm–Bonferroni corrected p values.
As shown in Figure , univariate Pearson correlations between 3He MRI VDP and carotid ultrasound measurements revealed a moderate significant relationship for VDP with carotid IMT (r = 0.42, p = 0.004), TPV (r = 0.41, p = 0.006) and VWV (r = 0.40, p = 0.007). Multivariate regression models for IMT, VWV and TPV are provided in Table . VDP was a significant determinant of IMT (β = 0.41, p = 0.001), VWV (β = 0.45, p = 0.003) and TPV (β = 0.38, p = 0.005). For the ex-smokers alone, CT-derived measurements including RA950 and airway count, WA%, LA and Pi10 did not correlate with carotid US measurements of IMT, TPV and VWV.
Figure 4. Relationships for carotid atherosclerosis and pulmonary VDP. Significant relationships between 3He MRI VDP and: A) carotid IMT (r = 0.42, p = 0.004), B) TPV (r = 0.41, p = 0.006), and, C) VWV (r = 0.40, p = 0.007).
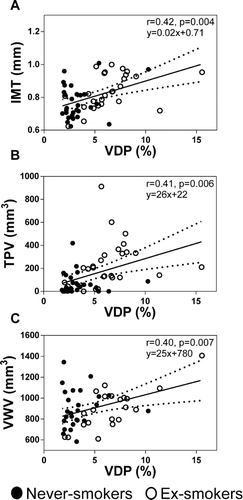
Table 2. Multiple regression models for IMT, VWV and TPV
Discussion
We tested the hypothesis that atherosclerosis and pulmonary structure-function measurements were significantly different in ex-smokers without airflow limitation or symptoms consistent with COPD than in never-smokers. We observed: 1) ex-smokers had significantly greater carotid TPV and worse 3He VDP than never-smokers, but were not significantly different with respect to pulmonary function test measurements, 2) 28 subjects including 15 ex- and 13 never-smokers with abnormally elevated IMT had significantly worse VDP but ADC, FEV1, DLCO and CT measurements were not different compared to subjects with normal IMT, 3) 3He VDP was significantly related to carotid atherosclerosis measurements (IMT, VWV and TPV) but pulmonary function tests were not, and 4), multivariate regression models showed that VDP, a measurement of small airway function, was the only significant determinant of carotid artery IMT, VWV and TPV.
In these older subjects without COPD, we observed, as expected, that spirometry and plethysmography measurements were not different in ex- and never-smokers, but importantly 3He VDP was significantly worse for ex-smokers. This finding is concordant with previous findings in asymptomatic ex-smokers that showed significant differences in 3He MRI ventilation defects compared to never-smokers (Citation22). Although we did not observe differences between subgroups for 3He MRI ADC, abnormally elevated 3He ADC has been previously reported in asymptomatic smokers (Citation21) and never-smokers with second-hand smoke exposure (Citation23). Our finding of abnormal 3He ventilation in the absence of abnormal DLCO, ADC or CT RA950 in ex-smokers is in agreement with the notion that ìsilentî airway disease (Citation20) and small airway remodeling (Citation52) may be the source of ventilation defects in these subjects.
We think that the significant differences observed for VDP in the absence of differences in DLCO, ADC or RA950 suggests mild, sub-clinical airway abnormalities, although we must point out that a definitive structure-function etiology for 3He ventilation defects (Citation46, Citation53, Citation54) is yet to be determined. It is also worth noting that for the healthy ex-smokers evaluated here, there was no CT evidence of emphysema or airways disease and such values were in agreement with those reported in healthy non-smokers in the COPDGene study (Citation49, 50). This finding underscores the sensitivity of 3He MRI ventilation measurements for detecting functional abnormalities that may not be apparent using CT or spirometry.
Previous work in COPD patients (3, 4, 6, 7, 17, 55, 56) reported a relationship for IMT with FEV1 and emphysema measurements, but this was not observed here in ex-smokers without COPD. We were likely underpowered to detect differences in IMT between subgroups as these previous studies investigating IMT in COPD and healthy older subjects used sample sizes ranging from 305 to 14,480 subjects. On the other hand, significantly elevated 3DUS TPV in the ex-smoker subgroup was consistent with previous work (Citation35, 36) that showed smaller sample sizes can be used when employing 3D measurements of plaque as compared to IMT. Although no difference was observed for IMT between ex- and never-smokers, when age-normalized IMT values were used to stratify subjects (Citation51), subjects with abnormal IMT had significantly worse VDP. This finding suggests that in both never- and ex-smokers with elevated IMT, there is evidence of mild, subclinical airways disease that may be related to factors other than cigarette smoking.
We also observed significant univariate relationships for 3He VDP with carotid artery IMT, TPV and VWV, consistent with previous studies that showed relationships between spirometry measurements and carotid IMT (3, 4, 6, 17, 55, 56), although this is the first report of such relationships in subjects with normal pulmonary function. Similarly, multivariate regression models that controlled for cardiovascular and pulmonary disease risk factors showed that VDP is a significant predictor of carotid artery IMT, VWV and TPV. It is also important to note that carotid TPV and VWV provide measurements of intima echogenic (TPV and VWV) and echolucent (VWV) plaque (Citation38, Citation57, Citation58). Hence, these relationships between VDP and IMT, VWV and TPV suggest that in this relatively small group of never- and ex-smokers, mild, subclinical airways disease may be related to carotid plaque burden and wall thickening.
Although it was recently shown that COPD patients may have atherosclerotic plaque characteristics that make them more vulnerable to rupture (Citation6), we did not evaluate carotid plaque composition here. The significant carotid plaque burden quantified in some of the ex-smokers investigated here may be amenable to more complex image methods (Citation59) to develop a better understanding of plaque composition/texture and outcomes. We acknowledge that the main limitation of this study was the relatively small sample size of never- and ex-smokers, and that this may have limited our power to detect any potential differences in IMT and FEV1 between subgroups.
Certainly, one of the strengths of 3D imaging is that significantly different measurements can be detected in small groups of subjects, because of the high dynamic range and sensitivity of 3D measurements to structure-function abnormalities. This is an important consideration when powering studies to detect differences in small groups of subjects. Finally, we cannot infer the temporal or causal nature of carotid and pulmonary abnormalities in this cross-sectional evaluation. Questions related to ìwhich came firstî still need to be answered in longitudinal natural disease and intervention studies (Citation60).
In conclusion, we acquired sensitive 3D imaging measurements of atherosclerosis and pulmonary structure-function in order to illuminate potential relationships between early or mild sub-clinical pulmonary disease with atherosclerosis in otherwise healthy ex-smokers. Although a number of studies have provided evidence that pulmonary and carotid abnormalities are both present and related in COPD patients, here, this important relationship was shown in never- and ex-smokers with normal pulmonary function. Although the clinical relevance of these observations is not yet clear, these data suggest that lung abnormalities and carotid atherosclerosis in never- and ex-smokers without airflow limitation may be directly related. As our knowledge of co-morbid lung and vascular disease increases, such findings may potentially have implications for patient management decision strategies in preclinical stages.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
We thank D. Buchanan and S. Blamires, CCRC, RPT, for 3DUS measurements, clinical coordination and clinical database management and T. Szekeres, RTMR, for MRI of research volunteers.
D. Pike was supported by a Canadian Institutes of Health Research (CIHR) Strategic Training Program and G. Parraga gratefully acknowledges support from a CIHR New Investigator Award. Ongoing research funding from a CIHR Team Grant CIF#97687 (Thoracic Imaging Network of Canada, TinCAN) is also gratefully acknowledged.
References
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005; 142(4):233–239.
- Sidney S, Sorel M, Quesenberry CP, Jr., DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 2005; 128(4):2068–2075.
- Iwamoto H, Yokoyama A, Kitahara Y, Ishikawa N, Haruta Y, Yamane K, et al. Airflow limitation in smokers is associated with subclinical atherosclerosis. Amer J Respir Crit Care Med 2009;179(1):35–40.
- Barr RG, Ahmed FS, Carr JJ, Hoffman EA, Jiang R, Kawut SM, et al. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Euro Respir J 2012; 39(4):846–854.
- Engstrom G, Hedblad B, Valind S, Janzon L. Asymptomatic leg and carotid atherosclerosis in smokers is related to degree of ventilatory capacity: longitudinal and cross-sectional results from ‘Men born in 1914’, Sweden. Atherosclerosis 2001; 155(1):237–243.
- Lahousse L, van den Bouwhuijsen QJ, Loth DW, Joos GF, Hofman A, Witteman JC, et al. Chronic obstructive pulmonary disease and lipid core carotid artery plaques in the elderly: the Rotterdam Study. Amer J Respir Crit Care Med 2013; 187(1):58–64.
- van Gestel YR, Flu WJ, van Kuijk JP, Hoeks SE, Bax JJ, Sin DD, et al. Association of COPD with carotid wall intima-media thickness in vascular surgery patients. Respir Med 2010; 104(5):712–716.
- Frantz S, Nihlen U, Dencker M, Engstrom G, Lofdahl CG, Wollmer P. Atherosclerotic plaques in the internal carotid artery and associations with lung function assessed by different methods. Clin Physiol Funct Imag 2012; 32(2):120–125.
- Dransfield MT, Huang F, Nath H, Singh SP, Bailey WC, Washko GR. CT emphysema predicts thoracic aortic calcification in smokers with and without COPD. COPD 2010; 7(6):404–410.
- Chae EJ, Seo JB, Oh YM, Lee JS, Jung Y, Lee SD. Severity of systemic calcified atherosclerosis is associated with airflow limitation and emphysema. J Comp Assist Tomogr 2013; 37(5):743–749.
- McAllister DA, MacNee W, Duprez D, Hoffman EA, Vogel-Claussen J, Criqui MH, et al. Pulmonary function is associated with distal aortic calcium, not proximal aortic distensibility. MESA lung study. COPD 2011; 8(2):71–78.
- Rasmussen T, Kober L, Pedersen JH, Dirksen A, Thomsen LH, Stender S, et al. Relationship between chronic obstructive pulmonary disease and subclinical coronary artery disease in long-term smokers. Euro Heart J Cardiovasc Imag 2013; 14(12):1159–1166.
- Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. New Engl J Med 2012; 367(10):913–921.
- Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Amer J Respir Crit Care Med 2007; 176(12):1200–1207.
- Cinarka H, Kayhan S, Gumus A, Durakoglugil ME, Erdogan T, Ezberci I, et al. Arterial stiffness measured by carotid femoral pulse wave velocity is associated with disease severity in chronic obstructive pulmonary disease. Respiratory Care 2014; 59(2):274–280.
- Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2007; 175(12):1259–1265.
- Kim SJ, Yoon DW, Lee EJ, Hur GY, Jung KH, Lee SY, et al. Carotid atherosclerosis in patients with untreated chronic obstructive pulmonary disease. Inter J Tubercul Lung Dis 2011; 15(9):1265–1270, i.
- Karkhanis VS, Joshi JM. Spirometry in chronic obstructive lung disease (COPD). J Asso Phys India 2012; 60 Suppl:22–26.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Euro Respir J 2005; 26(2):319–338.
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. New Engl J Med 2004; 350(26):2645–2653.
- Swift AJ, Wild JM, Fichele S, Woodhouse N, Fleming S, Waterhouse J, et al. Emphysematous changes and normal variation in smokers and COPD patients using diffusion 3He MRI. Euro J Radiol 2005; 54(3):352–358.
- Woodhouse N, Wild JM, Paley MN, Fichele S, Said Z, Swift AJ, et al. Combined helium-3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imag 2005; 21(4):365–369.
- Wang C, Mugler JP, 3rd, de Lange EE, Patrie JT, Mata JF, Altes TA. Lung injury induced by secondhand smoke exposure detected with hyperpolarized helium-3 diffusion MR. J Mag Reson Imaging 2014; 39(1):77–84.
- Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Euro Respir J 2008; 31(4):869–873.
- Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010; 7(1):32–43.
- Gietema HA, Muller NL, Fauerbach PV, Sharma S, Edwards LD, Camp PG, et al. Quantifying the extent of emphysema: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol 2011; 18(6):661–671.
- Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 2011; 261(1):274–282.
- Galban CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012; 18(11):1711–1715.
- Hersh CP, Washko GR, Estepar RS, Lutz S, Friedman PJ, Han MK, et al. Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir Res 2013; 14:42.
- Van Eeden S, Leipsic J, Paul Man SF, Sin DD. The relationship between lung inflammation and cardiovascular disease. Amer J Respir Crit Care Med 2012; 186(1):11–16.
- Man SF, Van Eeden S, Sin DD. Vascular risk in chronic obstructive pulmonary disease: role of inflammation and other mediators. Can J Cardiol 2012; 28(6):653–661.
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Amer Soc Echocardiogr 2008; 21(2):93–111; quiz 89–90.
- Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB, Sr. Carotid-wall intima-media thickness and cardiovascular events. New Engl J Med 2011; 365(3):213-221.
- Mallett C, House AA, Spence JD, Fenster A, Parraga G. Longitudinal ultrasound evaluation of carotid atherosclerosis in one, two and three dimensions. Ultrasound Med Biol 2009; 35(3):367–375.
- Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis 2012; 220(1):128–133.
- Spence JD. Carotid plaque measurement is superior to IMT Invited editorial comment on: carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis 2012; 220(1):34–35.
- Wannarong T, Parraga G, Buchanan D, Fenster A, House AA, Hackam DG, et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke J Cerebr Circul 2013; 44(7):1859–1865.
- Buchanan DN, Lindenmaier T, McKay S, Bureau Y, Hackam DG, Fenster A, et al. The relationship of carotid three-dimensional ultrasound vessel wall volume with age and sex: comparison to carotid intima-media thickness. Ultrasound Med Biol 2012; 38(7):1145–1153.
- Fenster A, Downey DB. Three-dimensional ultrasound imaging. Ann Rev Biomed Eng 2000; 2:457–475.
- Landry A, Fenster A. Theoretical and experimental quantification of carotid plaque volume measurements made by three-dimensional ultrasound using test phantoms. Med Phys 2002; 29(10):2319–2327.
- Kirby M, Svenningsen S, Owrangi A, Wheatley A, Farag A, Ouriadov A, et al. Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology 2012; 265(2):600–610.
- Shai I, Spence JD, Schwarzfuchs D, Henkin Y, Parraga G, Rudich A, et al. Dietary intervention to reverse carotid atherosclerosis. Circulation 2010; 121(10):1200–1208.
- Ukwatta E, Awad J, Ward AD, Buchanan D, Samarabandu J, Parraga G, et al. Three-dimensional ultrasound of carotid atherosclerosis: semiautomated segmentation using a level set-based method. Med Phys 2011; 38(5):2479–2493.
- Kirby M, Heydarian M, Svenningsen S, Wheatley A, McCormack DG, Etemad-Rezai R, et al. Hyperpolarized 3He magnetic resonance functional imaging semiautomated segmentation. Acad Radiol 2012; 19(2):141–152.
- Kirby M, Heydarian M, Wheatley A, McCormack DG, Parraga G. Evaluating bronchodilator effects in chronic obstructive pulmonary disease using diffusion-weighted hyperpolarized helium-3 magnetic resonance imaging. J Appl Physiol 2012; 112(4):651–657.
- Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. Amer J Respir Crit Care Med 2005; 171(2):142-6.
- Van Belle GF, L.; Heagerty, P.; Lumley, T. Multiple Comparisons Biostastistics: A Methodology for Health Sciences, 2nd ed. Seattle, Washington: Wiley-Interscience; 2004.
- van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC. Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam Study. Euro Heart J 2012; 33(2):221–229.
- Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, et al. Relationships between airflow obstruction and quantitative ct measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Amer J Roentgenol 2013; 201(3):W460–470.
- Zach JA, Newell JD, Jr., Schroeder J, Murphy JR, Curran-Everett D, Hoffman EA, et al. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Investig Radiol 2012; 47(10):596–602.
- Lim TK, Lim E, Dwivedi G, Kooner J, Senior R. Normal value of carotid intima-media thickness—a surrogate marker of atherosclerosis: quantitative assessment by B-mode carotid ultrasound. J Amer Soc Echocardiogr 2008; 21(2):112–116.
- McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. New Engl J Med 2011; 365(17):1567–1575.
- Coxson HO, Eastwood PR, Williamson JP, Sin DD. Phenotyping airway disease with optical coherence tomography. Respirology 2011; 16(1):34–43.
- Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, et al. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med 2006;56(6):1293–1300.
- Schroeder EB, Welch VL, Evans GW, Heiss G. Impaired lung function and subclinical atherosclerosis. The ARIC Study. Atherosclerosis 2005; 180(2):367–373.
- Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke J Cerebr Circul 1999; 30(4):841–850.
- Schminke U, Hilker L, Motsch L, Griewing B, Kessler C. Volumetric assessment of plaque progression with 3-dimensional ultrasonography under statin therapy. J Neuroimag 2002; 12(3):245–251.
- Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD. 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke J Cerebr Circ 2005; 36(9):1904–1909.
- Awad J, Krasinski A, Parraga G, Fenster A. Texture analysis of carotid artery atherosclerosis from three-dimensional ultrasound images. Med Phys 2010; 37(4):1382–1391.
- Sabit R, Shale DJ. Vascular structure and function in chronic obstructive pulmonary disease: a chicken and egg issue? Amer J Respir Crit Care Med 2007; 176(12):1175–1176.

