Abstract
We aimed at exploring whether the prevalence of co-morbidities of chronic obstructive pulmonary disease (COPD) increases with COPD severity. Analysis of medical records of outpatients with established diagnosis of COPD was retrospectively performed. The lower limit of normality (LLN) for FEV1/FVC was applied to establish the occurrence of airway obstruction in the elderly population. The prevalence of co-morbidities was calculated, and the proportion of patients with each co-morbidity along with GOLD stages was analysed by chi-square for trend. A total of 326 (M/F: 256/70) consecutive outpatients with COPD (stage GOLD I to IV), aging 71.8 ± 9.2 years, were included in the analysis. The most frequent co-morbidities in the entire sample were systemic hypertension (64.7%), diabetes (28.5%), coronary artery disease (19.9%), arrhythmias (16.6%) and congestive heart failure (13.8%). Underweight patients were 8.0% of the sample while obese patients were 22.4%. None of the analyzed co-morbidities showed a trend towards increasing prevalence with COPD severity, except for nutritional problems. The current findings suggest that the occurrence and prevalence of co-morbidities is independent from the COPD severity, and encourage to assess co-morbidities even in the early stages of the COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) shows high prevalence in general population, especially in the geriatric age (Citation1). It is known that most often COPD patients are affected by various coexisting diseases, or co-morbidities, including mainly, but not limited to, cardiovascular diseases, metabolic syndrome, skeletal muscle dysfunction, depression, osteoporosis, pulmonary infections, cancer and pulmonary vascular disease (Citation2). Moreover, the prospective evaluation of several co-morbidities allowed to identify those with higher prevalence in patients with COPD and those that are risk factors for increased mortality in COPD (Citation2). Among these co-morbidities, Divo and co-workers (Citation2) identified 12 diseases that could be used to create a quantitative risk stratification co-morbidity tool, named COTE index: lung, esophageal, pancreatic, and breast cancer, liver cirrhosis, atrial fibrillation, diabetes with neuropathy, pulmonary fibrosis, congestive heart failure, gastric/duodenal ulcers coronary artery disease and anxiety.
The reason for the high association between COPD and other diseases has not been fully elucidated. Describing COPD co-morbidities, the 2009 version of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Citation3) identified four modalities of co-morbidity occurrence: common pathway co-morbidities, complicating co-morbidities, co-incidental co-morbidities, and inter-current co-morbidities. However, such a classification was not reported in the subsequent editions of the document, while the interest was more centered on the treatment of co-morbidities, rather than their pathogenesis (Citation4).
In a recent study, 5 clusters of co-morbidities were identified (Citation5), showing comparable degree of COPD severity. Alternative approaches grouping co-morbidities in categories according to the relative number of associated chronic conditions were proposed (Citation6), showing that the distribution of staging and functional status was no different across the categories. However, it is not known whether co-morbidities increase with the severity of COPD. Although it is reasonable to presume that more severe degrees of respiratory impairment may facilitate the genesis or the clinical presentation of co-morbidities, the question whether co-morbid diseases are mostly present in the most severe patients (GOLD IV) or rather, their frequency is constant along with COPD stages is still a debatable issue. This is not a trivial point since these two scenarios could lead to different hypotheses on pathogenetic mechanisms and could imply a different impact on the clinical management.
We hypothesized that co-morbidities increase with COPD severity. Therefore, the present study was aimed at exploring the prevalence of co-morbidities of COPD in relation with COPD severity.
Materials and Methods
Subjects
We included subjects with an established diagnosis of COPD, confirmed by clinical assessment, as exposure to smoking habit and presence of dyspnea, cough and chronic sputum production in patients with lung functional values compatible with COPD, according to GOLD guidelines (Citation4); they were regularly followed for their disease at the outpatient clinics for respiratory diseases of the University of Palermo, Italy. All patients were under regular treatment for COPD commensurated with their degree of disease. The paucity of information on the rate of exacerbations and the lack of COPD Assessment Test (CAT) in the clinical charts did not allow to classify patients according to the new GOLD recommendations (Citation4). Spirometric tests were performed using a computerized water-sealed spirometer (Biomedin; Padua, Italy).
Criteria and procedures for measurement were selected in accordance to the ATS/ERS recommendations for standardisation of spirometry (Citation7). The diagnosis of airway obstruction in the elderly population was made by applying the lower limit of normality (LLN) for the FEV1/FVC ratio. In details, we used the equation provided by the study of Sorino and co-workers (Citation8), which identifies the LLN < 0.65 and < 0.67 for men and women, respectively. The local Ethic Committee approved the retrospective analysis of patients’ charts.
Study Design and Measurements
We performed a retrospective, observational study, on the basis of information contained in the clinical records of our specialist outpatient clinics. We collected the data on the frequency of some of the most common co-morbidities in patients with COPD, including arrhythmias (mainly atrial fibrillation), congestive heart failure (CHF), coronary artery disease (CAD), systemic hypertension, cerebrovascular disease, diabetes, chronic renal failure (CRF), depression (requiring treatment). The diagnosis of co-morbidities was done in retrospective way combining the information recorded in our electronic medical records. Therefore, the non-respiratory diagnoses were made by a combination of the available data, including: patients’ reported diagnosis, clinical charts/documents, non-respiratory drug use.
To analyze body mass index (BMI), we divided patients into three groups: malnourished or underweight patients (BMI < 21 Kg/m2), normal/overweight patients (BMI from 21 to 30 Kg/m2) and obese patients (BMI > 30 Kg/m2). This was based on previous literature that demonstrated that malnutrition, identified as BMI < 21 Kg/m2, is a risk factor for mortality in COPD (Citation9) and that a potential link between obesity and COPD also exists (Citation10).
Statistical Analysis
Statistical analysis was carried out using SPSS software (release 21 for Windows). Data are presented as mean ± SD, frequency distribution or proportion, as appropriate. Frequencies were analysed by chi-square test (χ2). Moreover, chi-square for trend analysis was carried out to assess the presence of linear trend in proportions of patients with co-morbidities, stratified by the four GOLD stages. Differences at probability values of p < 0.05 were considered to be statistically significant.
Results
A total of 326 consecutive outpatients with COPD, attending the clinic in the year 2012, were included in the analysis. The study sample consisted of 256 males and 70 females, with a mean age of 71.8 ± 9.2 years. Characteristics of the sample by GOLD stages are presented in Table .
Table 1. Characteristics of the sample
The prevalence of the analysed co-morbidities in the entire sample was 64.7% for systemic hypertension, 28.5% for diabetes, 19.9% for CAD, 16.6% for arrhythmias, 13.8% for CHF, 6.1% for cerebrovascular disease, 7.1% for CRF and 5.8% for depression. Underweight patients were 8.0% of the sample while obese patients were 22.4%.
When the trend in proportions of patients suffering from the analysed co-morbidities was evaluated according to the GOLD stages, the proportions did not show any linear trend in the cases of arrhythmias, cerebrovascular disease, diabetes, CRF, and depression (highest χ2 for trend = 3.5; lowest p = 0.071). These data are summarised in Table . Conversely, a linear trend in proportions along with GOLD stages was only found for underweight (χ2 for trend = 4.5; p = 0.039); (Figure ).
Figure 1. Proportions of patients suffering from underweight along with GOLD stages. For this co-morbidity a linear trend in proportions was demonstrated.
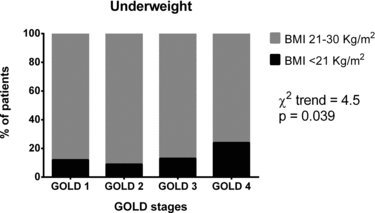
Table 2. Co-morbidities that did not show a linear trend in proportions with GOLD stages
Discussion
The main finding of this study is that the prevalence of co-morbidities does not tend to increase with severity of COPD, except for nutritional problems, thus implying that the occurrence of the explored co-morbidities is independent from the disease and therefore encouraging the multidisciplinary assessment of COPD even in early GOLD stages.
It has been largely demonstrated that patients with COPD show higher proportion of arrhythmias (Citation11), CHF (Citation12), CAD (Citation11, Citation13), systemic hypertension (Citation11, Citation13), cerebrovascular disease (Citation14), diabetes (Citation13, Citation15), CRF (Citation16), and depression (Citation17) compared to age-matched controls.
Our study is consistent with the analysis on the ECLIPSE cohort (Citation11), which revealed that co-morbidities were generally not associated with the severity of the airflow limitation (GOLD stage). Our study adds the analysis of the trend in proportions that in the ECLIPSE study (Citation11) was not systematically applied.
Several factors may explain our results. First, the co-morbidities that do not show any association with severity of the disease could occur because of age-associated changes. We favour the hypothesis that shared risk factors and common pathways in pathogenesis explain the lack of trend at least in CAD, arrhythmias, cerebrovascular disease. It is known that the presence of respiratory impairment is associated with a higher risk of these co-morbidities (Citation13, Citation18), but the reasons for this association are unclear: shared risk factors undoubtedly are involved (Citation19, 20). Although, this study was not designed to demonstrate which co-morbidities should be considered consequences or systemic effects of COPD, the current findings could contribute to design further studies specifically aiming at distinguishing the relationship of those co-morbid conditions with COPD.
We postulate that mechanisms explaining the association with underweight and obesity are different since these co-morbidities showed higher proportion of patients in advanced COPD. Underweight seems a consequence of COPD (Citation21), probably due to muscular deconditioning (Citation22), with clear linear trend from GOLD I to IV (Figure ).
The interpretation of the link between obesity and COPD is controversial (Citation23). The prevalence of obesity appears increased in patients with COPD (Citation24), although this finding is not constant (Citation25). The phenomenon referred to as a ‘‘reverse epidemiology of obesity’’ or the ‘‘obesity paradox’’ has been described (Citation10): the obesity is a factor decreasing mortality in COPD (Citation26), especially in GOLD stages III and IV (Citation10). In our sample, although a trend was not demonstrated, the prevalence of obesity in GOLD stage IV is higher than in some studies (Citation27), and comparable with others (Citation14). It is possible that obesity itself contributes to lower FEV1, leading to those subjects being classified as having severe COPD (Citation28).
Why is it important from a practical perspective to differentiate the co-morbidities? The lack of trend with COPD severity implies the possibility that co-morbidities may occur early during the course of the disease. It raises questions about their potential pathogenic mechanisms (Citation21) and emphasizes the clinical importance of identifying and treating them if present, early in the course of the disease. On the contrary, the importance of nutritional problems may rise in GOLD stage III and IV, however it is not known whether the early treatment of COPD may influence these co-morbidities. Decramer and Cooper (Citation29) elegantly discussed that at present that there is no irrefutable evidence that early treatment of COPD is better than later treatment. As they pointed out, there is evidence for dynamic hyperinflation being present early in the course of the disease, even in GOLD stage I: whether early treatment reduces inactivity and, hence, co-morbidities is not known.
This study has some limitations. The study was retrospective and therefore frequency of co-morbidities was not systematically recorded. This could have led to underestimation of further potentially important interactions. The study did not include a control group however, this should not influence the results, since it was aimed at exploring the proportions of patients with and without co-morbidities within the COPD and following the worsening of the disease, as accounted for by GOLD stages. Although the prevalence of co-morbidities varies among studies present results are in line with other recently reported in literature (Citation2), with the exception of CRF and depression, which were lower in the present study. This observation, along with the higher prevalence of obese patients, could be due to a bias caused by unpredictable and unlikely distinctive characteristics of the local outpatient clinic. Finally, we are aware that lung cancer prevalence is largely underestimated in our sample, because patients with lung cancer are usually referred to a different specialized clinic.
Unfortunately, detailed data on lung diffusion or imaging data (CT scan) for emphysema were only available in a small proportion of the study subjects, not allowing to draw conclusions with regard to the behaviours of different phenotypes of COPD.
The stratification of disease severity in this study led to a very high proportion of the very severe group. This derives from the “real life” nature of the study, which was not designed for an active recruitment, and reflects the population of patients usual referred to an outpatient clinics. Moreover, stratification problems have been observed even in observational, longitudinal and controlled studies (Citation30), where GOLD stage I were completely lacking, in comparison with 7% reported in the present study.
The proportion of female in our sample (about 20%) is lower compared with a recent study on co-morbidities of COPD (Citation11), which reported a female proportion of 35%. However, the lower proportion of females is consistent with epidemiology of smoking habit in elderly Italian population.
Conclusions
In conclusion, the present results favour the hypothesis that COPD and its co-morbidities develop in parallel. Under a clinical point of view, present results encourage to assess co-morbidities routinely, as part of the diagnostic workflow, even in the early stages of the COPD, since prevalence of several co-morbidities is not influenced by COPD severity.
Declaration of Interest Statement
All authors declare that they do not have any conflict of interest, which may affect the conduct or reporting of the present work. All authors are responsible for the content and the writing of this article.
References
- Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Euro Respir J 2007; 30(5):993–1013.
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Zulueta J, Cabrera C, Zagaceta J, Hunninghake G, Celli B, Group BC. Co-morbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2012; 186(2):155–161.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD (Updated 2009). 2009. p. Available from: http://www.goldcopd.org/.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD (Updated 2013). 2013. p. Available from: http://www.goldcopd.org/.
- Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, Rutten EP, Op ‘t Roodt J, Wouters EF, Franssen FM. Clusters of co-morbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 2013; 187(7):728–735.
- Crisafulli E, Costi S, Luppi F, Cirelli G, Cilione C, Coletti O, Fabbri LM, Clini EM. Role of co-morbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 2008; 63(6):487–492.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Euro Respir J 2005; 26(2):319–338.
- Sorino C, Battaglia S, Scichilone N, Pedone C, Antonelli-Incalzi R, Sherrill D, Bellia V. Diagnosis of airway obstruction in the elderly: contribution of the SARA study. Inter J Chron Obstruct Pulmon Dis 2012; 7:389–7395.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. The New England journal of medicine. 2004; 350(10):1005–1012.
- Franssen FM, O'Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008; 63(12):1110–1117.
- Miller J, Edwards LD, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, Miller BE, Rennard S, Silverman EK, Tal-Singer R, Vestbo J, Wouters E, Yates JC, Macnee W. Co-morbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013; 107(9):1376–1384.
- Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: An ignored combination? Euro J Heart Fail 2006; 8(7):706–711.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Euro Respir J 2008; 32(4):962–969.
- de Lucas-Ramos P, Izquierdo-Alonso JL, Rodriguez-Gonzalez Moro JM, Frances JF, Lozano PV, Bellon-Cano JM. Chronic obstructive pulmonary disease as a cardiovascular risk factor. Results of a case-control study (CONSISTE study). Inter J Chron Obstruct Pulmon Dis 2012; 7:679–86.
- Rana JS, Mittleman MA, Sheikh J, Hu FB, Manson JE, Colditz GA, Speizer FE, Barr RG, Camargo CA Jr. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 2004; 27(10):2478–2484.
- Incalzi RA, Corsonello A, Pedone C, Battaglia S, Paglino G, Bellia V. Chronic renal failure: a neglected co-morbidity of COPD. Chest 2010; 137(4):831–837.
- Hanania NA, Mullerova H, Locantore NW, Vestbo J, Watkins ML, Wouters EF, Rennard SI, Sharafkhaneh A. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. American journal of respiratory and critical care medicine. 2011; 183(5):604–611.
- Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proceedings of the American Thoracic Society. 2005;2(1):8-11.
- Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax 2008; 63(7):599–605.
- Kiechl S, Werner P, Egger G, Oberhollenzer F, Mayr M, Xu Q, Poewe W, Willeit J. Active and passive smoking, chronic infections, and the risk of carotid atherosclerosis: prospective results from the Bruneck Study. Stroke; J Cereb Circ 2002; 33(9):2170–2176.
- Barnes PJ, Celli BR. Systemic manifestations and co-morbidities of COPD. Euro Respir J 2009; 33(5):1165–1185.
- Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. The European respiratory journal. 2008 Mar;31(3):492-501.
- Rutten EP, Wouters E, Franssen FM. Malnutrition and obesity in COPD. In: Rabe KF, Wedzicha JA, Wouters E, editors. COPD Co-morbid Euro Respir Soc 2013; 80–92.
- Vozoris NT, O'Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J 2012; 19(3):e18–24.
- Montes de Oca M, Talamo C, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, Valdivia G, Pertuze J, Moreno D, Halbert RJ, Menezes AM, Team P. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med 2008; 102(5):642–650.
- Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PloS One 2012; 7(8):e43892.
- Burgel PR, Paillasseur JL, Peene B, Dusser D, Roche N, Coolen J, Troosters T, Decramer M, Janssens W. Two distinct chronic obstructive pulmonary disease (COPD) phenotypes are associated with high risk of mortality. PloS one. 2012;7(12):e51048.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 1999; 160(6):1856–1861.
- Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax 2010; 65(9):837–841.
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, Tal-Singer R, Wouters E, Yates JC, Vestbo J. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11:122.
