Abstract
It is unknown how interventions aimed at increasing physical activity (PA), other than traditional pulmonary rehabilitation, are structured and whether they are effective in increasing PA in chronic obstructive pulmonary disease (COPD). The primary aim of this review was to outline the typical components of PA interventions in patients with COPD. This review followed the PRISMA guidelines. A structured literature search of relevant electronic databases from inception to April 2014 was undertaken to outline typical components and examine outcome variables of PA interventions in patients with COPD. Over 12000 articles were screened and 20 relevant studies involving 31 PA interventions were included. Data extracted included patient demographics, components of the PA intervention, PA outcome measures and effects of the intervention. Quality was assessed using the PEDro and CASP scales. There were 13 randomised controlled trials and three randomised trials (PEDro score 5-7/10) and four cohort studies (CASP score 5/10). Interventions varied in duration, number of participant/researcher contacts and mode of delivery. The most common behaviour change techniques included information on when and where (n = 26/31) and how (n = 22/31) to perform PA behaviour and self-monitoring (n = 18/31). Significant between-group differences post-intervention in favour of the PA intervention, compared to a control group or to other PA interventions, in one or more PA assessments were found in 7/16 studies. All seven studies used walking as the main type of PA/exercise. In conclusion, although the components of PA interventions were variable, there is some evidence that PA interventions have the potential to increase PA in patients with COPD.
Introduction
Exercise training is a key component of the management of chronic obstructive pulmonary disease (COPD) and is frequently delivered in the context of pulmonary rehabilitation (PR). PR typically involves structured, supervised exercise sessions over 6–12 weeks, along with patient education, psychological support and nutritional interventions (Citation1–3). A Cochrane review has emphasised the benefits of PR in terms of statistically and clinically significant short-term improvements in exercise capacity and health-related quality of life (Citation4). However, there is still some uncertainty on the effects of PR on long-term modification of physical activity (PA) behaviour (Citation5). There is emerging evidence that patients with COPD only complete infrequent and short bouts of PA at moderate intensity (Citation6). In a recent meta-analysis, involving only randomised trials and single-group intervention studies, exercise training conducted ≥4 weeks as part of PR was shown to offer statistically significant but clinically small effects on PA levels in patients with COPD (Citation5). This suggests patients potentially need help in translating the benefits gained from PR into changes in PA behaviour (Citation7).
To help promote the adoption and maintenance of physically active lifestyles at an individual or population level, PA interventions have been developed (Citation8). Guidelines and position stands for clinical populations such as cancer (Citation9), type II diabetes (Citation10) and cardiovascular disease (Citation11) recommend that long-term maintenance of PA is at the core of effective rehabilitation programmes. Guidelines also highlight that PA counselling should be part of a multi-layered and multidisciplinary approach (Citation11). Although a number of PA interventions have been conducted in patients with COPD, there are no clear guidelines on the key components of PA interventions or how effective these have been in terms of long-term changes in PA levels and which PA outcome measures are most useful in assessing the efficacy of PA interventions.
The primary aim of this review was to outline the typical components of interventions other than PR focused on increasing PA levels in patients with COPD. The secondary aims were to examine the PA outcome measures and explore the efficacy of the interventions at increasing PA levels.
Methods
This comprehensive review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Citation12).
Eligibility criteria
The following inclusion criteria were used to select relevant studies: any intervention which aimed to increase PA in patients with COPD using one or more of the types of interventions highlighted by Cox et al. (Citation13) and included PA outcome measures: time spent in PA (measured in minutes per week, sessions per week, MET-minutes); energy expenditure (in calories or joules); activity counts; or step counts (Citation13). Exclusion criteria included: abstracts; study protocols; traditional PR as defined in recent guidelines (Citation3); maintenance programmes directly after PR which were structured similarly to PR and focused on maintaining functional capacity and outcomes of PR; and/or no PA outcome measure utilised. Studies that only recorded PA to determine adherence to the intervention were excluded.
Search and study selection
A structured literature search was undertaken on relevant electronic databases (AMED, CINAHL, Cochrane database, Embase and Medline) from inception (last search 17th April 2014). The following search terms were used to retrieve relevant studies: (“respiratory tract disease” OR “lung disease” OR “COPD” OR “chronic obstructive lung disease” OR “chronic bronchitis” OR “emphysema”) AND (“physical activity” OR “motor activity” OR “daily life activity” OR “exercise” OR “walking” OR “ambulation” OR “lifestyle activit”. OR “lifestyle physical activit”). Results were limited by human research and English language. Two authors (JW and JB) reviewed potential full-text articles against the eligibility criteria. Where the two authors disagreed, a third author (BO’N) made the final decision. The reference listings of the selected articles were then hand-searched for additionally relevant studies.
Study quality appraisal
Study quality was assessed independently by two authors (JW and JB) using the PEDro scale for randomised controlled trials (RCTs) and randomised trials (RTs) and the CASP scale for cohort studies. The PEDro scale is used by the Physiotherapy Evidence Database as a tool to rate the validity of over 25000 RTs, systematic reviews and clinical practice guidelines in physiotherapy (Citation14). The PEDro scale is an 11-item scale used to rate RCTs and RTs in terms of internal validity and also interpretability of study results. The PEDro scale is scored out of 10 (item 1 is not scored), with ‘Yes’ responses gaining 1 point and ‘No’ responses gaining 0 points. RCTs and RTs were scored as either low (≤3/10), medium (4–5/10) or high (≥6/10) quality (Citation15,16).
The CASP scale is provided by the Critical Appraisal Skills Programme to aid the appraisal of healthcare information underpinning decision making for reliability and validity in the United Kingdom (Citation17). The CASP scale is a 12-item scale used to rate cohort studies in terms of three broad areas including: Are the results of the study valid?; What are the results?; Will the results help locally? The questions in the CASP scale are answered with either ‘No’, ‘Can't tell’ or ‘Yes’. Currently, there is no recognised numbered scoring system for this scale, therefore the authors scored the scale out of 10, with ‘Yes’ responses gaining 1 point and ‘No’ or ‘Can't tell’ responses gaining 0 points. Questions 1 and 2 are screening questions so were not scored. Where the two authors disagreed on the quality appraisal for a study, a third author (BO’N) made the final decision.
Data collection process
Data extracted included participant demographic information, recruitment methods utilised, study design, quality, intervention groups, all assessment times from baseline to final follow-up and the attrition rates from the intervention groups. Other data extracted included details on the components of the intervention(s) such as: the duration of the intervention; how the intervention was delivered to participants; the number of contacts between participants and study staff; the frequency, intensity, time and type of PA/exercise; the setting where the PA/exercise took place; and the level of PA/exercise supervision. To identify the behaviour change techniques used in the interventions, a specific taxonomy related to changing PA behaviour was utilised (Citation18). Author JW independently chronicled the behaviour change techniques which appeared to be utilised in each intervention and identified the supporting text for each decision and this was independently verified by a second author (EC). Where the two authors disagreed, a third author (JB) made the final decision.
Data on the PA parameters used in the interventions, including between-group and within-group differences, were also extracted. Statistically significant effects of the PA interventions were measured as p ≤ 0.05. An attempt at a pooled estimate of the treatment effect of PA was unable to be conducted for all studies due to the heterogeneity of the PA interventions and PA parameters. However, to understand the magnitude of the intervention effects, effect sizes (d) were calculated for the most popular PA parameter (step counts) (Citation19). Effect sizes were classified as trivial (≤0.2), small (>0.2), medium (>0.5) and large (>0.8) (Citation19).
Results
Study selection
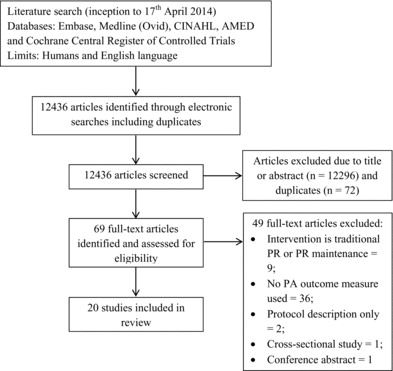
Figure 1 is a flow chart summarising how the final studies were selected. From the initial electronic searches, 12436 articles were identified. Removal of articles not relevant for the scope of this review and duplicates resulted in 69 full-text articles being selected for further reading. After reviewing the full-text articles, 49 studies were excluded after meeting one or more of the exclusion criteria. The most common exclusion criteria met were the intervention being either traditional PR or PR maintenance programmes (n = 9/48) and not using a PA outcome measure (n = 36/49). In total, 20 studies met the inclusion criteria and were included in the review (Citation20–39). Within these studies, 31 interventions were identified. The studies either incorporated a PA intervention group and a control group with no PA intervention (7/20), had two or more groups comparing different PA interventions (9/20), or had one PA intervention group with no control group (4/20). No other eligible articles were identified from the reference lists of the selected articles.
Study quality appraisal
provide details on the quality of the studies. There were 13 RCTs, three RTs and four cohort studies. The RCTs and RTs were of medium-high quality; ranging from 5–7 on the PEDro scale. The most common limitations included failing to conceal allocation and insufficient blinding of the patients/therapists. The cohort studies were all scored positively in 5/10 quality indicators on the CASP scale.
Table 1a. PEDro quality scores for the 16 RCT and RT studies
Table 1b. CASP quality scores for the four cohort studies
Study characteristics
Table provides details on the characteristics of the studies. The mean age of participants recorded across all the studies was ≥55 years old with 64% (n = 794) males and 36% (n = 449) females. Participants had either moderate or severe COPD according to the National Institute for Health and Care Excellence (NICE) guidelines (Citation2). Participants were recruited from a range of healthcare settings including hospitals (n = 11/20), PR sites (n = 5/20) and the internet (n = 3/20). Some participants were referrals from other unspecified settings (n = 5/20). The attrition rates by the end of the interventions ranged from 0–38%.
Table 2. Details of the 20 studies including recruitment, demographics, study design, quality, intervention groups, assessment times, attrition and PA instruments/parameters
Intervention components
Detailed overview of the different intervention components are provided in the online Supplementary Table S1. Intervention duration ranged from 10 days to 18 months. Interventions were short-term ≤ 3 months (n = 17/31); medium-term 4–6 months (n = 5/31); or long-term > 6 months (n = 9/31). Modes of delivery included one or more of the following: face-to-face exercise sessions (n = 13/31), telephone (n = 13/31), face-to-face PA/exercise counselling sessions (n =10/31), PA/exercise diaries (11/31) and internet (n = 6/31). Individual interventions included one mode (10/31), two modes (9/31), or three or more modes of delivery (12/31). The frequency of contacts with the health professional varied according to the intervention duration; ranging from one contact over an 8-week programme (Citation32) to 234 contacts over an 18-month programme (Citation35).
The frequency, intensity, time and type of PA/exercise were not comprehensively recorded and varied greatly between interventions. PA/exercise was generally prescribed for ≥ 3 times per week (n = 25/31). In the interventions which clearly reported on intensity (n =21/31), this was prescribed using Borg scale ratings from 3 to 6 (n = 12/21); intensity ratings of 60%–90% of maximal effort based on exercise test results (n = 9/21) or a combination of both (n = 4/21). When the length of the PA/exercise sessions was reported (n = 24/31), most sessions lasted 10–45 minutes (n = 17/24) with the longest sessions lasting 60 minutes (n = 7/24).
In the interventions which clearly reported on weekly prescribed PA/exercise time (n = 22/31), this was 60–119 minutes (n = 4/22), 120–180 minutes (n = 16/22) or >180 minutes (n = 2/22). The most popular types of PA/exercise prescribed included walking (n = 26/31), cycling (n = 14/31) and resistance/strength training (n = 14/31). A range of PA/exercise settings were used including home and community (n = 18/31); research centre/hospital (n = 9/31); or a combination of home and research centre/hospital (n = 4/31). The PA/exercise sessions were either fully supervised (n = 9/31), fully unsupervised (n = 18/31) or had a mixed level of supervision (n = 4/31).
The mean number of behavioural change techniques reported in the interventions was six (range 1–13). Table highlights the behaviour change techniques reported in the 31 interventions. The most popular included the following: information on where and when (n = 26/31) and instruction on how (n = 22/31) to perform the behaviour; prompt self-monitoring of behaviour (n = 18/31); goal setting (behaviour) (n = 15/31); setting graded tasks (n = 12/31); and facilitating social comparison (n = 11/31). Facilitating social comparison involves explicitly drawing attention to others’ performance to elicit comparisons (Citation18). Pedometers were used to enable self-monitoring in 8/31 interventions with 5/8 of these interventions also explicitly using the pedometer as a motivational/behavioural reinforcement tool. One or more psychological theories for PA behaviour change were explicitly identified in seven studies including: Social Cognitive Theory (n = 5/7); Self-Regulation Theory (n = 3/7) and Self-Efficacy Theory (n =2/7).
Table 3. Number of behaviour change techniquesTable Footnote used across the 31 interventions
PA outcomes and intervention effects
All studies measured PA at baseline and post-intervention (n = 20/20). Assessment of PA included objective (pedometers n = 7/20 and accelerometers n = 7/20) and subjective tools (questionnaires n = 6/20 and activity diaries n = 2/20). The PA parameters included objective (step counts n = 8/20, activity counts n = 3/20, energy expenditure n = 2/20 and time spent in different PA n = 4/20) and subjective parameters (estimated energy expenditure n = 1/20 and time spent in different PA n = 6/20).
Between-group differences in PA were measured in 16/20 studies. Statistically significant between-group differences post-intervention in favour of the PA intervention group (I1), compared to a control group or to other PA interventions, in one or more PA assessments were found in 7/16 studies (21,23,24,27,28,31,34) including the following PA parameters: step counts (2/7); activity counts (1/7); objective energy expenditure (1/7); time spent in different objective PA (2/7); time spent in different subjective PA (2/7). There were similarities in the intervention components; particularly regarding the type of PA/exercise with walking included in all seven studies. Other components of the interventions varied. The duration of the intervention lasted 12 weeks (3/7 studies) or 12 months (2/7 studies).
Modes of delivery included face-to-face exercise sessions (4/7 studies), paper diaries (4/7 studies), face-to-face instruction /education sessions (4/7 studies), telephone calls or texts (3/7 studies) and individual face-to-face exercise counselling (2/7 studies). Intensity of PA/exercise was 70–80% of maximum heart rate in 3/7 studies and in addition to walking (7/7 studies), other types of PA/exercise included cycling (2/7 studies) and resistance training (2/7 studies). Common BCTs included information on where and when to perform the behaviour (6/7 studies) and instruction on how to perform the behaviour (6/7 studies). Other BCTs included were prompt self-monitoring of behaviour (4/7 studies), use of follow-up prompts (3/7 studies), set-graded tasks (3/7 studies) and goal setting (behaviour) (3/7 studies). Pedometers were used as self-monitoring tools in 2/7 studies.
The frequency of contacts, choice of PA/exercise setting, frequency and time per week spent in PA/exercise and PA/exercise supervision were variable between the seven studies. In the most commonly used PA parameter (step counts), six studies allowed for the magnitude of the short-term intervention effects to be calculated. One study (Citation33) found a large effect (>0.8), three studies (Citation22,24,28) found small effects (>0.2) and two studies (Citation25,29) found trivial effects (≤ 0.2) of the PA intervention compared to the control/other PA intervention groups. Statistically significant differences in favour of the control group versus the PA intervention group (I1) were found in one study (Citation29). Statistically significant between-group differences with short-to-medium follow-up (≤ 6 months) post-intervention in favour of the PA intervention group were found in 1/2 studies (Citation27).
Within-group differences in PA were reported in 14/20 studies (10 RCTs and RTs and four cohort studies). Statistically significant improvements pre- versus post-intervention in one or more PA assessments were measured in 11/14 studies (Citation20,21,23,Citation26-28,Citation30,32,Citation36-38). A statistically significant decline in one or more PA assessments pre- versus post-intervention was measured in one study (Citation25). Statistically significant improvements with short-to-medium follow-up (≤ 6 months) post-intervention was measured in 1/2 studies (Citation27).
Discussion
This comprehensive review, which has followed a rigorous methodology, has highlighted a variety of components used in PA interventions in COPD and has identified a range of behaviour change techniques. A range of objective (pedometers and accelerometers) and subjective (questionnaires and activity diaries) PA outcomes were used and there is evidence that PA interventions have some effect on PA levels (step counts).
Patients with COPD are traditionally offered PR which has a strong evidence base for effectiveness (Citation1–4). Surveys of PR have highlighted that there are not enough programmes available; there are currently less than 1.5% of patients with COPD annually receiving PR in the UK (Citation40,41). Usually patients with moderate to severe COPD are targeted (Citation1,3). The majority of programmes are outpatient-based and are supervised by clinicians (40,41).
This structured and supervised format of PR does not meet the needs of all patients, with high numbers of drop-outs and non-adherence (Citation42–44), yet alternative options for increasing PA for patients with COPD currently do not seem to be offered. Studies have found that PR may not always lead to improved PA levels in COPD (Citation45) and the benefits can diminish towards baseline by 6 and 12 months (Citation1,42). Almost half of the studies in this review demonstrated a significant improvement in PA levels in the PA intervention group versus the control group. These positive results from PA interventions are supported by other reviews in different populations such as those being treated for cancer (Citation46), type 2 diabetes (Citation47) and cardiovascular disease (Citation48).
Whether these significant improvements in PA result in minimum clinically important differences (MCID) for patients with COPD is unknown, as no data on MCID and PA parameters in COPD currently exists. Data from other clinical populations may help provide estimates of MCID for some of the PA parameters. For example, Motl and colleagues (Citation49) have shown that a change of 779 steps per day appears to represent a MCID in patients with multiple sclerosis. Although it is unknown whether this MCID would be similar in patients with COPD, 5/8 studies that measured pre- versus post-intervention daily step counts in this review had an increase which may represent a clinically important difference (Citation24,28,33,36,37).
Future research needs to establish MCIDs for PA parameters in COPD. To quantify the magnitude of the treatment effects on step counts, we calculated effect sizes for six studies. One study measured a large effect of the PA intervention whereas the other five studies measured small or trivial effects of the PA intervention on step counts compared to the control. Considering most patients with COPD typically exhibit low levels of daily PA (Citation50), it is important to recognise that relatively small increases in PA are still likely to be effective in conferring important health benefits compared with larger PA increases in already more active populations (Citation51).
The components of all the PA interventions were variable in terms of duration, format of delivery, contacts and the behaviour change techniques utilised. This makes it difficult to distinguish which components are likely to optimise the benefit of the PA interventions. However, focusing on the intervention components in the seven studies measuring significant between-group differences in favour of the PA intervention appeared to show some patterns. It is currently unclear what intensity, length of intervention and mode of delivery (e.g., internet or face-to-face) is required to support change in PA behaviour in COPD. A whole diversity of behaviour change techniques were used with the most common including providing information and instruction on the behaviour, self-monitoring and goal setting in terms of the PA behaviour. It is not feasible to include every behaviour change technique so careful attention should be given to the theoretical framework of the PA intervention to ensure compatibility between the intervention format and planned behaviour change techniques (Citation18). Investment is important in future studies that explore optimal PA intervention/models.
The prescribed/recommended PA/exercise appeared to be based on established guidelines in COPD (Citation1,3) and/or using general PA recommendations (Citation52). In general, this included moderate aerobic PA/exercise for 150–180 minutes per week. Almost half of the interventions also included resistance training to provide a more holistic approach to PA/exercise training. Most of the interventions promoted walking which has proven physical and psychological health benefits (Citation53). Walking can be completed in a range of settings, has a very low injury risk and can be completed at variable speeds. The approach taken by Berry and colleagues in gradually reducing patients’ dependency on staff and structured centre-based exercise training towards independently self-regulating daily life PA could be practical and potentially help in the long-term adoption of PA behaviour change (Citation26).
The majority of studies in this review used an objective tool to assess PA (pedometer or accelerometer). They report variable parameters including step counts, activity counts, energy expenditure and time spent in different PA and this heterogeneity in reporting has limited the ability to conduct a pooled estimate of the treatment effect on PA levels. In order to provide a more comprehensive assessment of treatment effects, studies should include an objective outcome measure and report step counts and/or time spent in different intensities (including sedentary behaviour) at a minimum, and also energy expenditure when available (Citation54).
Pedometers can be used to help patients monitor themselves to promote self-regulation and set goals by giving direct feedback (Citation55), whereas most accelerometers do not provide direct feedback. Conversely pedometers can only enable estimation of daily step counts, whereas accelerometers provide a more accurate and comprehensive estimate of PA and sedentary behaviour in terms of volume and intensity. The subjective measures used in studies in this review included mostly in-house questionnaires and diaries. The limitations of questionnaires and diaries as outcome measures is well documented and subjective estimates of PA usually do not relate well to objective assessment (Citation56, 57). Within COPD, it is likely that questionnaires and diaries are more useful as an aid for goal setting and providing supplementary information on PA rather than providing an accurate PA assessment (Citation58).
This review updates published reviews in this area (Citation5,59,60) and provides a current synthesis of the literature related to PA interventions specifically in COPD. The strength of this review was that it followed the PRISMA systematic approach in terms of having a detailed search strategy, independent assessment of study eligibility, data extraction and quality assessment by two research team members. Study quality was moderate-high with no low quality studies which also helped to strengthen the review findings.
We were unable to pool the results of all studies together due to the large heterogeneity, in terms of the PA intervention and the reporting of the PA outcome assessment. However, we were able to calculate effect sizes for some studies to calculate the magnitude of the PA intervention treatment effects. This review focused on PA interventions and PA outcomes but did not explore the impact of PA on other important outcomes such as quality of life and exercise capacity. The lack of details provided in most of the papers made it difficult in some circumstances to determine whether certain behaviour change techniques were being utilised and resulted in reporting bias due to potentially relevant techniques not being classified (Citation61).
Globally there is a clear message that PA is important for improving health in chronic disease (Citation62). However, patients with COPD face specific challenges when trying to break the cycle of breathlessness and physical inactivity. Support to adopt a more physically active lifestyle could lead to improvements in symptoms such as reduced exercise capacity, breathlessness and depression (Citation63,64). The potential for low cost, accessible PA interventions is an attractive option for healthcare clinicians.
Conclusions
This review has shown that although the components of PA interventions were variable, there is some evidence that PA interventions have the potential to increase PA in patients with COPD. This review justifies investment in research that would explore the role of PA interventions in patients with COPD.
Declaration of Interest Statement
Author JW is funded by the Department for Employment and Learning Northern Ireland (DELNI) as part of his PhD. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary materials are available in the online version of this article.
Detailed characteristics of included studies
Download MS Word (73.6 KB)References
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013 Oct 15; 188(8):e13–64.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre; 2010 Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English (accessed on 4th November 2013).
- Bolton CE, Bevan-Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, Greening NJ, Heslop K, Hull JH, Man WD, Morgan MD, Proud D, Roberts CM, Sewell L, Singh SJ, Walker PP, Walmsley S. British Thoracic Society guideline on pulmonary rehabilitation in adults: accredited by NICE. Thorax 2013 Sep; 68(Suppl 2):ii1–ii30.
- Lacasse Y, Goldstein R, Lasserson T, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009 Jul 8; 3.
- Ng LWC, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis 2012 Feb; 9(1):17–26.
- Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, Rodríguez DA, Farrero E, de Batlle J, Benet M, Ferrer A, Barberà JA, Gea J, Rodriguez-Roisin R, Antó JM, Garcia-Aymerich J. Physical activity in COPD patients: Patterns and bouts. Eur Respir J 2013 Oct 1; 42(4):993–1002.
- Zwerink M, van der Palen J, van der Valk P, Brusse-Keizer M, Effing T. Relationship between daily physical activity and exercise capacity in patients with COPD. Respir Med 2013 Feb; 107(2):242–248.
- Marcus BH, Williams DM, Dubbert PM, Sallis JF, King AC, Yancey AK, Franklin BA, Buchner D, Daniels SR, Claytor RP. Physical activity intervention studies—What we know and what we need to know: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (subcommittee on physical activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006 Dec 12; 114(24):2739–2752.
- Rock CL, Doyle C, Demark–Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Cli. 2012 Jul-Aug; 62(4):242–274.
- Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes - the American College of Sports Medicine and the American Diabetes Association: Joint position statement. Med Sci Sports Exerc 2010 Dec; 42(12):2282–2303.
- Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2007 May 22; 115(20):2675–2682.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009 Jul 21; 6(7):e1000097.
- Cox NS, Alison JA, Holland AE. Interventions for promoting physical activity in people with cystic fibrosis. Cochrane Database Syst Rev 2011 Dec 7:12.
- Physiotherapy Evidence Database. Welcome to PEDro [Internet]. NSW, Australia: The George Institute for Global Health; 2013 Nov 4; [about 2 screens]. Available from: http://www.pedro.org.au/ (accessed 4 November 2013).
- de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 2009; 55(2):129–133.
- Rand D, Miller WC, Yiu J, Eng JJ. Interventions for addressing low balance confidence in older adults: A systematic review and meta-analysis. Age Ageing 2011 May; 40(3):297–306.
- Critical Appraisal Skills Programme. About CASP [Internet]. Oxford, United Kingdom: Critical Appraisal Skills Programme; 2010 Oct; [about 2 screens]. Available from: http://www.casp-uk.net/about-casp/ (accessed 4 November 2013).
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALORE taxonomy. Psychol Health 2011 Jul 14; 26(11):1479–1498.
- Cohen J. Statistical Power for the Behavioral Sciences, 2nd ed. Hillsdale, New Jersey: Lawrence Erlbaum, 1988.
- Nguyen HQ, Donesky D, Reinke LF, Wolpin S, Chyall L, Benditt JO, Paul SM, Carrieri-Kohlman V. Internet-based dyspnea self-management support for patients with chronic obstructive pulmonary disease. J Pain Symptom Manage 2013 Aug; 46(1):43–55.
- Pleguezuelos E, Pérez, ME, Guirao L, Samitier B, Ortega P, Vila X, Solans M, Riera A, Moreno E, Meri A, Miravitlles, M. Improving physical activity in patients with COPD with urban walking circuits. Respir Med 2013 Dec; 107(12):1948–1956.
- Tabak M, Vollenbroek-Hutten MM, van der Valk PD, van der Palen J, Hermens HJ. A telerehabilitation intervention for patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot trial. Clin Rehabil 2013 Nov 2013; 28(6):582–591.
- Pomidori L, Contoli M, Mandolesi G, Cogo A. A simple method for home exercise training in patients with chronic obstructive pulmonary disease: one-year study. J Cardiopulm Rehabil Prev 2012 Jan–Feb; 32(1):53–57.
- Effing T, Zielhuis G, Kerstjens H, van der Valk P, van der Palen J. Community based physiotherapeutic exercise in COPD self-management: A randomised controlled trial. Respir Med 2011 Mar; 105(3):418–426.
- Probst VS, Kovelis D, Hernandes NA, Camillo CA, Cavalheri V, Pitta F. Effects of 2 exercise training programs on physical activity in daily life in patients with COPD. Respir Care 2011 Nov; 56(11):1799–1807.
- Berry MJ, Rejeski WJ, Miller ME, Adair NE, Lang W, Foy CG, Katula JA. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respir Med 2010 Jun; 104(6):829–839.
- Breyer M, Breyer-Kohansal R, Funk G, Dornhofer N, Spruit MA, Wouters EF, Burghuber OC, Hartl S. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res 2010 Aug 22; 11(1):112.
- Hospes G, Bossenbroek L, ten Hacken NH, van Hengel P, de Greef MH. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns 2009 May; 75(2):274–278.
- Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis 2009 Aug; 4:301–313.
- Nguyen HQ, Donesky-Cuenco D, Wolpin S, Reinke LF, Benditt JO, Paul SM, Carrieri-Kohlman V. Randomized controlled trial of an internet-based versus face-to-face dyspnea self-management program for patients with chronic obstructive pulmonary disease: Pilot study. J Med Internet Res 2008 Apr 16; 10(2):e9.
- Steele BG, Belza B, Cain KC, Coppersmith J, Lakshminarayan S, Howard J, Haselkorn JK. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil 2008 Mar; 89(3):404–412.
- Varga J, Porszasz J, Boda K, Casaburi R, Somfay A. Supervised high intensity continuous and interval training vs. self-paced training in COPD. Respir Med 2007 Nov; 101(11):2297–2304.
- de Blok BM, de Greef MH, ten Hacken NH, Sprenger SR, Postema K, Wempe JB. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot study. Patient Educ Couns 2006 Apr; 61(1):48–55.
- Behnke M, Wewel AR, Kirsten D, Jörres RA, Magnussen H. Exercise training raises daily activity stronger than predicted from exercise capacity in patients with COPD. Respir Med 2005 Jun; 99(6):711–717.
- Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2003 Jan-Feb; 23(1):60–68.
- Moy ML, Weston NA, Wilson EJ, Hess ML, Richardson CR. A pilot study of an internet walking program and pedometer in COPD. Respir Med 2012 Sep; 106(9):1342–1350.
- Moy ML, Janney AW, Nguyen HQ, Matthess KR, Cohen M, Garshick E, Richardson CR. Use of pedometer and internet-mediated walking program in patients with chronic obstructive pulmonary disease. J Rehabil Res Dev 2010 Aug 2; 47(5):485–496.
- Wewel AR, Gellermann I, Schwertfeger I, Morfeld M, Magnussen H, Jörres RA. Intervention by phone calls raises domiciliary activity and exercise capacity in patients with severe COPD. Respir Med 2008 Jan; 102(1):20–26.
- Nguyen HQ, Carrieri-Kohlman V, Rankin SH, Slaughter R, Stulbarg MS. Is internet-based support for dyspnea self-management in patients with chronic obstructive pulmonary disease possible? Results of a pilot study. Heart Lung 2005 Jan-Feb; 34(1):51–62.
- Yohannes AM, Connolly MJ. Pulmonary rehabilitation programmes in the UK: a national representative survey. Clin Rehabil 2004 Jun; 18(4):444–449.
- O’Neill B, Elborn JS, MacMahon J, Bradley JM. Pulmonary rehabilitation and follow-on services: A Northern Ireland survey. Chron Respir Dis 2008 Aug; 5(3):149–154.
- O’Neill B, McKevitt A, Rafferty S, Bradley JM, Johnston D, Bradbury I, McMahon J. A comparison of twice-versus once-weekly supervision during pulmonary rehabilitation in chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2007 Feb; 88(2):167–172.
- Al Moamary MS. Health care utilization among chronic obstructive pulmonary disease patients and the effect of pulmonary rehabilitation. Med Princ Pract 2010 Jul 2010; 19(5):373–378.
- Earley D, MacMahon J, Bourbeau J, Bradley JM, O’Neill B. The adaptation and evaluation of the Living Well with COPD programme for pulmonary rehabilitation. In: British Thoracic Society Winter Meeting; 2011 Dec 7–9; London, United Kingdom. 2011. p. A127.
- Troosters T, Gosselink R, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: New insights and remaining challenges. Eur Respir Rev 2010 Mar 1; 19(115):24–29.
- Conn VS, Hafdahl AR, Porock DC, McDaniel R, Nielsen PJ. A meta-analysis of exercise interventions among people treated for cancer. Support Care Cancer 2006 Jul; 14(7):699–712.
- Avery L, Flynn D, van Wersch A, Sniehotta FF, Trenell MI. Changing physical activity behavior in type 2 diabetes: A systematic review and meta-analysis of behavioral interventions. Diabetes Care 2012 Dec; 35(12):2681–2689.
- Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, Meininger JC, Banks J, Stuart-Shor EM, Fletcher BJ, Miller TD, Hughes S, Braun LT, Kopin LA, Berra K, Hayman LL, Ewing LJ, Ades PA, Durstine JL, Houston-Miller N, Burke LE. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation 2010 Jul 27; 122(4):406–441.
- Motl RW, Pilutti LA, Learmonth YC, Goldman MD, Brown T. Clinical importance of steps taken per day among persons with multiple sclerosis. PLoS ONE 2013 Sep 4; 8(9):e73247.
- Bossenbroek L, de Greef MH, Wempe JB, Krijnen WP, ten Hacken NH. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD 2011 Aug; 8(4):306–319.
- Minton J, Dimairo M, Everson-Hock E, Scott E, Goyder E. Exploring the relationship between baseline physical activity levels and mortality reduction associated with increases in physical activity: a modelling study. BMJ Open 2013 Oct 18; 3(10):e003509.
- O’Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, Fox KR, Gately P, Giles-Corti B, Gill JM, Hamer M, McDermott I, Murphy M, Mutrie N, Reilly J, Saxton J, Stamatakis E. The ABC of physical activity for health: A consensus statement from the British Association of Sport and Exercise Sciences. J Sports Sci 2010 Apr; 28(6):573–591.
- Morris JN, Hardman AE. Walking to health. Sports Med 1997 May; 23(5):306–332.
- Van Remoortel H, Giavedoni S, Raste Y, Burtin C, Louvaris Z, Gimeno-Santos E, Langer D, Glendenning A, Hopkinson NS, Vogiatzis I, Peterson BT, Wilson F, Mann B, Rabinovich R, Puhan Milo A, Troosters T. Validity of activity monitors in health and chronic disease: a systematic review. Int J Behav Nutr Phys Acta 2012 Jul 9; 9:84.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007 Nov 21; 298(19):2296–2304.
- Prince S, Adamo K, Hamel M, Hardt J, Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 2008 Nov; 5:56.
- Shephard R. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med 2003 Jun; 37(3):197–206.
- Pitta F, Troosters T, Probst V, Spruit M, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J 2006 May; 27(5):1040–1055.
- Leidy NK, Kimel M, Ajagbe L, Kim K, Hamilton A, Becker K. Designing trials of behavioral interventions to increase physical activity in patients with COPD: Insights from the chronic disease literature. Respir Med 2014 Mar; 108(3):472–481.
- Vaes AW, Cheung A, Atakhorrami M, Groenen MT, Amft O, Franssen FM, Wouters EF, Spruit MA. Effect of ‘activity monitor-based’ counseling on physical activity and health-related outcomes in patients with chronic diseases: A systematic review and meta-analysis. Ann Med 2013; 45(5–6):397–412.
- McGauran N, Wieseler B, Kreis J, Schüler Y, Kölsch H, Kaiser T. Reporting bias in medical research—A narrative review. Trials 2010 Apr 13; 11:37.
- Lee I, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012 Jul 21–27; 380(9838):219–229.
- Mitchell KE, Warrington V, Sewell L, Bankart J, Williams JE, Steiner M, Morgan M, Singh SJ. A randomised controlled trial of a self-management programme of activity coping and education—SPACE FOR COPD: Impact on physical activity at 6 weeks [abstract]. Am J Respir Crit Care Med 2013 May 19; 187:A5952.
- Watts S, Ng C, Mckeough Z, Jenkins S, Hill K, Eastwood P, Hillman D, Jenkins C, Cecins N, Spencer L, Alison J. Effects of ground walking training in COPD: A Randomised Controlled Trial [abstract]. Respirology 2013 Apr; 18(S2):O064.