Abstract
Background: Exposure to secondhand tobacco smoke (SHS) can be a risk factor for chronic obstructive pulmonary disease (COPD), but its role among relatively heavy smokers with potential co-exposure to workplace vapors, gas, dust, and fumes (VGDF) has not been studied. Methods: To estimate the contribution of SHS exposure to COPD risk, taking into account smoking effects and work-related exposures to VGDF, we quantified SHS based on survey responses for 1400 ever-employed subjects enrolled in the COPDGene study, all current or former smokers with or without COPD. Occupational exposures to VGDF were quantified based on a job exposure matrix. The associations between SHS and COPD were tested in multivariate logistic regression analyses adjusted for age, sex, VGDF exposure, and cumulative smoking. Results and Discussion: Exposures to SHS at work and at home during adulthood were associated with increased COPD risk: odds ratio (OR) = 1.12 (95% confidence interval [CI]: 1.02–1.23; p = 0.01) and OR = 1.09 (95%CI: 1.00–1.18; p = 0.04) per 10 years of exposure adjusted for smoking and other covariates, respectively. In addition, subjects with employment histories likely to entail exposure to VGDF were more likely to have COPD: OR = 1.52 (95%CI: 1.16–1.98; p < 0.01) (adjusted for other covariates). While adult home SHS COPD risk was attenuated among the heaviest smokers within the cohort, workplace SHS and job VGDF risks persisted in that stratum. Conclusion: Among smokers all with at least 10 pack-years, adult home and work SHS exposures and occupational VGDF exposure are all associated with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common and costly lung disease accounting for major worldwide morbidity and mortality (Citation1–4). Although the bulk of such disease is attributable to tobacco smoking, there is growing recognition that other risk factors are important contributors to COPD as well (Citation5). Among these, exposure to various occupational pollutants is believed to account for approximately 15% of the population attributable fraction (PAF) of COPD (Citation6–11). Work-related exposure to various particular- and vapor-phase moieties, although heterogeneous, is often grouped collectively as vapors, gas, dust, or fumes (VGDF)(Citation12). In addition to VGDF, exposure to tobacco via secondhand tobacco smoke (SHS) has also been identified as a COPD risk factor, including both occupational and non-occupational sources of SHS (Citation8, Citation13, Citation14).
One particular challenge in estimating and even in perceiving COPD risk due to multiple factors can be the relative dominance of smoking in disease causation. Thus, estimating risk over and above smoking among persons with a heavy burden of current or past smoking is especially important. Epidemiological studies have shown that the combined smoking and VGDF occupational exposure carry an additive or modestly supra-additive COPD risk (Citation15, 16). It may be reasonable, therefore, to extrapolate a similar pattern when SHS is combined with smoking, VGDF exposure, or both, but this scenario has not been extensively investigated.
In this analysis, we wished to estimate the separate and combined risks of COPD associated with exposure to SHS in a cohort notable for a relatively heavy burden of cigarette smoking (with a median of 40 pack-year smoking). We did so by analyzing SHS-associated risk for COPD taking into account not only cumulative cigarette smoking but also work-related VGDF exposure as well. We did this using data from a subgroup of the COPDGene cohort that had occupational information relevant to job exposure. All COPDGene cohort members were required to have at least a 10 pack-year history of cigarette use as a study entry criterion. Because the population was heavily enriched with disease and all experienced the predominant risk factor for COPD (that is, smoking), this provided a unique opportunity to examine the superimposed incremental risks associated with exposure to SHS as well as work-related VGDF.
Materials and Methods
Study Design and Subjects
This is a cross-sectional analysis of data for a subgroup of subjects enrolled in COPDGene study. The design for COPDGene study has been previously described (Citation17). Briefly, the COPDGene study is a U.S.-based multicenter observational prospective study designed to identify genetic factors associated with COPD that has enrolled 10,300 current and former smokers with or without a reported COPD diagnosis. The COPDGene study inclusion criteria were: non-Hispanic White or African-American, current or former smokers (≥10 pack-years), and age 45 to 80 years. Subjects reporting a medical diagnosis of selected active lung diseases other than asthma, emphysema, chronic bronchitis, or COPD were excluded (e.g., lung cancer). The study goals of the COPDGene study have been to characterize phenotypes of tobacco smokers using spirometry, exercise tests, chest computerized tomographic (CT) scans, medical history, medical questionnaires regarding respiratory symptoms, and to perform genome-wide association studies (GWAS).
The data for the current study derives from a subgroup of 1,400 subjects who were enrolled in the COPDGene study at a single study site, (National Jewish Health [NJH]) in Denver, Colorado and further limited to those who had a history of current or past labor force participation. This single study site uniquely elicited SHS exposure along with occupational histories from its participants. Local institutional review board (IRB) approval to enroll participants in this project was obtained and all subjects provided informed consent to participate in the study. The dataset we analyzed was cleared of personal identifiers.
A key feature of the COPDGene study was to enroll a large cohort (10,000) of subjects, over a range of COPD disease severity, including smokers without COPD at the time of enrollment (although additional nonsmokers were also enrolled, they were not included in the present analysis). For smokers in the COPDGene study, a minimum history of 10 pack-years of smoking was required as a study entry criterion with the rationale that less exposure than that would be inconsistent with smoking-related COPD, even mild disease. Other cohort studies of COPD have also used minimum exposure thresholds of either 10 or 20 pack-years to define cohort eligibility (Citation18, 19).
Study Procedures
Questionnaire including SHS and job histories were administered to all subjects recruited between January 2008 and April of 2011. These questionnaire items adapted from previously validated SHS and job history batteries developed by our research group (Citation8, Citation20, Citation21) and were used as tools to quantify subjects’ SHS exposure at home and work and to identify subjects’ occupation and industry of employment targeting the longest job held. In addition, as part of the general COPDGene protocol, all subjects underwent spirometry to determine the presence of COPD consistent with Global Initiative for Chronic Obstructive Lung Diseases (GOLD) guidelines (Citation22).
Smoking and Secondhand Tobacco Smoke Exposure Assessment
Smoking history was quantified by calculation of the total number of pack-years (number of packs of cigarettes smoked per day multiplied by the number of years smoked). Exposure to SHS history was quantified by the number of years that each subject reported being exposed regularly to SHS at their home or work environments. Childhood and adult SHS exposures at home were quantified by asking “Growing up until age 18, for how many years in total did you live in the same household with someone else who smoked tobacco products?’’ and “Since age 18, for how many years in total have you lived in the same household with someone else who smoked tobacco products?’’ Exposure at work to SHS was quantified by asking “Thinking about all of the jobs you have had, for how many years of your employment have you been regularly exposed to another person's cigarette smoke inside your workplace?’’
Occupational Vapors, Gas, Dust, and Fumes (VGDF) Exposure Assessment
To examine the risk of development of COPD due to occupational exposures, we chose to use a previously validated job exposure matrix (JEM) scoring system that allows for risk assessment due to occupational VGDF exposure (Citation6). Rather than depending on subject-reported exposures that might be prone to report bias, the JEM approach uses open-ended responses for the occupation and industry of subjects’ longest held job and assigns ordinal exposure likelihoods of low, intermediate, or high probability of inherent job exposures associated with COPD. The exposure categorization of this JEM is adapted from a European job-code-based classification originally used to analyze asthma data from the Swedish component of the European Community Respiratory Health Survey (Citation23) and was later adapted for COPD and applied to U.S. job data (Citation9). It is important to note that the JEM categorization captures a heterogeneous group of potential risk factors for the development of COPD, collectively considered as likely exposure to VGDF.
Reported Respiratory Conditions
In addition to standard demographics, the survey questionnaire also elicited subject self-report and report of a physician's diagnosis of COPD and emphysema. Although other health-related information was queried, we did not include additional variables (e.g., other medical co-morbidities) in this analysis.
Spirometry
Spirometry was performed before and 15 minutes after administration of 180 mcg of albuterol by metered dose inhaler using a ndd EasyOne Spirometer (ndd Medical Technologies, Andover, MA) and according to American Thoracic Society performance criteria (Citation24). The best values for forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were used from three acceptable FVC maneuvers (Citation24) obtained approximately 30 seconds apart. Post-bronchodilator FEV1 and FVC were obtained consistent with GOLD guidelines (Citation22). We used the lower limit of normal (LLN) to define a reduced FEV1 to FVC ratio (Citation22, Citation25) rather than less than a fixed ratio of 0.70 to be consistent with the practice used in the parent COPDGene study as well as other recent large cohort investigations. For similar reasons, because higher GOLD stages are standardly defined by the FEV1 being < 80% predicted, we retained this convention rather than apply a LLN approach to that criterion.
Diagnosis of COPD was defined as having a post-bronchodilator FEV1 to FVC ratio less than the LLN and a FEV1 < 80% predicted (that is, GOLD stage 2 severity or higher as defined by FEV1, but including the LLN criterion for FEV1 to FVC ratio as a basis for establishing COPD). All other subjects were grouped together as smokers without COPD; that is, subjects with normal FEV1 to FVC ratio by LLN and subjects with abnormal (low) FEV1 to FVC ratio by LLN but with FEV1 ≥80% predicted (that is, GOLD stage 1 severity). Individuals with GOLD stage 1 COPD are hypothesized to include a subgroup with early disease and a putative “normal” subgroup (Citation17), and thus all the recent major COPD cohort studies have evaluated those with GOLD stage 1 COPD separately from those with GOLD stages 2–4 COPD (Citation17–19). To ensure the correct determination of our outcome variable (spirometric diagnosis of COPD), we elected to adopt this approach and group the subjects with GOLD stage 1 COPD together with those smokers with normal FEV1 to FVC ratio.
Data Analysis
Data from questionnaires were then entered into a database in Microsoft Excel 2007, and analyzed using STATA 12.0 (StataCorp LP, College Station, Texas, USA). As a primary analytic endpoint, we defined the presence or absence of COPD as a dichotomous spirometry-based outcome (as defined above). For secondary analyses, we also utilized subject-reported condition data by redefining COPD as the presence of spirometric obstruction as defined and a self-reported physician's diagnosis of COPD or emphysema.
The primary predictor variable of interest was self-reported SHS exposure. Three separate predictor variables of SHS exposure (childhood-home, adult-home, and work) were created by calculating the sum of total number years of SHS exposure at home during childhood, at home as an adult, and at work, respectively. We generated descriptive statistics for age, sex, cumulative smoking, SHS, COPD by spirometry, and COPD by self-reported physician diagnosis for all subjects, stratifying by low compared with intermediate or high VGDF exposure likelihood (the latter two combined) for longest held job (defined by JEM, as noted above), testing differences with chi-square or t-test. We used logistic regression analysis to test the associations between COPD as a dichotomous dependent variable and different SHS exposures, occupational VGDF exposure (intermediate and high JEM likelihood exposure), tobacco smoking, age, and sex. We initially tested each independent variable without adjustment for covariates. To assess whether age was collinear with pack-years of smoking or with years of childhood-home, adult-home, and work SHS exposure in our cohort, we tested their correlations with age: smoking, r = 0.30 [ p < 0.01]; childhood-home SHS exposure, r = −0.05 [ p = 0.08]; adult-home SHS exposure, r = 0.15 [ p < 0.01]; and work SHS exposure, r = 0.31 [ p < 0.01]). Because the correlations were only moderate (< 0.40), we included them in the same multivariate models. Since childhood-home SHS exposure was not significantly associated with COPD in any preliminary models and neither its inclusion or exclusion substantively altered multivariate estimates for the other covariates, we did not include it in the final modeling. Odds ratios (ORs) were expressed per 10 years SHS exposure for the two other categories of adult home and work, 10 pack-years smoking, and 10 years of age.
As a sensitivity analysis, we re-estimated this model redefining COPD as the presence of spirometric obstruction as defined above along with concomitant self-reported physician diagnosis of COPD/emphysema, excluding from this analysis all those meeting one but not both criteria.
We also carried out further logistic regression analyses stratifying by sex, age (stratified by the median age of 62 years for the study group), and pack-years of smoking (stratified by the median value of 40 pack-years for the group). The median values for age and smoking were chosen as the cut-points for stratification to generate relatively equal numbers of subjects in each group, thus maximizing analytic power.
To estimate the proportion of COPD prevalence attributable to each of occupational VGDF exposure, SHS exposure, and smoking with adjustment for all other predictors in the model, we calculated the PAF using STATA 12.0 and consistent with the method of Greenland and Drescher (Citation9, Citation15, Citation26). The PAF is an estimate of the proportion of all cases of a disease in a given population that would not have occurred in the absence of the exposure of interest. The PAF for each variable was estimated from the multivariate logistic models using all binary variables for adult-home and work SHS exposures, smoking, and occupational VGDF exposure, and adjusted for age and sex. For these PAF estimates, smoking, adult, and workplace SHS exposures were dichotomized to generate binary variables.
For the PAF calculations we chose breakpoints for dichotomization of smoking and secondhand smoke exposure that captured a substantial real-world exposure but not an unrealistically high value. Twenty pack-years of smoking is a commonly accepted cut-point of clinical significance and we extended this to workplace secondhand smoke exposure. Because the overall adult home exposure in our cohort was of somewhat longer duration, for this metric, we chose a somewhat higher breakpoint at 30 years. In the final analysis, smoking was dichotomized at ≥20 years vs. <20 (noting that for smoking the minimum for entry into the cohort was 10 pack-years), while work and adult home SHS exposures were dichotomized at the median (20 years) and upper quartile (30 years) of exposure, respectively.
Results
Subject characteristics
Of the 1,400 subjects who completed the questionnaire, 114 subjects (8.1%) had incomplete key data, (missing information on SHS exposure, occupation, or smoking history) and thus were excluded from the analysis. Altogether, data from 1,286 subjects were retained for analysis. All subjects were current or former smokers with at least 10 pack years of exposure, by COPDGene study design entry criteria. Overall, 97.9% of subjects reported at least some history of regular occupational or home SHS exposure (83.7% and 93.2% exposure to SHS at work and outside work environment, respectively). Childhood exposure to SHS at home was reported by 81.3%.
The prevalence of COPD in the cohort defined by an FEV1/FVC ratio < LLN and FEV1 < 80% predicted was 47.3% (n = 608), with a relative, but non-significant, higher proportion of men (49.8%; 335 of 673) than women (44.5%; 273 of 613) having COPD (p = 0.06). The prevalence of self-reported diagnosis of COPD in the cohort was 51.9% (n = 667), distributed relatively evenly between men (52.9%; 356 of 673) and women (50.7%; 311 of 613). Overall, self-report of COPD misclassified 17.0% of all subjects: 12.9% (80 of 619) who did not report having COPD or emphysema yet met the criteria (a post-bronchodilator FEV1 to FVC ratio less than the LLN and a FEV1 <80% predicted) for COPD by spirometry (false negatives), and 20.8% (139 out of 667) who reported having COPD or emphysema that did not meet criteria for COPD by spirometry (false positives) (Table S1 in supplemental material).
Of all 1,286 subjects, 736 (57.2%) were classified based on the JEM as having low likelihood of occupational exposures (largely white collar workers), while 550 (42.8%) were categorized as having jobs with an intermediate or high likelihood of exposure to work-related VGDF (Table ). Smoking and work SHS exposure differed significantly (p < 0.05) by occupational status, with greater exposure to both sources of tobacco smoke exposure stepping up by likelihood of occupational VGDF exposure. Home exposure to SHS was not significantly different between the low and intermediate/high VGDF exposure groups. There was a non-statistically significant association (p = 0.10) between job exposure to VGDF and self-reported disease vs. spirometric obstruction mismatch (50.0% of false negatives and 61.2% of false positives had low VGDF exposure risk exposure jobs; data not shown in Table).
Table 1. Subject characteristics by job exposure matrix likelihood of exposure among 1286 study participants, all at reported longest-held job
Associations of SHS, occupational VGDF exposure, and cumulative pack-years with COPD
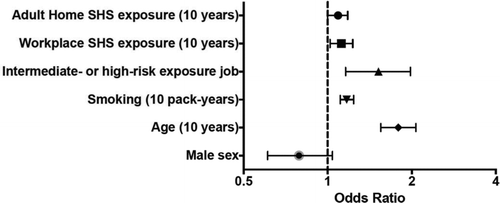
Childhood home exposure to SHS was not significantly associated with COPD risk (OR [95% CI]: univariate, 1.11 [0.95–1.30], p < 0.15; multivariate analysis including the other exposure risk factors, 1.08 [0.88–1.23], p = 0.63). The results from univariate and multivariate logistic regression models for the remaining risk factors are presented in Table and illustrated graphically in Figure 1. Exposure to SHS at work and at home as an adult were associated with COPD in both univariate and multivariate models, although the point estimates of risk per 10 years exposure were decreased after adjustment for covariates (OR [95% CI]: 1.09 [1.00–1.18], p = 0.04 and 1.12 [1.02–1.23], p = 0.01 for adult home and work SHS exposure, respectively). Occupational VGDF exposure group was also a significant risk factor for COPD in both univariate and multivariate models; however, in the multivariate modeling, the point estimate of risk of COPD increased (OR [95% CI]: 1.52 [1.16-1.98], p < 0.01). The cumulative cigarette smoking burden (in this all smoking cohort) was also a significant risk factor in both models.
Table 2. Association between SHS Exposure by Source and spirometrically defined COPD
Re-analysis excluding all self-reported diagnosis-spirometry mismatches (n =1067 remaining) yielded similar point estimates for the relative odds of COPD associated with work SHS, occupational VGDF exposure, and pack years of smoking, although adult home SHS exposure was less strongly associated (p = 0.07) (Table S2 in supplemental material).
Population attributable risk fraction of COPD
The PAF estimates for adulthood home SHS (30 years; the highest exposure quartile), workplace SHS (20 years; median of exposure), and occupational VGDF exposure in the study population (all persons with > 10 pack-years history of smoking), after adjustment for age, sex, and smoking intensity, were 4.5% [95%CI: 1.2%–7.7%], 9.0% [95%CI: 2.7%–14.9%] and 8.9% [95%CI: 3.7%–13.9%], respectively (Table ). In the same multivariate modeling, 20 pack-years of smoking was associated with a PAF of 34.8% [95% CI: 17.7%–48.3%], relative to less smoking (that is, from 10 [the minimum by definition in this cohort] to up to 20 pack-years).
Table 3. Multivariate analysis of the population attributable risk fraction (PAF) for COPD associated with secondhand smoke and work-related vapors, gas, dust, or fume (VGDF) exposure
Stratification by age, sex, and smoking intensity
To determine whether age or sex affects the associations between SHS or occupational VGDF exposure and COPD, we re-estimated the regression models after age and sex stratification (Table S3 and S4 in the Supplemental material). Stratification by age at the cohort median of 62 years indicated that adult home SHS exposure was only a significant risk factor for COPD in the younger stratum (OR [95%CI]: 1.18 [1.04–1.35], p = 0.01). The estimated effects for workplace SHS exposure stratified by age are similar to the non-stratified estimate, although the confidence intervals are wider in the older group. Occupational VGDF exposure, although yielding similar point estimates of risk, was only significantly associated with COPD in the younger stratum (OR [95%CI; p value]: 1.46 [1.00–2.13; p = 0.04]; versus 1.34 [0.94–1.93; p = 0.10] in younger and older subjects, respectively).
Figure 1. COPD risk factors in a subgroup of the COPDGene study cohort in adjusted modeling. Forest plot of odds ratios for presence of COPD (FEV1 to FVC ratio less than LLN and FEV1 <80% predicted) of adult home and workplace SHS exposure after adjustment for vapors, gas, dust, and fumes (VGDF) occupational exposure and other covariates.
Among women, adult home SHS exposure was significantly associated with COPD (OR [95%CI]: 1.23 [1.09–1.39], p < 0.01), while in men, the association was substantially attenuated. By sex, workplace SHS was somewhat stronger and statistically significant in men.
We further retested the univariate and multivariate logistic regression models (Table S5 in supplemental material) stratified by smoking history of more or less than the median smoking history of 40 pack-years. Exposure to SHS at work was a statistically significant predictor of diagnosis of COPD even among relatively heavier smokers (OR [95%CI]: 1.13 [1.00–1.27], p = 0.04), while adulthood home SHS exposure risk was attenuated in that group and was not statistically significant. On the other hand, the cumulative burden of smoking did not impact the estimated association between VGDF exposure and COPD risk (OR [95%CI; p value]: 1.52 [1.03–2.23; p = 0.03] and 1.53 [1.07–2.20; p = 0.02], for ≤ 40 and > 40 pack-years of smoking, respectively).
Discussion
This relatively large, all-smoking cohort with a high prevalence of COPD provides a unique opportunity to assess the associations between work and home exposures to SHS and disease, while adjusting for demographics, occupational VGDF exposure, and, most importantly, smoking-associated risk. Taking into account cumulative smoking, we found that those with a history of SHS exposure at home during adulthood and at work had 9% and 12% increased odds of having COPD per 10 years of exposure, respectively. Given the overall high-level of average adulthood home and work exposures of the cohort (over 18 years), these SHS exposures seem to have substantially contributed to COPD risk in this cohort. Consistent with a previous report from the COPDGene study cohort, childhood home exposure to SHS was not a significant contributor to COPD (Citation27). In addition, we found that subjects with intermediate and high likelihood of VGDF exposure in their longest-held jobs had over 50% greater odds of COPD after adjustment for smoking.
The PAF estimates provide an alternate evaluation of the contribution of SHS and VGDF exposures to COPD risk. The PAF estimates the proportional reduction in population disease that would occur if the exposure in question were eliminated. Our PAF estimates suggest that the contribution of SHS exposure to development of COPD is not trivial compared to that of smoking (4.5% and 9% reduction in COPD for adult home and work SHS exposures, respectively). Altogether, the association of SHS exposure and COPD diagnosis that we observed in our cohort indicates that, even among relatively heavy smokers (median smoking history of 40 pack-years), exposure to SHS contributes to COPD risk. This is biologically consistent with the observations that, while the concentration of SHS exposure is less than that of inhaled mainstream smoke, SHS can be more toxic than the mainstream smoke because of its differing constituents (Citation28, 29).
In addition, the PAF estimate for VGDF exposure indicates that it also makes a contribution to COPD risk (8.9% reduction in COPD for intermediate and high likelihood exposure). Of note, the PAF associated with occupational VGDF exposure in this cohort was within the range of other reported estimates and is consistent with the observation that the work-related PAF for COPD is typically higher among non-smokers (and thus likely to be higher in cohorts that include non-smokers along with smokers, unlike this study population)(Citation9, Citation15, Citation30, 31).
The associations we observed appeared to differ by age, sex, and burden of smoking exposure. In stratified analyses, COPD risk associated with adult home exposure to SHS was manifested among the younger members of the cohort and in women, but not to the same degree among the older age stratum or among men. On the other hand, work exposure to SHS seemed to be more important among the older members of cohort and in men. In addition to temporal trends, this very well may reflect age and sex differences in patterns of home and work SHS exposure and have implications for targeted SHS prevention efforts and disease surveillance. Interestingly, and in contrast to the adult home SHS exposure pattern of risk, occupational exposure to SHS and VGDF were both risk factors for COPD, even in subjects with a heavier cumulative burden of smoking. In terms of prevention, this supports both workplace smoking bans and the enforcement of protective occupational permissible exposure limits.
Recall bias and misclassification error are limitations in the survey-based measures of cigarette smoking, SHS, and VGDF that we used. Of note, the JEM approach to assessing VGDF exposure minimizes recall bias (Citation6, Citation21). All study participants, by entry criteria, were smokers and the group, overall, was heavily exposed. As such, the findings of the study are only generalizable to smokers. We view this as an inherent strength of this analysis, however, as it reflects the smoking burden consistent in a group with a high prevalence of COPD. This is a cross-sectional study that cannot assess the prospective risk of COPD development. It is also a subgroup from only one study site and thus cannot be generalized geographically nor even to the entire COPDGene study group.
This also limits our power in terms of stratification although we maximized strata size by using median cut-points that divided the cohort in half. We did not, for example, stratify by current vs. former smokers for this reason. Our subgroup analysis provides an opportunity for validation in the entire cohort (over 10,000 subjects in total instead of 1,400) which might also allow the inclusion of additional lung function data, symptom reporting, and radiographic findings to more thoroughly grade COPD severity as well as identifying a subset with emphysema. Finally, we defined COPD by the lower limit of normal (LLN) approach rather than using the fixed ratio of FEV1 to FVC (e.g., which, combined with FEV1 < 80% predicted is the GOLD Stage 2 severity or higher), but this should not have led to associations that we observed. In fact, since persons with GOLD Stage 1 COPD were included in the disease-free referent category for these analyses, this would tend to attenuate any associations with the risk factors we examined and, if anything, underestimate the PAF contributions that they make to the burden of disease. Of further note, our sensitivity analysis redefining disease as requiring both spirometric obstruction and a report of clinically-defined COPD did not yield substantially different risk estimates.
In conclusion, we found that even in a group characterized by relatively heavy smoking, adult SHS exposure is significantly associated with diagnosis of COPD. While the heavier smoking accounted for more than a third of the attributable COPD risk, adult home and workplace SHS exposures together accounted for about a third as much of that burden of risk.
Declaration of Interest Statement
We would like to thank Dr. John Hokanson from University of Colorado Health Sciences with help in preparation of manuscript. Authors’ contributions were as follows. Conceived and designed the experiment: RB. Collected data: RB. Designed analytical approach: MA, PB. Analyzed and interpreted the data: IvK, MA, PB. Wrote the manuscript: IvK, MA. Provided feedback on analyses and manuscript: PB, RB. Guarantor of the manuscript: MA, RB. The authors do not have any conflict of interest to disclose. This study was supported by NHLBI (U01 HL08-9856, U01 HL08-9897, Arjomandi K23 HL08-3099), NCRR/HIH (UL1 RR025780), the Butcher Foundation, and the Flight Attendants Medical Research Institute (FAMRI). The sponsors of the study had no role in the design and conduct of this study.
Supplementary Materials
Download MS Word (88 KB)Supplementary materials are available in the online version of this article.
References
- Feenstra TL, van Genugten ML, Hoogenveen RT, Wouters EF, Rutten-van Molken MP. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Amer J Respir Crit Care Med 2001; 164(4):590–596.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765–773.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364(9434):613–620.
- GOLD. Global Initiative for Chronic Obstructive Lung Disease: Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (Revised 2011). 2011 [ 12/29/2012]; Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf..
- Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med; 182(5):693–718.
- Blanc PD, Eisner MD, Balmes JR, Trupin L, Yelin EH, Katz PP. Exposure to vapors, gas, dust, or fumes: assessment by a single survey item compared to a detailed exposure battery and a job exposure matrix. Am J Ind Med 2005; 48(2):110–117.
- Blanc PD, Iribarren C, Trupin L, Earnest G, Katz PP, Balmes J, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax 2009; 64(1):6–12.
- Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health 2005; 4(1):7.
- Trupin L, Earnest G, San Pedro M, Balmes JR, Eisner MD, Yelin E, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J 2003; 22(3):462–469.
- Mehta AJ, Miedinger D, Keidel D, Bettschart R, Bircher A, Bridevaux PO, et al. Occupational exposure to dusts, gases, and fumes and incidence of chronic obstructive pulmonary disease in the Swiss cohort study on air pollution and lung and heart diseases in adults. Am J Respir Crit Care Med 2012; 185(12):1292–1300.
- Blanc PD, Eisner MD, Trupin L, Yelin EH, Katz PP, Balmes JR. The association between occupational factors and adverse health outcomes in chronic obstructive pulmonary disease. Occup Environ Med 2004; 61(8):661–667.
- Quinlan PJ, Earnest G, Eisner MD, Yelin EH, Katz PP, Balmes JR, et al. Performance of self-reported occupational exposure compared to a job-exposure matrix approach in asthma and chronic rhinitis. Occup Environ Med 2009; 66(3):154–160.
- Yin P, Jiang CQ, Cheng KK, Lam TH, Lam KH, Miller MR, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet 2007; 370(9589):751–757.
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009; 374(9691):733–743.
- Blanc PD, Eisner MD, Earnest G, Trupin L, Balmes JR, Yelin EH, et al. Further exploration of the links between occupational exposure and chronic obstructive pulmonary disease. J Occup Environ Med 2009; 51(7):804–810.
- Darby AC, Waterhouse JC, Stevens V, Billings CG, Burton CM, Young C, et al. Chronic obstructive pulmonary disease among residents of an historically industrialised area. Thorax 2012; 67(10):901–907.
- Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010; 7(1):32–43.
- Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J 2008; 31(4):869–873.
- Couper D, Lavange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax 2014; 69(5): 491–494.
- Eisner MD, Iribarren C, Yelin EH, Sidney S, Katz PP, Sanchez G, et al. The impact of SHS exposure on health status and exacerbations among patients with COPD. Int J Chron Obstruct Pulmon Dis 2009; 4:169–176.
- Eisner MD, Katz PP, Yelin EH, Hammond SK, Blanc PD. Measurement of environmental tobacco smoke exposure among adults with asthma. Environ Health Perspect 2001; 109(8):809–814.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Amer J Respir Crit Care Med 2001; 163(5):1256–1276.
- Blanc PD, Ellbjar S, Janson C, Norback D, Norrman E, Plaschke P, et al. Asthma-related work disability in Sweden. The impact of workplace exposures. Am J Respir Crit Care Med 1999; 160(6):2028–2033.
- Standardization of Spirometry, 1994 Update. American Thoracic Society. American journal of respiratory and critical care medicine. Amer Thorac Soc 1995; 152(3):1107–1136.
- Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax [Multicenter Study] 2008; 63(12):1046–1051.
- Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics [Research Support, U.S. Gov’t, P.H.S.] 1993; 49(3):865–872.
- Hersh CP, Hokanson JE, Lynch DA, Washko GR, Make BJ, Crapo JD, et al. Family history is a risk factor for COPD. Chest [Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] 2011; 140(2):343–350.
- Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control 2005; 14(6):396–404.
- Schick S, Glantz SA. Sidestream cigarette smoke toxicity increases with aging and exposure duration. Tob Control 2006; 15(6):424–429.
- Blanc PD. Occupation and COPD: a brief review. J Asthma 2011; 49(1):2–4.
- Balmes JR, Earnest G, Katz PP, Yelin EH, Eisner MD, Chen H, et al. Exposure to traffic: lung function and health status in adults with asthma. J Allergy Clin Immunol 2009; 123(3):626–631.
