Abstract
Tiotropium bromide, a long-acting anticholinergic agent, improves pulmonary function and quality of life of patients suffering from chronic obstructive pulmonary disease (COPD). We retrospectively examined the factors that determine the long-term persistence with tiotropium bromide. Among 6,301 patients who underwent pulmonary function tests in our pulmonary clinic between 2006 and 2009, 644 met the following criteria: 1) age > 40 years, 2) ≥ 20 pack-years smoking history, and 3) forced expiratory volume in 1 sec / forced vital capacity ratio < 0.7. The clinical information, including the prescription of tiotropium, was obtained from the patients’ records. Tiotropium was administered to 255 patients (40%), of whom 48 (19%) discontinued treatment within 1 year, and 65 (25%) discontinued treatment within the median observation period of 32 months. The drug was discontinued because of ineffectiveness in 35 patients (73%), and because of adverse drug effects in 13 patients (27%). Young age, current smoking, absence of respiratory symptoms alleviation, and less severe disease characterized by a) mild airflow limitation, b) mild to moderate emphysema, or c) no exacerbation of COPD during the 1st year of treatment were predictors of drug discontinuation.
Introduction
Tiotropium bromide, a long-acting anticholinergic agent, improves pulmonary function and quality of life, slows the progression of disease, and lowers the rates of disease exacerbation and mortality in patients suffering from chronic obstructive pulmonary disease (COPD) (Citation1–7). In addition, its adverse effects are relatively mild (Citation5). Therefore, in the clinical guidelines issued by the Global Initiative for Chronic Obstructive Lung Disease, tiotropium bromide has been placed on the frontline of pharmacotherapy for patients presenting with symptomatic COPD (Citation8). However, high rates of treatment discontinuation have been reported during long-term management of COPD (Citation9). Even during clinical trials, despite the strong motivation of participants and caregivers to continue the study medication, the reported rates of tiotropium bromide discontinuation have ranged between 16 and 42% (Citation5, Citation10, Citation11). In the UPLIFT study, over one third of nearly 3,000 patients who received tiotropium bromide discontinued the drug during the 4-year study period (Citation5). Although adverse events were the leading cause of premature discontinuation of the drug in clinical trials (Citation5, Citation10, Citation11), the determinants of its withdrawal in real-life practice remain poorly known (Citation12–14).
The aim of this study, conducted in a pulmonary clinic of a tertiary medical centre, was to examine the characteristics of patients treated for COPD with tiotropium bromide, and compare the characteristics of patients who remained on long-term treatment with the patients whose therapy was discontinued.
Patient population and methods
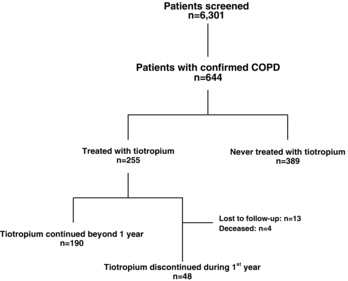
Out of 6,301 patients who underwent spirometry between years 2006 and 2009 at the Pulmonary Medicine clinic of Keio University Hospital, we identified 644 who fulfilled the following criteria: 1) age >40 years, 2) ≥ 20 pack-year history of smoking, and 3) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.7. We reviewed their medical records and ultimately retained in this analysis the 255 patients who were prescribed and received ≥ 1 dose of tiotropium bromide (Figure Citation1).
The severity of airflow limitation was graded according to the 2009 Global Initiative for Chronic Obstructive Lung Disease classification (Citation8). Clinical information, including smoking habits, modified Medical Research Council dyspnoea scale and other respiratory disease manifestations, body mass index, medication, and long-term oxygen therapy, was obtained from the patient's medical records. The transcripts of the visits made at 1–3 months intervals between 1 year before the beginning of tiotropium bromide therapy and March 2012, were examined. Patients who, at the first visit after the initiation of tiotropium bromide, answered positively the query “does tiotropium bromide improve your respiratory symptoms?”, were classified as “responders.” Exacerbation of COPD within 12 months before or after the introduction of tiotropium bromide, defined as worsening of respiratory symptoms requiring 1) treatment with systemic corticosteroids, antimicrobials, or both, or 2) hospitalization, was also recorded. The groups of patients who discontinued therapy or whose physicians discontinued its prescription were compared with the group who remained on long-term treatment.
The protocol of this study was approved by the Institutional Review Board of Keio University School of Medicine.
Pulmonary function tests
Vital capacity, FVC, and FEV1 were measured using an MFR-8200 electronic spirometer (Nihon Koden, Tokyo, Japan), which met the specifications recommended by the American Thoracic Society (Citation15). Diffusing capacity for carbon monoxide per alveolar volume was measured by a 10-second breath holding, using a Chestac-55V volume-type spirometer (Chest MI Co., Tokyo, Japan).
Pulmonary emphysema score
Pulmonary emphysematous lesions were identified as low attenuation areas (LAA) on computed tomography scan (ProSeed, GE Healthcare Japan, Tokyo, Japan), and evaluated according to the Goddard classification (Citation16). The whole lung was divided into upper, mid and lower left and right lung fields. LAA were visually scored from 0 to 4 in each field, where grade 0 corresponded to no LAA, grade 1 indicated LAA in up to 25%, grade 2 between 26 and 50%, grade 3 between 51 and 75%, and grade 4 between 76 and 100% of the lung area under examination. Two pulmonologists unaware of the patient's clinical status graded the areas, and assigned an overall score of the emphysematous changes between 0 and 24. Emphysema was scored as mild [1–2], moderate [3–6], severe [7–12], and most severe [13–24].
Statistical analysis
The data are presented as means ± standard deviations. The characteristics of the patients who continued versus patients who discontinued treatment with tiotropium bromide were compared, using chi-square and non-paired t-tests. Single and multiple variable logistic regression analyses were performed to identify the factors associated with discontinuation of tiotropium bromide during the first year of treatment. Variables with p values < 0.1 in the single variable regression analysis were entered in a multiple variable regression model. Continuation of treatment with tiotropium bromide was also analysed by Kaplan–Meier estimates, log-rank tests, and Cox proportional hazards regression model. Odds (OR) and hazard (HR) ratios were calculated. Statistical analyses were performed using GraphPad Prism, version 4.0c (GraphPad Software, Inc., La Jolla, CA) and SPSS Statistics, version 19 (IBM Corporation, Armonk, NY). A p value < 0.05 was considered statistically significant.
Results
The characteristics of the 644 patients diagnosed with COPD are shown in Table . Their mean age was 69 years and the majority of patients were men. Mild (FEV1 ≥ 80% of predicted), moderate (50–79%), severe (30–49%), and very severe (<30%) airflow limitation was observed in 176 (27%), 329 (51%), 95 (15%), and 44 (7%) patients, respectively. The 255 patients (39.5%) who were treated with tiotropium bromide during the 4-year period suffered more severe dyspnoea, airflow limitation and pulmonary emphysema than the patients who were not treated with tiotropium bromide (Table ). Prior to the prescription of tiotropium bromide, 12.5% of patients had been treated with a long-acting b-adrenergic agonist alone, 5.1% with an inhaled corticosteroid alone, and 12.9% had been treated with both, while 69.4% had received no specific treatment for COPD.
Table 1. Characteristics of the study groups
Within 1 year of onset of treatment with tiotropium bromide, 4 patients died, 13 were lost to follow-up, and 48 discontinued the drug. The remaining 190 patients stayed on treatment for >1 year (Figure ). Table compares the patients who remained on tiotropium bromide for >1 year versus those who discontinued treatment. Out of 16 patients who developed adverse effects, including cardiac complications (n = 2), urinary disturbance (n = 8), glaucoma (n = 1), dry mouth (n = 2) or worsened respiratory symptoms (n = 3), 13 (80%) discontinued tiotropium bromide. Other factors associated with its discontinuation within the first year were young age ( p = 0.001), current smoking ( p = 0.001), mild airflow limitation ( p = 0.001–0.002), and less extensive emphysema ( p = 0.01).
Table 2. Single variable logistic regression analysis of the factors correlated with continuation of tiotropium bromide beyond 1 year after its introduction
Approximately 75% of the patients with mild-to-moderate airflow limitation (FEV1 ≥ 50% of predicted) continued to use tiotropium bromide, whereas 89% and 95% of patients with severe and very severe airflow limitation, respectively, remained on treatment with tiotropium bromide for ≥ 1 year. At 1 year, 45, 67, 82, and 81% of patients with no or mild, moderate, severe, and very severe emphysema ( p = 0.002), continued treatment. Alleviation of respiratory symptoms by tiotropium bromide was reported at the first visit by 93 patients (36%), of whom >90% continued the medication for >1 year. It is noteworthy that the patients who experienced exacerbation of COPD during the first year of treatment were less likely to discontinue treatment ( p = 0.03), whereas exacerbation occurring in the year before institution of treatment was not associated with long-term continuation of therapy.
By multiple variable logistic regression analysis (), current smoking (OR 0.28; p = 0.001), FEV1 < 50% of predicted (OR 2.54; p = 0.033), and presence of any adverse effect (OR 0.04; p < 0.001) were correlated with the continuation of tiotropium bromide beyond 1 year. A response to treatment at the first visit after its initiation (OR 2.28; p = 0.05), and exacerbation of the disease within the first year of treatment (OR 3.18; p = 0.09) were weakly correlated with continuation of the drug for > 1 year. These observations were confirmed when the analysis was limited to patients with a FEV1 < 80% predicted (Supplemental Table 1).
Table 3. Multiple variable logistic regression analysis of the factors correlated with continuation of treatment with tiotropium bromide beyond 1 year after its introduction
During the median 32 months of observation (range 1–75), tiotropium bromide was discontinued in 65 of the 255 patients (25.5%). The drug was continued in 82.1% of patients at 1 year and 77.8% of patients at 3 years after its initiation. Over 50% of drug discontinuations occurred within the first year (Figure ). The factors associated with continuation of tiotropium bromide were examined, using Kaplan-Meier estimate and log-rank test (Figure ). The rate of drug discontinuation was significantly higher among a) current than former smokers ( p = 0.01; Figure a), b) patients with ≥ 50% versus < 50% predicted FEV1 ( p = 0.01; Figure b), c) non-responders than responders to treatment ( p = 0.04; Figure c), and d) patients who suffered exacerbation versus no exacerbation of COPD in the first year after its prescription ( p = 0.003; Figure d). By Cox proportional hazards regression analysis (Table ), current smoking (HR 0.52, p = 0.013) and adverse effects (HR 0.11, p < 0.001) were negatively correlated with the long-term continuation of treatment, while exacerbation of COPD during the first year of treatment was positively correlated with the long-term administration of tiotropium bromide (HR 3.37; p = 0.042). The correlation with <50% predicted FEV1 was weak (HR 1.35; p = 0.052).
Figure 2. Kaplan–Meier estimates of cumulative probability of discontinuation of tiotropium bromide in 255 patients.
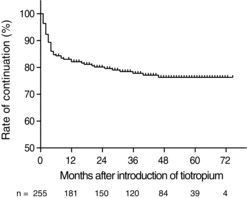
Figure 3. Kaplan–Meier estimates of cumulative probability of discontinuation of tiotropium bromide according to a) former versus current smoker status, b) ≥50% versus <50% FEV1, c) alleviation versus no alleviation of symptoms, and d) exacerbation versus no exacerbation of COPD during first year of treatment with tiotropium bromide. The p-values were calculated by log-rank test.
Table 4. Cox proportional hazards regression analysis of factors correlated with continuation of treatment with tiotropium bromide beyond 1 year
Discussion
In this study, the rate of long-term treatment with tiotropium bromide, an inhaled, long-acting anti-cholinergic agent for maintenance treatment of COPD was low in young patients, current smokers, and patients who experienced adverse effects. On the other hand, FEV1 < 50% of predicted, severe emphysema, and exacerbation of COPD occurring within 12 months after the initiation of therapy were factors correlated with long-term acceptance of treatment. The alleviation of subjective symptoms was another noteworthy predictor of long-term treatment continuation.
The >80% and >70% rates of persistent treatment with tiotropium bromide at 1 and 3 years, respectively, observed in the present study are as high as those observed in randomized clinical trials, such as UPLIFT (Citation5). In contrast, observational studies of 5,330 and over 31,000 patients reported 37% and 53% rates, respectively, of long-term treatment for COPD with tiotropium bromide (Citation9, Citation20). The high persistence of treatment with tiotropium bromide observed in our study may be due to the higher acceptance by patients suffering from COPD of medications prescribed by pulmonologists than by general practitioners (Citation20, 21). The Japanese health care system, which offers affordable universal coverage, may also facilitate the long-term continuation of treatment.
Our analysis of the factors associated with long-term tiotropium bromide therapy is consistent with earlier reports of lower rates of long-term pharmacotherapy of COPD associated with younger age (Citation9, Citation20–22) and current smoking (Citation12, 13). In addition, we found that disease severity was associated with high rates of long-term therapy, which is concordant with reports of a high likelihood of long-term treatment continuation in patients with more severe disease, manifest by a) the administration of multiple drugs, b) emergency room visits, and c) interim hospitalizations (Citation20, 21, Citation23). It is noteworthy that exacerbations of COPD during, but not before treatment with tiotropium bromide, predicted the long-term continuation of treatment.
The alleviation of symptoms was also an important incentive for patients to stay on treatment of COPD with tiotropium bromide. The reported superiority of tiotropium bromide compared with salmeterol alone or in combination with inhaled corticosteroids in the treatment of breathlessness (Citation24) may explain the higher long-term acceptance of tiotropium bromide than of long-acting b-adrenergic agonists, inhaled corticosteroids, or both for the management of COPD (Citation20). Since tiotropium bromide combined with long-acting b-adrenergic agonists, such as salmeterol or indacaterol, is more effective in the alleviation of dyspnoea than tiotropium bromide alone (Citation25, 26), we expect even higher rates of long-term treatment continuation associated with these combined therapies.
Limitations of our study
Because COPD is more prevalent in Japanese men, due to the higher rate of smoking among men than women (Citation27), our data were mostly collected from men and may not be applicable to women. Second, under the Japanese universal coverage of prescriptions, economical factors may have a lesser impact on the patients’ response to the medical treatment than in other parts of the world. Third, symptomatic alleviation due to the treatment was evaluated with a simple query, instead of with a more quantitative and validated measurement such as a quality-of-life questionnaire or COPD assessment test. The patients who experienced adverse effects with tiotropium bromide reported the effectiveness of treatment less frequently (13%) than the patients who remained free from adverse effects (40%); however, a re-analysis of the data in 239 patients who remained free from adverse effects continued to reveal a significant impact of symptom alleviation on the persistence of treatment with tiotropium bromide ( p = 0.03).
In conclusion, our observational, “real-life,” clinical study identified important factors that influence the treatment of COPD with tiotropium bromide, and suggests the need to further increase the long-term rate of treatment continuation.
Declaration of Interest Statement
The authors have no potential conflict of interest to disclose. The authors alone are responsible for the content and writing of the paper.
Supplementary materials are available in the online version of this article.
Acknowledgment
The authors thank H. Sugiura, MD, from the Department of Radiology at Keio University School of Medicine, for his contributions with the imaging studies.
References
- Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19:217–224.
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23:832–840.
- Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 2005; 128:1168–1178.
- Anzueto A, Tashkin D, Menjoge S, et al. One-year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropium. Pulm Pharmacol Ther 2005; 18:75–81.
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med 2005; 143:317–326.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2011 update. http://www.goldcopd.com
- Cramer JA, Bradley-Kennedy C, Scalera A. Treatment persistence and compliance with medications for chronic obstructive pulmonary disease. Can Respir J 2007; 14:25–29.
- Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008; 177:19–26.
- Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364:1093–1103.
- Ágh T, Inotai A, Mészáros Á. Factors associated with medication adherence in patients with chronic obstructive pulmonary disease. Respiration 2011; 82:328–334.
- Decramer M, Molenberghs G, Liu D, et al. Premature discontinuation during the UPLIFT study. Respir Med 2011; 105:1523–1530.
- Laforest L, Denis F, Van Ganse E, et al. Correlates of adherence to respiratory drugs in COPD patients. Prim Care Respir J 2010; 19:148–154.
- American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Goddard PR, Nicholson EM, Laszlo G, et al. Computed tomography in pulmonary emphysema. Clin Radiol 1982; 33:379–387.
- Smith BM, Schwartzman K, Kovacina B, et al. Lung cancer histologies associated with emphysema on computed tomography. Lung Cancer 2012; 76:61–66.
- Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008; 178:738–744.
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001; 345:1075–1083.
- Breekveldt-Postma NS, Koerselman J, Erkens JA, et al. Enhanced persistence with tiotropium compared with other respiratory drugs in COPD. Respir Med 2007; 101:1398–1405.
- Breekveldt-Postma NS, Gerrits CM, Lammers JW, et al. Persistence with inhaled corticosteroid therapy in daily practice. Respir Med 2004; 98:752–759.
- Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol 2006; 118:899–904.
- Jung E, Pickard AS, Salmon JW, et al. Medication adherence and persistence in the last year of life in COPD patients. Respir Med 2009; 103:525–534.
- Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002; 122:47–55.
- van Noord JA, Aumann JL, Janssens E, et al. Combining tiotropium and salmeterol in COPD: Effects on airflow obstruction and symptoms. Respir Med 2010; 104:995–1004.
- Mahler DA, D’Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax 2012; 67:781–788.
- Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: The Nippon COPD Epidemiology study. Respirology 2004; 9:458–465.