Abstract
Background: Knowledge on factors associated with mortality can help identify patients with COPD that might benefit from close monitoring and intervention. Arterial blood gases (ABGs) are related to mortality, but both arterial tension of oxygen (PaO2) and arterial tension of carbon dioxide (PaCO2) vary over time. The aim of our study was to investigate the association between repeatedly measured ABGs and mortality in men and women with COPD. Methods: A cohort of 419 Norwegian subjects with COPD, GOLD stage II-IV, aged 40–75, was followed up with up to seven ABGs, measured during stable phase for three years. Cox proportional hazard models were used to quantify the relationship between both single and repeatedly measured ABGs and all-cause mortality after five years, adjusting for age, sex, and the updated BODE index. Results: A total of 64 subjects died during follow-up. Mean initial arterial oxygen tension (standard deviation) was significantly higher in survivors compared to deceased, with PaO2 (in kPa) 9.4 (1.1) versus 8.8 (1.2), p<0.001. Corresponding numbers for PaCO2 were 5.3 (0.5) and 5.5 (0.7), p < 0.001. In analyses adjusting for age, sex, and the updated BODE index hazard ratios – HR(95% confidence intervals) - for all-cause mortality were 0.73 (0.55, 0.97) and 1.58 (0.90, 2.76) for repeated measures of PaO2 and PaCO2, respectively. Conclusion: Both arterial oxygen and carbon dioxide tension were related to mortality in this study, and arterial oxygen tension added prognostic information to the updated BODE index in COPD.
Introduction
COPD is the fifth-leading cause of death world wide, and is estimated to rank as the third most frequent cause of death in 2020 (Citation1). Knowledge on factors associated with mortality can help identify COPD patients that might benefit from close monitoring and intervention. The multidimensional BODE index is a composite measure that predicts survival better than each of its components (dyspnoea, lung function, exercise capacity, and body mass index) (Citation2, 3). However, exercise capacity testing is not always feasible in a clinical setting.
Assessment of arterial blood gases (ABGs) are recommended in stable COPD patients with peripheral oxygen saturation < 92% (Citation4), so that long-term continuous oxygen treatment can be initiated in patients with respiratory failure. Despite the documented effect of oxygen treatment on survival in respiratory failure (Citation5, 6), the reports concerning the relationship between partial pressure of arterial oxygen (PaO2) and survival in studies comprising COPD patients both with and without hypoxemia have been conflicting (Citation7–10).
The 2007 GOLD guidelines categorized subjects with respiratory failure in GOLD stage IV together with subjects with forced expiratory volume in one second (FEV1) < 30%. In the updated GOLD guidelines assessment of COPD is based on severity of disease (airflow limitation), symptoms, and history of exacerbation, but the inclusion of subjects with respiratory failure into the category with most severe airflow limitation as “this seemed to be an arbitrary inclusion” without further documentation of the statement (Citation4).
Hypercapnia (partial pressure of arterial carbon dioxide (PaCO2) > 6.7 kPa) has also been related to mortality in COPD (Citation11–13), but it has been difficult to disentangle the relationship between mortality and hypercapnia per se from hypoxemia combined with hypercapnia (Citation5–7, Citation14, 15).
Since levels of PaO2 and PaCO2 may vary with disease activity, we hypothesized that temporal trajectories of arterial blood gases could improve the predictive value of arterial blood gases of mortality in COPD. Using the Bergen COPD study cohort including both male and female subjects in GOLD stages II-IV, we aimed to evaluate the association between repeated measures of arterial blood gases measured in the stable phase and all-cause mortality, and to investigate whether ABGs add prognostic information to an established multi dimensional measure as the BODE index.
Methods
Study population and outcome
The study sample comprised 419 patients with COPD aged 40-75 included in the Bergen COPD cohort study. COPD was defined as post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.70 and FEV1 < 80% predicted, corresponding to GOLD stage II (Citation16). Norwegian pre-bronchodilator reference values for spirometry were used (Citation17). The subjects were recruited from hospital outpatient clinics, private pulmonary specialists practices, and through advertisements in local newspapers during 2006.
Exclusion criteria comprised having other known respiratory disease; long-term oral corticosteroid treatment; former lung surgery; inflammatory bowel disease; rheumatoid arthritis, blood transfusion in the last four weeks; cancer in the last five years, or a smoking history of less than 10 pack-years. The selection of the study participants is reported previously (Citation18). Briefly, of a total of 928 potential study participants, 286 refused to participate in the study, and 220 subjects did not meet inclusion criteria. Of the 433 remaining subjects, 14 were not assessed with arterial blood gases.
A total of 419 patients were followed up with arterial blood gases drawn during visits at the study centre every six months for three years. If acute exacerbation of COPD coincided with a scheduled visit, the visit was postponed until four weeks after symptom relief or end of medical treatment of the exacerbation. If a patient did not show up to a visit, he or she was contacted by phone.
If neither contact with the patient nor its kin was established, survival status was obtained from the Norwegian patient registry. All subjects were monitored until death or end of follow-up, 25th of August 2011. All-cause mortality during the follow-up period was used as the outcome measure. Written informed consent was obtained prior to inclusion, and the regional ethical committee approved the study (the Western Norway Regional Research Ethics Committee, approval number 165.08.).
Arterial blood gas measurements
With the patient sitting, breathing room air, the study physician drew 1.5 ml blood from the radial artery with a heparinised self-filling syringe (Radiometer PICO 70 arterial sampler, Radiometer, Copenhagen, Denmark). In patients with long-term oxygen therapy, oxygen was discontinued 30 minutes prior to arterial blood gas sampling. The samples were analysed in duplicates within a few minutes, with a daily-calibrated apparatus (Radiometer ABL 520, Radiometer, Copenhagen, Denmark). PaO2 tended to drift upward and PaCO2 downward from the first to the second analysis. Hence, we used the first duplicate in the analyses. Nevertheless, if the discrepancy between the first and second measurement exceeded 1 kPa, the values were checked up against the hospital's medical archives, and the values closest to the patient's former arterial blood gas values were chosen. This was the case for 20 samples from 20 different subjects.
Covariates
Dyspnoea was measured using the modified Medical Council Dyspnoea scale (MRC) (Citation19). Exercise capacity was evaluated through six minutes walking distance (6MWD) in meters. Body mass index (BMI, kg/m2) was calculated using weight and height measured during the first visit. The 6MWD, MRC scale, FEV1, and BMI were used to calculate the updated BODE index (Citation3), which was used as a measure of disease severity.
Statistical analyses
The statistical analyses were conducted using Stata version 12. Continuous variables were described as mean and standard deviation (SD) when normally distributed, or as median and 25th–75th percentiles when having skewed distributions. Bivariate tests were performed using Student's t test for normally distributed outcomes, Kruskal-Wallis and Mann–Whitney U-tests for skewed outcomes, Pearsons χ2 test for two categorical variables, and the log rank test for equality of survival functions. P values less than 0.05 were considered significant.
The associations of arterial blood gases and all-cause mortality were assessed using Cox proportional hazards models. Several modelling approaches were followed in order to assess effects of different temporal patterns for arterial blood gases on mortality. First, a single measure of PaO2 and PaCO2 was considered for each patient. Single-measure models were fitted using the usual fixed-covariate Cox proportional hazards model taking as single predictor measure the first available value, worst (lowest PaO2 and highest PaCO2), or last value of arterial blood gases.
The highest/lowest and the last were expected to capture short-term associations between mortality and arterial blood gases, while baseline would describe long-term associations. Single-measure models assume that individuals had the same value of arterial blood gases for the entire follow-up period, which may lead to incorrect inferences if variations in PaO2 and PaCO2 over time are related to mortality. Thus, the data was also analysed treating PaO2 and PaCO2 as repeated time-dependent covariates (Citation20), where a hazard ratio was calculated based upon the risks of death in all the time periods following all blood gas measurements.
Finally, the possibility that the shape of the blood gas trajectories over time (decreasing trend, stable, and increasing trend) could provide additional information about the risk of death. This model was fit in two steps. In the first step, the slopes of PaO2 and PaCO2 against time were calculated for each participant, provided that they had two or more blood gas measurements. The slopes were categorized into three categories according to tertiles, and this variable was included as a predictor in a Cox regression model along with the corresponding baseline level of PaO2 or PaCO2.
All models were adjusted for sex, age, and the updated BODE index at baseline. All models were first fitted separately for PaO2 and PaCO2. As a sensitivity analysis, we performed the analyses excluding subjects with long-term oxygen therapy, since it is related to survival. Results were expressed as hazard ratios (HR) with 95% confidence intervals (CI).
Results
During a median follow-up time of 5.12 years, 64 individuals (15.3%) died.
The deceased were older and leaner; had lower lung function and exercise capacity; had more dyspnoea and a longer history of tobacco exposure (Table ). Current smoking status, sex, and number of exacerbations the year prior to inclusion did not differ between survivors and deceased. Altogether 2062 blood gas measurements were conducted. With 100% attendance rate to the visits the potential number of blood gas measurements in survivors was 2250.
Table 1. Baseline demographic-, clinical-, and physiological characteristics of COPD patients by survival status after five years
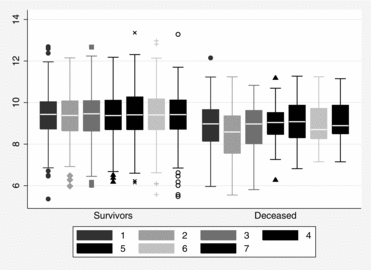
There were 358 blood gases (16%) missing due to nonattendance, and 22 (1%) blood gases missing due to patient refusal or technical failure. A total of 100 subjects (23.9%) had one or more visits with PaO2 < 8.0 kPa, and 19 subjects (4.5%) had one or more visits with PaCO2 > 6.7 kPa. The deceased had on average significantly lower measures of PaO2 than the surviving (Table ) both in first (p < 0.001), lowest (p < 0.01), and last (p < 0.001) blood gas measurements. Mean PaO2 was rather stable through the study period in survivors, but the variability was greater and the median lower in the deceased (Figure ).
Table 2. Distribution of arterial blood gases in kPa by survival status after five years
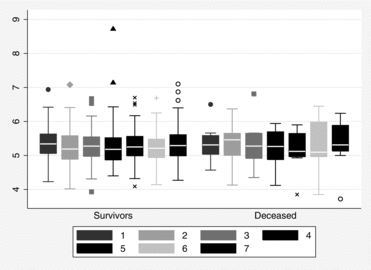
PaCO2 was significantly higher in the first (p < 0.001), highest (p < 0.05), and last (p < 0.01) measurements among deceased than survivors (Table ), but the visual difference in the repeated measurements was not obvious (Figure ). Univariate Cox proportional hazard models identified increased risk of death with higher age; lower PaO2; higher PaCO2; higher score in the updated BODE index and each of its individual components; higher tobacco exposure; and presence of co-morbidity (the Charlson index and coronary heart disease) (Table ). Using the Cox proportional hazard method with adjustments for age and sex, both PaO2 and PaCO2 were associated with mortality (Table ). The hazard ratio estimates did not change overtly when cluster analyses for repeated measurements were applied. Taking the updated BODE index into account, only PaO2, and not PaCO2 remained associated with mortality, with a 23–27% reduced risk for death by each kPa increase in arterial oxygen tension.
Table 3. Crude hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality after five years by baseline demographic, clinical, and physiological characteristics from Cox proportional hazard models
Table 4. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality after five years with respect to first and repeated measurements of PaO2 and PaCO2
The slope of PaO2 for those with two or more arterial blood gases during follow up ranged from −0.83 to 0.79, with tertile cut-offs at −0.08 and 0.07. The corresponding numbers for the PaCO2 slope were −0.57 and 1.42; −0.04 and 0.02, respectively. When the slopes of PaO2 and PaCO2 were included as predictors in a Cox proportional hazards model, having a decreasing trend of PaO2 was associated with a four-fold increase in mortality (Table ). Trends in CO2 trajectories were not related to morality.
Table 5. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality after five years with respect to variation in blood gas values expressed as slopes for PaO2 and PaCO2 (regressed linearily on time)
Discussion
In this COPD cohort including both men and women with moderate to very severe stages of COPD, we found that PaO2 was associated with all-cause mortality, independent of age, sex, and the updated BODE index. Furthermore, models using single, repeated, and trajectory measurements of PaO2 all suggested long-term effects of PaO2 on mortality. PaCO2 was not associated with mortality in COPD when age, sex, the updated BODE index were taken into account. This implies that measurements of ABG adds prognostic information to the updated BODE index, and that in settings where measurement of the BODE component is not feasible, ABG can convey information regarding prognosis.
Previous studies have relied on one or maximum two measurements of arterial blood gases, and to our knowledge, this is the first study that follows up a cohort of COPD patients with repeated blood gas measurements in relation to mortality. Former studies have not found a relationship between PaO2 and mortality in multivariate analyses (Citation21–24). These studies however, have investigated mortality in selected patient cohorts, with either mostly (Citation24) or solely (Citation21) males, or in patients with very severe COPD (Citation23).
Our finding that PaO2 in repeated measurements is related to mortality in a cohort of patients of both sexes, with a wide age span and varying disease severity, taking other prognostic variables into account, suggests that PaO2 carries prognostic information in heterogeneous COPD cohorts. The repeated measurements enabled us to evaluate the relationship between mortality and arterial blood gases in a long-term versus short-term perspective.
The results from the repeated measurements analysis in our study were similar to the results from the baseline single measurements, which indicates that PaO2 in COPD in the stable phase can give information on prognosis. As opposed to the GOLD classification of COPD severity from 2007, evaluation of disease severity in the present GOLD guidelines do not take respiratory failure into account (Citation4), as “this seemed to be an arbitrary inclusion.” The present article contradicts this statement.
We did not find any association between PaCO2 and mortality when we adjusted for the updated bode index in our study. This is in contrast with the findings in a study of patients receiving non-invasive home ventilation (Citation25), and with some studies of patients discharged after hospital treatment of acute exacerbation where PaCO2 have been associated with mortality (Citation22, Citation26–28). Two other studies of hospitalized COPD patients failed to find such a relationship (Citation29, 30), and no such relationship was found in a study of severe emphysema patients.
In our study, PaCO2 was rather stable, and there were few patients with hypercapnic respiratory failure, leaving little strength to detect associations between PaCO2 and mortality. On the other hand, there are many other factors related to hospitalization that might confound the relationship between PaCO2 and mortality, like severity of disease, co-morbidity, age, and functional capacity.
There are some limitations that should be acknowledged. We analysed repeated measurements of arterial blood gases without taking into account the changes in the BODE index. These measures were not available. This could represent a problem if the change in the BODE index variables were a result of a change in the arterial blood gases. If this is true, the association between arterial blood gases and mortality is overestimated. On the other hand, if the changes in the BODE index variables influences the blood gas levels there is no risk of overestimation of the association between blood gas levels and mortality. Another potential limitation is the missing blood gases, altogether 358 (16%) due to nonattendance. The subjects with one or more unattended visits were younger, more likely to be women, and had higher FEV1 than the ones that attended to all scheduled visits.
However, the baseline blood gas values of the ones that did not show up to all visits were not significantly different from the ones that attended to all. There were 22 blood gases that were not obtained. Some were missing completely at random (due to technical problems), but some of the patients did not allow arterial blood gases to be taken at all points in time. Sampling of arterial blood gas can be unpleasant, but yet another reason to refuse is the fear of the consequences of hypoxemia. In Norway, patients with hypoxemia are not allowed to drive a car without supplement of oxygen, and that is not given to patients that smoke. However, the number of missing blood gas values in the smoking subjects with hypoxemia was low, and not significantly different from the number of missing blood gases in non-smoking subjects with hypoxemia. Hence, this is not likely to have biased our results.
Conclusions
In this study we found that PaO2, but not PaCO2, was independently associated with long-term mortality even after disease severity was taken into account. Our results suggest that arterial blood gas measurements add information to composite prognostic indices regarding the prognosis in COPD. Further, this article is a counterargument to the latest GOLD report stating that inclusion of respiratory failure in the most severe GOLD grade seemed arbitrary, as we found that respiratory failure grade I in COPD was associated with mortality.
Declaration of Interest Statement
The authors have neither received financial support nor have been involved with organizations with financial interest in the subject matter. The authors have no relevant financial activities outside the submitted work during the last 36 months to report.
The sampling of the cohort study was funded by unrestricted grants from The Foundation for Respiratory Research, University of Bergen, Bergen, Norway and by grants from the Centre for Clinical Research, Haukeland University Hospital, Bergen, Norway.
MAa wrote the article, and performed the statistical analyses together with MB and XB. TMLE, JAH, and PSB planned the study. EWS contributed to data collection. XB, MB, and JMA planned the analyses together with MAa. All authors contributed actively in the writing process.
Acknowledgments
The authors thank Lene Svendsen, Eli Nordeide, Tina Andersen, and Inge Zwetzig for help with data collection.
References
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997 May 3; 349:1269–1276.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004 Mar 4; 350:1005–1012.
- Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Antó JM, Agusti AG, Gómez FP, Rodriguez-Roisin R, Moons KG, Kessels AG, Held U. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009 Aug 29; 374:704–711.
- Global Strategy for Diagnosis, Management, and Prevention of COPD. Available from: http://www.goldcopd.org (accessed 18 June 2014).
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980 Sep; 93:391–398.
- Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981 Mar 28; 1:681–686.
- Chailleux E, Fauroux B, Binet F, Dautzenberg B, Polu JM. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. A 10-year analysis of ANTADIR Observatory. Chest 1996 Mar; 109:741–749.
- Coleta KD, Silveira LV, Lima DF, Rampinelli EA, Godov I, Godov I. Predictors of first-year survival in patients with advanced COPD treated using long-term oxygen therapy. Respir Med 2008 Apr; 102:512–518.
- Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Clin Chest Med 1990 Sep; 11:555–569.
- Terzano C, Conti V, Di Stefano F, Petroianni A, Ceccarelli D, Graziani E, Mariotta S, Ricci A, Vitarelli A, Puglisi G, De Vito C, Villari P, Allegra L. Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 2010 Aug; 188:321–329.
- Boushy SF, Thompson HKJ, North LB, Beale AR, Snow TR. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1973 Dec; 108:1373–1383.
- Burrows B, Earle RH. Prediction of survival in patients with chronic airway obstruction. Am Rev Respir Dis 1969 Jun; 99:865–871.
- Postma DS, Burema J, Gimeno F, May JF, SMit JM, Steenhuis EJ, Weele LT, Sluiter HJ. Prognosis in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1979 Mar; 119:357–367.
- Aida A, Miyamoto K, Nishimura M, Aiba M, Kira S, Kawakami Y. Prognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapy. Am J Respir Crit Care Med 1998 Jul; 158:188–193.
- Soler-Cataluna JJ, Sanchez-Sanchez L, Martinez-Garcia MA, Sánchez PR, Salcedo E, Navarro M. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest. 2005 Oct; 128:2108–2115.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverly P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J, Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007 Sep 15; 176:532–555.
- Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke P, Gulsvik A. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med. 2006 Jun 15; 173:1316–1325.
- Eagan TM, Ueland T, Wagner PD, Hardie JA, Mollnes TE, Damás JK, Aukrust P, Bakke PS. Systemic inflammatory markers in COPD: results from the Bergen COPD Cohort Study. Eur Respir J. 2010 Mar; 35:540–548.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988 Mar; 93:580–586.
- Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health 1999; 20:145-157.
- Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, Félez M, Khalaf A, Marrades RM, Monsó E, Serra-Battles J, Antó JM. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002 Sep 1; 166:680–685.
- Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996 Mar; 153:961–966.
- Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, DeCamp MM, Benditt J, Sciurba F, Make B, Mohsenifar Z, Diaz P, Hoffman E, Wise R, NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006 Jun 15; 173:1326–1334.
- Nishimura K, Izumi T, Tsukino M et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002 May; 121:1434–1440.
- Budweiser S, Jorres RA, Riedl T, Heinemann F, Hitzl AP, Windisch W, Pfeifer M. Predictors of survival in COPD patients with chronic hypercapnic respiratory failure receiving noninvasive home ventilation. Chest 2007 Jun; 131:1650–1658.
- Goel A, Pinckney RG, Littenberg B. APACHE II predicts long-term survival in COPD patients admitted to a general medical ward. J Gen Intern Med 2003 Oct; 18:824–830.
- Sanjaume M, Almagro P, Rodriguez-Carballeira M, Cuchi E, Torres J, Heredia JL. [Post-hospital mortality in patients re-admitted due to COPD. Utility of BODE index]. Rev Clin Esp 2009 Sep; 209:364–370.
- Slenter RH, Sprooten RT, Kotz D, Wesseling G, Wouters EF, Rohde GG. Predictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary disease. Respiration 2013; 85:15–26.
- Chung LP, Winship P, Phung S, Lake F, Waterer G. Five-year outcome in COPD patients after their first episode of acute exacerbation treated with non-invasive ventilation. Respirology. 2010 Oct; 15:1084–1091.
- Roca B, Almagro P, López F, Cabrera FJ, Montero L, Morchón D, Diez J, de la Iglesía F, Fernández M, Castiella J, Zubillaga E, Recio J, Soriano JB, for the ECCO Working Group on COPD, Spanish Society of Internal Medicine. Factors associated with mortality in patients with exacerbation of chronic obstructive pulmonary disease hospitalized in General Medicine departments. Intern Emerg Med 2011 Feb; 6:47–54.