Abstract
Background: The prevalence of COPD among individuals with acute coronary syndrome (ACS) is estimated at 5% to 18%, and COPD appears to be a predictor of poor outcome. Diagnosis of COPD has mostly been based on medical records without spirometry. As COPD is largely undiagnosed and misdiagnosed, the prevalence and clinical significance of COPD in the ACS population has not been reliably assessed. The present study aimed to estimate the prevalence of COPD in patients with ACS and evaluate the accuracy of medical record-based COPD diagnoses. Methods: This was a single-centre spirometry screening study for COPD in patients admitted for ACS in the county of Jämtland, Sweden. Patient medical records were reviewed to register previous medical history. Spirometry was performed prior to discharge or at the first follow-up outpatient visit after discharge. COPD was defined as a post-bronchodilator FEV1/FVC of <0.7 or below lower limit of normal. Results: Of 743 eligible patients, 407 performed spirometry. Five percent had COPD according to medical records; 11% and 5% fulfilled spirometric criteria of COPD according to FEV1/FVC of < 0.7 (p = 0.002) and below lower limit of normal definitions, respectively. “COPD according to medical history” had a sensitivity of 23%, specificity of 98%, positive predictive value of 53%, and negative predictive value of 91% compared with spirometric COPD FEV1/FVC of < 0.7 Conclusions: In patients with ACS, COPD is underdiagnosed and misdiagnosed. We raise concerns regarding the validity of medical record-based COPD in evaluating the biological and clinical association between COPD and coronary disease. Clinical Trial Registration: ISRCTN number 05697808 (www. controlled-trials.com)
Introduction
Chronic obstructive pulmonary disease (COPD) was the sixth most-common cause of death in the world in 1990, and is projected to become third by 2020 (Citation1). Population-based studies estimate the prevalence of COPD at 5% to 20%, of whom 80% are undiagnosed (Citation2).
COPD shares a common risk factor, tobacco, with coronary heart disease, which is currently the leading cause of death globally. Studies based on population and primary care records show that individuals with COPD have an increased prevalence of cardiovascular disease (CVD) compared with non-COPD individuals, even after controlling for age, sex, smoking status, and co-morbidity (Citation3,4).
COPD-related systemic inflammation and vascular dysfunction have been suggested as potential mechanisms linking COPD to increased cardiovascular morbidity. In people with COPD, elevated inflammatory biomarkers in peripheral blood are associated with CVD (Citation5,6). Case-control studies have shown that COPD is associated with increased radial arterial stiffness (Citation7), stenotic lesions on coronary angiography (Citation8), coronary artery calcium detected with cardiac computed tomography scans, and carotid artery wall thickening (Citation9,10).
The prevalence of COPD in patients admitted for acute coronary syndrome (ACS) has been estimated at 5% to 18%, and in all but two studies (Citation11,12) , COPD was an independent predictor of poor outcome (Citation13–18). When estimating the prevalence and significance of COPD in ACS, these previous studies have limitations. The diagnosis of COPD has not been based on chronic obstructive airflow limitation, the prerequisite for COPD. Instead, the diagnosis of COPD has been based on medical records, and has sometimes been restricted to patients on COPD-related pharmacotherapy. Individuals with asthma have also been included.
As approximately 80% of people with COPD are undiagnosed (Citation2,Citation19) and 20% to 50% of people with self-reported COPD do not fulfil the spirometric criteria for COPD (Citation20–22), the prevalence and clinical significance of COPD in ACS has not been reliably assessed. Soriano et al. addressed this knowledge gap and obtained spirometry in 450 individuals without CVD, 52 individuals with CVD, and 119 hospital patients with coronary heart disease (Citation23). The respective prevalences of spirometry-verified COPD in these three groups were 17.5%, 19.2%, and 33.6%. The prevalences of self-reported COPD in these groups were 5.8%, 11.5%, and 7.6%, respectively, indicating a high proportion of undiagnosed COPD in patients with CVD and that COPD is more common among those with CVD than among those without CVD (Citation23).
The present study aimed to estimate the prevalence of COPD, as defined by chronic obstructive airflow limitation, among individuals with ACS and to evaluate the accuracy of medical record-based COPD diagnosis in this study population.
METHODS
Design
This was a single-centre spirometry screening study for COPD in patients admitted for ACS. The study was registered with ISRCTN number 05697808 (http://www.controlled-trials.com/isrctn/).
Study participants
All patients living in the county of Jämtland, Sweden, and hospitalised with a diagnosis of ACS (myocardial infarction or unstable angina) were assessed for inclusion. An acute myocardial infarction was defined according to the Universal definition of myocardial infarction (an elevation of cardiac troponin T and evidence of myocardial ischemia; type 1 (Citation24, 25). Unstable angina was defined as typical ischemic symptoms combined with ECG changes indicative of ischemia. Östersund Hospital is the only hospital in the county, and all patients (terminal care excluded) with symptoms of suspected ACS are referred for diagnostic evaluation. Study participants were recruited in conjunction with the study “Secondary preventive, nurse based, telephone follow-up for risk factor control after an acute coronary syndrome” (ISRCTN 96595458). The inclusion period was from 1 January 2010 to 8 June 2012. The Regional Ethical Review Board, Umeå University, Umeå, Sweden approved the study on 16 December 2009 (reference number Dnr 09-142M).
Study variables
Patient age, smoking status, exposure to other noxious substances, body mass index, in-hospital mortality, and length of hospital stay were recorded. Patient medical records were reviewed by three research nurses to register previous medical history and medication. The type of ACS was registered.
Dynamic spirometry was performed immediately prior to discharge or at the first follow-up outpatient visit after discharge. Spirometry was conducted using Spirare3 (Diagnostica, Norway). At least 3 acceptable manoeuvres of forced expiratory volume in one second (FEV1) and forced expiratory volume (FVC) were done. If the two best measurements were not within 150 mL of each other, further manoeuvres (up to a total of 8) were done. After this, the best FEV1 and FVC was chosen. Participants with forced expiratory volume in 1 second (FEV1) < 80% of predicted, forced expiratory volume (FVC) < 80%, or FEV1/FVC < 0.7 received ipratropium bromide (dry powder inhaler, 4 × 40 ug), and post-bronchodilator values were measured after 30 minutes. COPD was defined according to the Global initiative for Obstructive Lung Disease (GOLD) guidelines (Citation26) as a post-bronchodilator FEV1/FVC < 0.7.
The severity of COPD was thereafter defined as stage I (FEV1 > 79% of predicted), stage II (50% to 79%), stage III (30% to 49%), or stage IV (<30%). In subjects without spirometric COPD, restrictive spirometric pattern was defined as FEV1/FVC ≥ 0.7 and FVC < 80% of predicted. For this, pre bronchodilation values were used if post-bronchodilation values were not done. The prevalence of subjects with FEV1/FVC below lower limit of normal post bronchodilatation was also calculated. All spirometric measurements were individually reviewed by the corresponding author. Swedish reference values for lung function tests were used (Citation27,28).
Statistical analysis
The primary hypothesis was that the prevalence of spirometry-verified COPD would be higher than the prevalence of medical record-based COPD in participants with ACS. The ability to detect an assumed prevalence of spirometry-verified COPD of 15 ± 4% would require that at least 306 participants be screened. With a significance level of 0.05 and a power of 80%, the ability to detect a 5% difference in the primary hypothesis would require the screening of 350 participants. The t-test and Pearson chi-square test were used for group comparisons of continuous and categorical variables, respectively.
Results
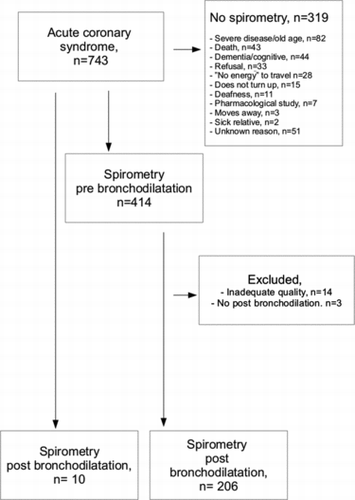
A flowchart of the study participants is presented in Figure . A total of 743 patients with ACS were admitted to Östersund Hospital during the study period. In short, their mean age was 71 years, 36% were women and 59% were current or former smokers. Main co-morbidities were hypertension and diabetes. In-hospital mortality was 6% (Table ).
Table 1. Baseline characteristics, co-morbidity, and in-hospital outcomes of 743 patients with ACS
Of the 743 participating subjects, 424 were able to perform spirometry (Table ). Compared to those not performing spirometry, the 424 patients completing spirometry were younger, had less co-morbidity and had more smoking history. Ten subjects had taken bronchodilation prior to spirometry and did thus not perform spirometry pre bronchodilation. Seventeen participants were excluded because their spirometric data was incomplete. A description of the remaining 407 patients is presented in Table .
Table 2. Study participants without and with COPD according to spirometry (FEV1/FVC <0.7)
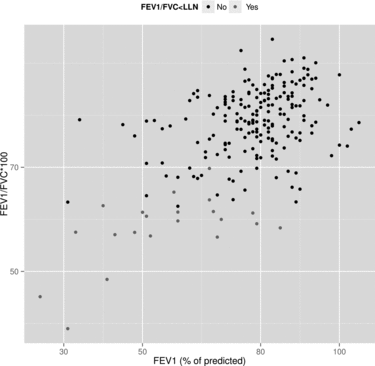
Of these 407 patients, 19 (5%, 95% CI 3–7%) had COPD according to medical records. In comparison, 43 (11%, 95% CI 8–14%) study participants fulfilled spirometric criteria for COPD. The 95% CI for the difference in prevalence was 2–10% (p = 0.002). The subjects with COPD were older, had more smoking history, lower BMI, and similar extent of co-morbidity. The prevalence of spirometric COPD among women was 9% and among men 11%, above the age of 67 years 14% and below 67 years 6%. Of those who fulfilled spirometric criteria for COPD, seven had stage I COPD, 28 had stage II, seven had stage III, and one had stage IV (Figure ). Of the nine never-smokers with spirometric COPD, three had asthma, four had a history of exposure to noxious substances (passive smoking, construction work, indoors wood smoking or farming), one had no known exposure history and in one case no exposure history was available. Of the 33 subjects with undiagnosed COPD, 8 had respiratory treatment for asthma.
Figure 2. The distribution of airflow obstruction after bronchodilation in patients with ACS. Grey dots denotes subjects with FEV1/FVC below lower limit of normal.
The prevalence of undiagnosed COPD among men was 10% and among women 5%. The prevalence of undiagnosed COPD above the age of 67 years was 11% and among those younger than 67 years 5%. When the prevalence of COPD in the study population was defined according to the lower limit of normal of FEV1/FVC, only 21 subjects had COPD (Table ). Their demography and extent of co-morbidity was similar to the group defined using the “fixed-ratio” definition of COPD.
Table 3. Study participants according to the lower limit of normal (LLN) of FEV1/FVC
Using FEV1/FVC below the lower limit of normal post bronchodilatation as definition for COPD, one new subject not identified using fixed ratio was found (Table A). As 20 patients fulfilled both spirometric definitions (Figure ), a formal comparison of the two defined groups cannot be made.
Table 4 A. A comparison of subjects fulfilling COPD according to fixed ratio vs lower limit of normal after bronchodilation
The diagnostic accuracy of “COPD according to medical history” compared with “COPD based on spirometric criteria” is presented in Table B. The sensitivity was 23%; specificity was 98%; positive predictive value was 53%; negative predictive value was 91%; and the diagnostic accuracy was 92%. The diagnostic accuracy of “COPD according to medical history” compared to “COPD according to lower limit of normal” is presented in Table C. The sensitivity was 33%; specificity was 97%; positive predictive value was 37%; negative predictive value was 97%; and the diagnostic accuracy was 94%. Among subjects without spirometric COPD, many had a restrictive spirometric pattern (Table ).
Table 4 B. The accuracy of medical record-based COPD diagnosis versus COPD according to fixed ratio obstructive airflow limitation after bronchodilation.
Table 4 C. The accuracy of medical record-based COPD diagnosis versus COPD according to lower limit of normal after bronchodilation
Table 5. Study participants without spirometric COPD according to restrictive spirometric pattern
Discussion
Summary
During this 30-month study, spirometry was performed to estimate the prevalence of COPD among consecutive patients admitted for ACS to Östersund Hospital, Sweden. Spirometry was feasible in a subset of the study population, predominantly younger patients with more smoking history and less co-morbidity. In the study population, 5% of the patients had prior COPD; the prevalence of patients fulfilling spirometric criteria for COPD was 11% (p = 0.002). The study revealed widespread underdiagnosis and misdiagnosis of COPD in individuals with ACS.
Limitations
Only 57% of the patients with ACS performed spirometry, an expected proportion considering an elderly study population with significant co-morbidity. The main causes were refusals, death, severe disease, and dementia/cognitive impairment. Many older subjects living far from the hospital refused to participate, as spirometry would require an extra outpatient follow-up at the hospital. As the diagnosis of COPD requires spirometry, it is impossible to examine the prevalence of COPD in patients who are unable to perform lung function measurements. However, we assume that a similar sampling bias would occur if the study were repeated elsewhere or if targeted COPD case finding for COPD among patients with ACS was conducted.
Patients who performed spirometry were younger and a higher proportion were ever-smokers compared with those who did not measure lung function. Whether this selection bias led to an under- or overestimation of the “true” prevalence of COPD among individuals with ACS is uncertain. According to their medical history, COPD was not more common among those not performing spirometry. As the prevalence of COPD increases with age, a selection bias towards younger patients would lead to an underestimation of disease prevalence. However, as smoking history is a very strong predictor of COPD, a selection predominantly comprising smokers and/or former smokers would lead to an overestimation of COPD prevalence.
Taken together, we are inclined to believe that the present study may have underestimated the prevalence of COPD. In the present study, COPD was defined according to the GOLD guidelines (Citation26) as a post-bronchodilator FEV1/FVC < 0.7. Almost all subjects with airflow obstruction had a history of exposure to cigarette smoking or other noxious substances. The proportion of subjects with clinical symptomatic COPD was probably lower.
The majority of subjects were on beta blockers at the time point of spirometry. As treatment with cardioselective beta-blockers does not significantly change FEV1 in patients with COPD (Citation29), we assume that the medication did not affect the proportion of subjects with airflow obstruction.
Interpretation
In the present study population, the prevalence of prior COPD based on medical records was 5%, similar to a recent study conducted in Middle East, even though their study population were considerably younger (Citation12).
The present prevalence appears to be significantly lower than the prevalence of 11.5% reported in Denmark in 6669 patients with myocardial infarction (Citation13). The difference may partly be explained by the higher proportion (59% vs. 73%) of ever-smokers in the Danish study. Salisbury et al. reported a 16% prevalence of prior COPD or asthma in 2481 patients with myocardial infarction (Citation14). Wakabayashi (Citation18) (11% COPD prevalence) and Campo (Citation17) (18% prevalence) included only subjects with STEMI, only 36% of all subjects in the present study.
Bursi et al. studied the prevalence of medical record-based COPD in an US population with myocardial infarction, and found that the prevalence increased from 7% in 1979–1985 to 15% in 2000–2007 (Citation16). Their study population appears to be similar in age and smoking history to the population of the present study. Whether the difference in COPD prevalence between the study by Bursi et al. and the present study is true or a consequence of a study variable with poor external validity is uncertain.
Soriano et al. investigated the prevalence of obstructive airflow limitation fulfilling spirometric criteria for COPD in a Spanish population-based study (Citation23). In 52 people with CVD, 11.5% had a prior diagnosis of COPD and 19.2% exhibited airflow limitation. In 119 individuals with coronary artery disease confirmed by coronary angiography, 7.6% had prior COPD and 33.6% exhibited obstructive airflow limitation. This is a higher proportion of obstructive airflow limitation than in the present study, likely a result of a larger proportion of current smokers (46% vs. 26%) in the study by Soriano et al. In addition, the study populations were different (CVD vs. ACS).
In the present study, the prevalence of obstructive airflow limitation fulfilling spirometric criteria for COPD among patients with ACS was 11%. A crude comparison with the prevalence of chronic airflow obstruction in a general Swedish population might be done. In 1994–1995, lung function was measured in a random sample of 666 Swedes, and 14% fulfilled spirometric criteria for COPD. The cohort had a mean age of 49 years, 26% were current smokers, 28% were former smokers, and 45% had never smoked (Citation30). Another more recent Swedish population-based study of 548 individuals older than 40 years of age reported a COPD prevalence of 16%. That study cohort included fewer current smokers and more former smokers than the present study (Citation19). As expected, the group with spirometric COPD were older and included fewer never-smokers than the group without COPD. Compared to these two population-based screening studies, the prevalence of spirometric COPD among patients with ACS was surprisingly low, considering that the present study population was older. Had all patients performed spirometry, it is probable that the prevalence of COPD had increased in the study population.
Spirometric screening revealed widespread underdiagnosis of COPD among patients with ACS. Some of these subjects were on treatment for asthma. This underdiagnosis is in agreement with previous population-based screening studies (Citation2). However, instead of general screening, targeted case-finding of COPD has been advocated. COPD case-finding through a screening questionnaire and a hand-held “screening spirometer” appear to be more feasible (Citation31).
It is reasonable to assume that detection and treatment of significant co-morbidities, such as COPD, among patients with ACS may have positive effects on patient prognosis. Treatment with roflumilast, an oral phosphodiesterase 4 inhibitor, which reduces COPD exacerbations, has in a retrospective post-hoc analysis been shown to reduce the risk of major adverse cardiovascular events, although more substantially in patients without baseline cardiovascular diseases (Citation32). Diabetes is another common co-morbidity, present in 28% of those with spirometric COPD in this study. Intensive insulin treatment of diabetic patients suffering a myocardial infarction has in one study been shown to reduce long-term mortality (Citation33).
Spirometric screening revealed widespread misdiagnosis of COPD in the study population. This finding has been demonstrated previously in studies from primary and secondary care (Citation20–22). We suspect that the present finding is a consequence of a physician not using or incorrectly interpreting spirometry in the diagnosis of COPD. We suggest that a medical record-based diagnosis of COPD should be supported by spirometric data, and if data is unavailable then spirometry should be performed.
As we expected due to the known age-related decrease in FEV1/FVC, in this elderly study population the prevalence of COPD decreased considerably by defining airway obstruction using FEV1/FVC below lower limit of normal instead of a fixed ratio. Surprisingly, the prevalence of smoking history was similar in those with and without COPD using the lower limit of normal. This may be due to a type II false negative error, due to a small sample size. We hesitate to further compare the clinical characteristics of the two groups (fixed-ratio vs lower limit of normal), due to small sample sizes and a high proportion of subjects that fulfill both criteria.
The validity of medical records based COPD was poor irrespective of which spirometric COPD criteria were used, as indicated in Tables A to C. We want to emphasize than in Sweden, the fixed ratio definition for COPD is recommended so far. Using the LLN as spirometric criteria for COPD, 12 of 19 (63%) patients with a prior diagnosis of COPD would lose the diagnosis and treatment with unnecessary bronchodilators to ACS-patients without COPD would be significantly decreased. Current beta-2-selective bronchodilators may have cardiac side-effects. Fourteen (4%) previously undiagnosed patients with obstructive spirometry would be identified. Using fixed-ratio, 9 of 19 (47%) subjects with prior COPD would lose the diagnosis and 33 (9%) previously undiagnosed patients with obstructive spirometry would be found. The concordance and discordance between the two spirometric definitions of airflow obstruction in COPD has been reviewed and highlights the lack of longitudinal studies (Citation34).
Still, we lack a diagnostic test for COPD that with complete accuracy reflects the pathophysiology of the disease. Meanwhile, our data highlights the importance of spirometry in the diagnosis of COPD irrespective ofspirometric criteria . Spirometric screening, using either fixed-ratio or lower limit of normal, for COPD after an acute coronary syndrome would reduce the number of subjects with incorrectly diagnosed as well as undiagnosed COPD. As a consequence, incorrect over-treatment would be reduced. Also, we raise concerns regarding the use of medical-records based COPD in studies intended to evaluate if and how COPD predicts clinical outcomes after ACS.
Among subjects without spirometric COPD, as many as 50% had a restrictive spirometric pattern. An explanation to the finding would be the presence of obesity, indicated by an increased BMI in the “restrictive” patients. The proportion of patients with restrictive spirometric pattern was larger than previously shown among subjects with ischemic heart disease (Citation35). The association between restrictive spirometric pattern and cardiovascular or ischemic heart disease is still uncertain (Citation36,37).
Conclusions
In summary, the present spirometry-based screening study revealed widespread underdiagnosis and misdiagnosis of COPD among patients admitted for ACS. Additional studies are needed to evaluate the significance of spirometry-verified COPD or obstructive airflow limitation in ACS.
Acknowledgments
We thank the research nurses at the Unit of Cardiology for their assistance with this project. This study was financially supported by the Research & Development Unit of the Jämtland County Council (Sweden), and The Swedish Heart and Lung Association. The sponsors had no influence on the study design, data collection, data analysis, interpretation of data, writing of the manuscript or in the decision to submit the manuscript for publication.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
| Abbreviations | ||
| ACS, acute coronary syndrome | = | |
| CI, confidence interval | = | |
| COPD, chronic obstructive pulmonary disease | = | |
| CVD, cardiovascular disease | = | |
| FEV1, forced expiratory volume in one second | = | |
| FVC, forced expiratory volume | = | |
| GOLD, Global initiative for Obstructive Lung Disease | = | |
References
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global burden of disease study. Lancet 1997; 349:1498–1504.
- Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009; 374:721–732.
- Finkelstein J, Cha E, Scharf SM. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruct Pulmon Dis 2009; 4:337–349.
- Feary JR, Rodrigues LC, Smith CJ, et al. Prevalence of major co-morbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax 2010; 65:956–962.
- Sin DD, Man SFP. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107: 1514–1519.
- Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012;7:e374–383.
- Mills NL, Miller JJ, Anand A, et al. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular risk. Thorax 2008; 63:306–111.
- Topsakal R, Kalay N, Ozdogru I, et al. Effects of chronic obstructive pulmonary disease on coronary atherosclerosis. Heart Vessels 2009; 24:164–168.
- Lahousse L, van den Bouwhuijsen QJA, Loth DW, et al. Chronic obstructive pulmonary disease and lipid core carotid artery plaques in the elderly: the Rotterdam study. Am J Respir Crit Care Med 2013: 187:58–64.
- Iwamoto H, Yokoyama A, Kitahara Y, et al. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med 2009; 179(1):35–40.
- Behar S, Panosh A, Reicher-Reiss H, et al. Prevalence and prognosis of chronic obstructive pulmonary disease among 5,839 consecutive patients with acute myocardial infarction. sprint study group. Am J Med 1992; 93:637–641.
- Hadi HA, Zubaid M, Al Mahmeed W, et al. Prevalence and prognosis of chronic obstructive pulmonary disease among 8167 Middle Eastern patients with acute coronary syndrome. Clin Cardiol 2010; 33(4):228–235.
- Kjøller E, Køber L, Iversen K, et al. Importance of chronic obstructive pulmonary disease for prognosis and diagnosis of congestive heart failure in patients with acute myocardial infarction. Eur J Heart Fail 2004; 6:71–77.
- Salisbury AC, Reid KJ, Spertus JA. Impact of chronic obstructive pulmonary disease on post-myocardial infarction outcomes. Am J Cardiol 2007; 99:636–641.
- Hawkins NM, Huang Z, Pieper KS, et al. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: analysis of the valsartan in acute myocardial infarction trial (valiant). Eur J Heart Fail 2009; 1:292–298.
- Bursi F, Vassallo R, Weston SA, et al. Chronic obstructive pulmonary disease after myocardial infarction in the community. Am Heart J 2010; 160:95–101.
- Campo G, Guastaroba P, Marzocchi A, et al. Impact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest 2013; 144(3):750–757.
- Wakabayashi K, Gonzalez MA, Delhaye C, et al. Impact of chronic obstructive pulmonary disease on acute-phase outcome of myocardial infarction. Am J Cardiol. 2010; 106(3):305–309.
- Danielsson P, Olafsdóttir IS, Benediktsdóttir B, et al. The prevalence of chronic obstructive pulmonary disease in Uppsala, Sweden- the burden of obstructive lung disease (BOLD) study: cross-sectional population-based study. Clin Respir J 2012; 6:120–127.
- Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2009; 99:493–500.
- Walker PP, Mitchell P, Diamantea F, et al. Effect of primary care spirometry on the diagnosis and management of . Eur Respir J 2008; 28:945–952.
- Arne M, Lisspers K, Ställberg B, et al. How often is diagnosis of COPD confirmed with spirometry? Respir Med 2010; 104:550–556.
- Soriano JB, Rigo F, Guerrero D, et al. High prevalence of undiagnosed airow limitation in patients with cardiovascular disease. Chest 2010; 137:333–340.
- Thygesen K, Alpert JS, White HD et al. Universal definition of myocardial infarction. Circulation 2007; 116:2634–2653
- Thygesen K, Alpert JS, Jaffe AS et al. Third universal definition of myocardial infarction. Eur Heart J 2012; 33:2551–2567
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–355.
- Hedenström H, Malmberg P, Agarwal K. Reference values for lung function tests in females. regression equations with smoking variables. Bull Eur Physiopathol Respir 1985; 21:551–557.
- Hedenström H, Malmberg P, Fridriksson HV. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci 1986; 91:299–310.
- Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005 Issue 4. Art. No.: CD003566. DOI: 10.1002/14651858.CD003566.pub2.
- Lindberg A, Jonsson AC, Rönmark E, et al. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor's diagnosis, symptoms, age, gender, and smoking habits. Respiration 2005; 72:471–479.
- Wada H, Nakano Y, Nagao T, et al. Detection and prevalence of chronic obstructive pulmonary disease in a cardiovascular clinic: evaluation using a hand held FEV1/FEV6 meter and questionnaire. Respirology 2010; 15:1252–1258.
- White WB, Cooke GE, Kowey PR, et al. Cardiovascular Safety in Patients Receiving Roflumilast for the Treatment of COPD. Chest 2013; 144(3):758–765.
- Malmberg K, Rydén L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMIstudy): effects on mortality at 1 year. J Am Coll Cardiol 1995; 26(1):57–65.
- Mohamed Hoesein FA, Zanen P, Lammers JW. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: an evidence-based review. Respir Med 2011; 105(6):907–915.
- Eriksson B, Lindberg A, Müllerova H, et al. Association of heart diseases with COPD and restrictive lung function–results from a population survey. Respir Med 2013; 107(1):98–106.
- Johnston AK, Mannino DM, Hagan GW, et al. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax 2008; 63(7):599–605.
- Guerra S, Sherrill DL, Venker C, et al. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax 2010; 65(6):499–504.