Abstract
The novel long-acting β2-agonist olodaterol demonstrated an acceptable safety profile in short-term phase II clinical studies. This analysis of four randomized, double-blind, placebo-controlled, parallel-group, phase III studies (1222.11, NCT00782210; 1222.12, NCT00782509; 1222.13, NCT00793624; 1222.14, NCT00796653) evaluated the long-term safety of olodaterol once daily (QD) in a large cohort of patients with moderate to very severe (Global initiative for chronic Obstructive Lung Disease 2–4) chronic obstructive pulmonary disease (COPD). The studies compared olodaterol (5 or 10 μg) QD via Respimat®, formoterol 12 μg twice daily (BID) via Aerolizer® (1222.13 and 1222.14), and placebo for 48 weeks. Patients continued receiving background maintenance therapy, with ∼60% receiving concomitant cardiovascular therapy and 25% having a history of concomitant cardiac disease. Pre-specified analyses of pooled data assessed the adverse events (AEs) and serious AEs in the whole population, and in subgroups with cardiac disease, along with in-depth electrocardiogram and Holter monitoring. In total, 3104 patients were included in the safety analysis: 876 received olodaterol 5 μg, 883 received olodaterol 10 μg, 885 received placebos, and 460 received formoterol 12 μg BID. Overall incidence of on-treatment AEs (71.2%), serious AEs (16.1%), and deaths (1.7%) were balanced across treatment groups. Respiratory and cardiovascular AEs, including major adverse cardiac events, were reported at similar frequencies in placebo and active treatment groups. The safety profiles of both olodaterol 5 μg (marketed and registered dose) and 10 μg QD delivered via Respimat® are comparable to placebo and formoterol BID in this population, with no safety signals identified.
Introduction
The novel long-acting β2-agonist (LABA) olodaterol has high β2-selectivity and a near full-agonist profile at β2-adrenoceptors (Citation1,2). Phase II clinical studies of olodaterol in patients with chronic obstructive pulmonary disease (COPD) have demonstrated a 24-hour duration of action and effective bronchodilation over a 24-hour dosing interval (Citation3–5). These studies also showed a satisfactory safety profile with up to 4 weeks of treatment for doses of up to 20 μg olodaterol once daily. The data supported further investigation of the efficacy and safety of 5 and 10 μg olodaterol once daily in longer-term studies in patients with COPD.
The olodaterol phase III clinical program in COPD was specifically designed to assess long-term effects on lung function, symptomatic benefit, and safety in 48-week pivotal studies, supplemented by evaluation of additional efficacy parameters in 6-week studies (Citation6–11). Similar adverse-event (AE) profiles for olodaterol compared to placebo and active comparators (tiotropium and formoterol) were observed in the individual studies within the phase III program.
The phase III pivotal studies were designed to permit evaluation of the efficacy and safety of olodaterol in a population closely representative of those seen in clinical practice, including patients with very severe COPD (Global initiative for chronic Obstructive Lung Disease [GOLD] 4), those receiving background pulmonary medication, and those with co-morbidities (Citation12,13).
This article presents the results of a pre-specified pooled safety analysis of olodaterol 5 and 10 μg from the large database of patients in the combined phase III 48-week olodaterol studies that formed the basis of the safety assessment for olodaterol registration.
Methods
Study designs
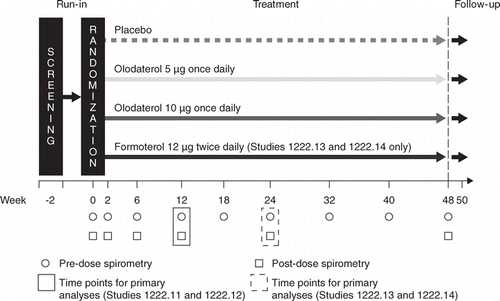
There were two sets of global, replicate, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group studies registered with ClinicalTrials.gov (1222.11: NCT00782210; 1222.12: NCT00782509; 1222.13: NCT00793624; 1222.14: NCT00796653) (Figure ). Following an initial screening visit and 2-week baseline period, eligible patients were randomized to receive either 5 or 10 μg olodaterol once daily, formoterol 12 μg twice daily (Studies 1222.13 and 1222.14 only; double-dummy studies), or placebo. Olodaterol inhalation solution was delivered via the Respimat® inhaler, with each administration comprising two actuations, and formoterol was delivered via the Aerolizer® inhaler, with each administration comprising one actuation.
Patients
Patients were included if they: were current or ex-smokers with a smoking history of >10 pack-years; were aged ≥40 years with a diagnosis of COPD according to GOLD (Citation14); had a post-bronchodilator forced expiratory volume in 1 second (FEV1) <80% of predicted normal; and had post-bronchodilator FEV1/forced vital capacity <70%. Patients continued with usual background maintenance therapy, other than LABAs, for the study duration, including long-acting muscarinic antagonists (LAMAs) and short-acting muscarinic antagonists (SAMAs), inhaled corticosteroids, and xanthines. Patients on LABAs were allowed to switch to SAMAs. All patients were provided with salbutamol for use as rescue medication, as needed, during the baseline, treatment, and follow-up periods. Patients were also allowed to continue using any cardiovascular maintenance medication including both non-selective and cardio-selective β-blockers, providing they were taking a stable dose for at least 6 weeks prior to Visit 1.
Patients were excluded if they had: a history of asthma; myocardial infarction within 1 year of screening; unstable or life-threatening cardiac arrhythmia; known active tuberculosis; cystic fibrosis or life-threatening pulmonary obstruction; or hospitalization for heart failure within the past year.
The studies were performed in accordance with the Declaration of Helsinki, International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice, and local regulations. The protocol was approved by the ethics research board of the respective institutions and signed, informed consent was obtained from all patients.
Safety end points
All randomized patients from each of the four studies who received at least one dose of treatment were included in the safety analysis set (the treated set). Electrocardiogram (ECG), vital signs, laboratory parameters, and frequency of AEs and serious AEs were monitored throughout all four studies. Additionally, vital status follow-up of all prematurely discontinued patients was requested up to day 351 (planned final study visit; 337 days on treatment plus 14 days post-treatment follow-up).
Frequency of AEs
AEs and serious AEs were recorded using the Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC) and Preferred Terms (PTs). The analysis of AEs was based on treatment-emergent AEs using the standard definition of those events with an onset any time following the first dose of study drug up to 12 days after the last study drug intake, and results were summarized descriptively. In addition, specific aggregate terms, Special MedDRA Query (SMQ) and a number of predefined sponsor-customized MedDRA queries, were used to examine categories of AEs. Furthermore, the incidence of cardiac and vascular AEs with olodaterol and placebo was compared in a subgroup of patients with a history of cardiac disease, and in a separate subgroup of patients who were concomitantly taking β-blockers. Exposure-adjusted incidence rates for major adverse cardiac events (MACE) were calculated and rate ratios estimated for treatment comparisons of all active therapies versus placebo based on a Cochran−Mantel−Haenszel test stratified by study.
Evaluation of primary cause of death
The primary cause of death for all recorded fatalities was independently adjudicated by a Mortality Adjudication Committee (MAC) that was blinded to treatment. A full list of the safety analyses carried out can be found in Appendix 1 (see the online supplement). This committee was comprised of two experts in pulmonology (L.M.G., D.E.N.) and one in cardiology (S.M.) not linked to any of the phase III pivotal studies. The committee followed similar principles to those previously reported (Citation15).
The MAC systematically assessed all reported fatalities at the Verbatim Term/PT level. Details of the MAC-adjudication process, principles of operation, and copies of the forms associated with adjudication can be found in Appendices 2–5 (see the online supplement).
Following MAC adjudication of all fatalities within the studies, the MAC-adjudicated primary cause of death and the AEs recorded as having a fatal outcome on the case report form by study investigators were compared at the level of the MedDRA SOC and PT.
Vital signs and laboratory monitoring
During the course of these studies, vital signs (heart rate and blood pressure), physical examinations, clinical laboratory testing (clinical chemistry, hematology, and urinalysis), and pre-dose ECGs were monitored. Olodaterol exposure for patients within each group was assessed using plasma concentration monitoring, pooled for Studies 1222.11, 1222.12, 1222.13, and 1222.14, and reported per therapy. Serum potassium was analyzed as a systemic pharmacodynamic end point, assessed both pre-dose and 1 and 3 hours post-dose at weeks 6 and 12. In addition, selective safety evaluations included ECGs pre-dose and 40 minutes post-dose in all patients (weeks 0, 6, 12, 24, and 48) and 24-hour Holter monitoring in a subset of 775 patients (weeks 0, 12, 24, 40, and 48).
Results
In total, 3104 patients with COPD (2377 male; 727 female) were included in this pre-defined pooled safety analysis (Table ). The mean drug exposure for olodaterol 5 μg and 10 μg groups was 308.4 and 304.7 days, respectively, and 287.5 and 299.0 days for the placebo and formoterol groups, respectively. Overall, 18.0% of patients discontinued prematurely, with a higher rate reported for placebo compared to active treatment: 22.5% with placebo, 15.1% with olodaterol 5 μg, 16.5% with olodaterol 10 μg, and 18.0% with formoterol.
Table 1. Demographic and baseline patient characteristics by treatment group for 48-week, parallel-group trials (treated patient population)
Co-medications and co-morbidities in the 48-week studies in COPD were balanced across all groups at screening (Table ). At baseline, 45.4% of patients were receiving inhaled corticosteroids, 23.8% were receiving LAMAs, and 24.9% were receiving SAMAs; 65.1% of the population were receiving cardiovascular medication, with 24.0% having co-morbid cardiac disorders and 33.0% with hypertension.
AEs, serious AEs, and treatment-related AEs
Incidence of AEs was generally balanced across groups, with an overall incidence of 71.2% (Table and Appendix 6 [see the online supplement]). The majority of AEs were respiratory, thoracic, and mediastinal disorders, and infections and infestations; only three AE PTs occurred with a frequency >5%, namely COPD (representing exacerbations and disease worsening), upper respiratory tract infection, and nasopharyngitis (Figure ).
Figure 2. AEs ≥2% in olodaterol 48-week studies in chronic obstructive pulmonary disease. On-treatment including 12-day washout period unless specified otherwise. AE = adverse event; GI = gastrointestinal.
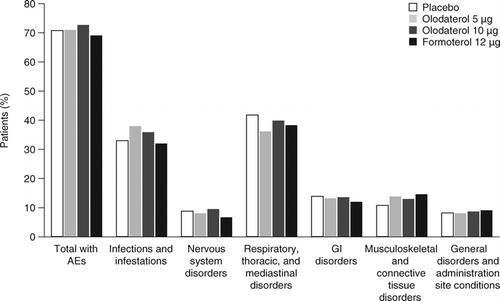
Table 2. Overview of AEs for all 48-week, olodaterol parallel-group trials
Total serious AEs were also balanced across treatment groups (Table ). Overall incidence was 16.1% and was comparable between all treatment groups for the majority of SOCs; the most frequent were reported as respiratory, thoracic, and mediastinal disorders, followed by infections and infestations. A numerically higher incidence of neoplasms as serious AEs was seen with all active treatments (highest with 10 μg) compared to placebo. The most frequent type of malignant neoplasm observed was lung cancer. There were 53 (1.7%) on-treatment deaths recorded during the course of the 48-week studies (Table ). The most frequent of these were due to COPD exacerbations and cardiac and vascular disorders.
Vital status follow-up identified 21 deaths that occurred post-treatment or post-study (solicited) and two deaths were recorded post-study without solicitation (10 in the placebo group, six with olodaterol 5 μg, four with olodaterol 10 μg, and three with formoterol [see Appendix 7 in the online supplement]). This gave a total of 76 deaths. More than 98% of patients had satisfactory vital status follow-up across the 48-week trials. Incidences of fatal AEs reported were comparable between groups.
Treatment-related AEs were also comparable between all groups, including respiratory and cardiac disorders (Appendix 8 [see the online supplement]). COPD and cough were the most frequently described AEs, with only small differences noted in frequencies between treatment groups.
Primary cause of death
All 53 on-treatment deaths underwent external, independent adjudication by the MAC to determine the primary cause of death (Table and Appendix 7 [see the online supplement]).
Table 3. Fatal AEs, adjudicated cause 48-week studies in COPD (on-treatment)
MAC primary cause of death assessment was in complete concordance with 35 (66%) fatal AEs reported by the site investigators; both incomplete concordance (disagreement at either SOC or PT level) and complete disagreement (at both levels) were reported in nine cases (17%) each. The level of concordance was 83% between MAC-determined cause of death and site investigator-determined AE leading to death at the level of SOC and 70% at the level of PT. For the eight (15%) fatal AEs reported as cardiac disorders by the study investigators, primary causes of death assessed by the MAC were: COPD exacerbation (n = 3), unknown cause of death (n = 3), and sudden cardiac death (n = 2).
ECG and Holter monitoring and other safety parameters
Overall, there were no clinically significant changes associated with active treatment for any of the six ECG parameters (QT interval, QT interval corrected according to Bazett's formula, QT interval with Fridericia correction, heart rate, PR interval, and QRS interval) and seven ECG abnormalities investigated (ST segment, T wave, U wave, rhythm, myocardial infarction, ECG conduction, and morphology [see Appendix 1 in the online supplement for parameter details]). Likewise, in the subset of patients in which Holter-ECG evaluation was carried out, analysis of results identified no clinically relevant dose-related effect or effect at any specific time point in heart rate, supraventricular premature beats, or ventricular premature beats.
Although small differences were noted between groups in terms of changes in blood pressure and pulse rate, frequencies of increases and decreases were generally similar between all active treatments and placebo, with no discernible trend.
Compared to placebo, a higher proportion of patients in the active treatment groups had a shift in maximum creatinine phosphokinase to exceed the upper limit of normal: 14.1% and 17.7% of patients receiving olodaterol 5 and 10 μg, respectively, 21.4% receiving formoterol, and 8.2% on placebo. There was no difference in the pattern of AEs in those patients who had a shift in creatinine phosphokinase to greater than the upper limit of normal versus the overall population. No relationship between the plasma concentration of olodaterol and potassium was observed, and no differences were reported between treatment groups.
Cardiovascular AE profile
Incidence of MACE
Incidences of MACE were balanced across treatment groups: placebo, 24/885 (2.7%); olodaterol 5 μg, 10/876 (1.1%); olodaterol 10 μg, 16/883 (1.8%); formoterol, 9/460 (2.0%) (Table ). In total, 59 patients experienced fatal and non-fatal MACE during the course of the 48-week studies. Of these, 16 patients had fatal MACE, with four additional patients having events coded to death due to an unknown origin. Of the 59 MACE, 29 events were coded to the SMQ end point of ischemic heart disease, sub-SMQ myocardial infarction (broad) (any); incidences of these were also similar between treatment groups (Table ).
Table 4. Frequency of patients with MACE by treatment group in all 48-week studies
Cardiovascular AEs and sub-group analyses
Within the whole patient population, incidence of “cardiac disorders” and “vascular disorders” was comparable with olodaterol and placebo (Table ). Hypertension was the only individual cardiac or vascular PT occurring at an incidence of >2%, experienced by 3.4%, 2.6%, 2.9%, and 1.7% of patients in the placebo, olodaterol 5 μg, olodaterol 10 μg, and formoterol groups, respectively. Serious AEs were reported infrequently, with no individual term occurring at an incidence of >2%. Numerical differences were observed for atrial fibrillation and myocardial infarction. Atrial fibrillation was reported in 0.3% (n = 3), 0.6% (n = 5), 0.6% (n = 5), and 0.2% (n = 1), while myocardial infarction was reported in 0.2% (n = 2), 0.1% (n = 1), 0.5% (n = 4), and in 0 patients receiving placebo, olodaterol 5 μg, olodaterol 10 μg, and formoterol, respectively. “Cardiac arrhythmias” was the most frequently reported category of cardiovascular system adverse event as assessed by (aggregated) SMQ and predefined sponsor-customized MedDRA queries, reported by 145 patients in total, with no differences between treatment groups (Table ).
Table 5. Frequency of patients with AEs related to the cardiovascular system in all 48-week studies
Analysis of the subgroup of patients with cardiac history revealed a higher incidence of AEs overall (Table ) versus those with no history and, unsurprisingly, a higher total incidence of cardiac AEs. Cardiac AEs were similar between groups, and all confidence intervals (CIs) included one; however, analysis of the rate ratios of respective incidence rates per 100 patient-years of events in both olodaterol treatment groups versus placebo revealed no statistically significant differences in risk of any cardiac or cardiovascular AE. Risk ratio with olodaterol 5 μg versus placebo was 0.97 (95% CI 0.70, 1.36) for “cardiac disorders” and 1.01 (95% CI 0.68, 1.51) for “vascular disorders”, and 0.90 (95% CI 0.64, 1.27) and 0.91 (95% CI 0.60, 1.36), respectively, for olodaterol 10 μg versus placebo.
Table 6. Incidence of cardiac and vascular AEs and serious AEs in patients with cardiac disorder at baseline or cardiac history
Similar results were seen in the subgroup of patients receiving β-blockers during the trial. A higher incidence of AEs was reported versus those who were not taking β-blockers (Table ), largely distributed over various organ classes and balanced across treatment groups. Cardiac AEs were not increased with olodaterol 5 or 10 μg versus placebo. In addition, no increase in risk estimates of fatal or any MACE end points was observed in the cardiac history subgroup with olodaterol versus placebo (Appendix 9 [see the online supplement]).
Table 7. Incidence of cardiac and vascular AEs and serious AEs in concomitant β-blocker medication subgroup
Discussion
In this pooled analysis of 3104 patients from four phase III pivotal studies, we sought to determine the safety of the novel once-daily LABA olodaterol via the Respimat® inhaler over 48 weeks in patients with moderate to very severe COPD, based on evaluation of AEs, laboratory assessments, vital signs, and 12-lead ECG for all patients. Vital status follow-up was requested and planned to ensure a more robust approach for analysis of fatalities by recording any potential influence of a differential discontinuation bias.
Incidence of AEs, serious AEs, and on-treatment deaths was balanced between olodaterol, active comparator, and placebo, with the characteristics and observed frequencies consistent with those expected for a 1-year COPD clinical trial (Citation16,17). As would be anticipated in this patient population, the most frequent AEs were in the respiratory, thoracic, and mediastinal disorders categories, with a similar incidence across treatment groups. Cardiovascular AEs including MACE were reported less frequently, with comparable incidences across groups. Numerically lower values were observed in the olodaterol-treated population.
Given the pharmacologic properties of LABAs, there is a potential risk of serious adverse cardiovascular effects (Citation18); furthermore, there is a risk of serious exacerbations of asthma and asthma-related deaths in patients with asthma (Citation19). Therefore, detailed investigation of adverse cardiovascular and respiratory safety signals is of particular importance when evaluating the benefit:risk ratio for a novel LABA.
In addition to safety data gathered during the course of individual clinical trials, it is important to gain a detailed understanding of the long-term safety of new therapies by in-depth analysis of the total available safety database across trials. These data are vital, not only to satisfy drug developmental and regulatory requirements (Citation12,13), but also to ensure the availability of an adequate bank of evidence for practicing clinicians that is sufficiently representative of their patient population to assist them in making informed treatment decisions.
This pre-specified pooled analysis of the four pivotal 48-week olodaterol trials represents a rigorous and comprehensive safety evaluation for a new LABA, and provides robust evidence for the long-term safety of 5 μg olodaterol once daily, the submitted and registered dose for clinical use, as well as a higher dose of 10 μg, in a large population of patients (n = 3104) with moderate to very severe COPD. In addition, a relatively high proportion of the population had very severe COPD (GOLD 4: 8.3–12.2%), with 82.2% having one or more co-morbidities and over two-thirds were receiving cardiovascular medication. This represents a patient profile similar to patients treated in clinical practice (Citation20,21). It should, however, be borne in mind that given the exclusion criteria, our data do not apply to patients with recent myocardial infarction or unstable/life-threatening cardiac arrhythmia.
Evaluation of the pooled olodaterol and active-comparator results from Studies 1222.13 and 1222.14 demonstrates a similar safety profile for olodaterol in comparison to formoterol, an established twice-daily LABA. These data are in line with the individual findings of the constituent trials of this analysis, plus those of the wider phase III clinical program (Citation6,7,Citation9,10).
Overall, the incidence of on-treatment fatal events reported here (1.7%) was consistent with that expected for a 1-year trial in a patient population with moderate to very severe COPD. Although not directly comparable, it was also in line with those reported for tiotropium Respimat® in two 1-year studies (Citation22) and lower than the overall annualized death rates for other large COPD trials, which ranged from an average of 2.6% in the TIOSPIR study (mean follow-up 2.3 years) (Citation23) to 3.9% in the 4-year UPLIFT study (Citation24) and 4.7% in the 3-year TORCH study (Citation25).
The causes of fatal AEs determined in the 48-week “on-treatment” population were similar across treatment groups. During the olodaterol pivotal studies, investigators were asked to record all on-treatment AEs as well as identifying the outcome of the event (one option being “fatal”). In the pooled analysis, all deaths across the four pivotal studies underwent a further independent assessment by an external MAC of three physicians to ascertain the primary cause of death. Independent adjudication of primary cause of death by committee can provide valuable additional detail to aid in the better understanding of on-treatment fatalities (Citation15,Citation23,Citation26,27).
Due to the complex sequence of AEs that can occur at the time of a fatal event, and to minimize between-site variation in determination of cause of death, the utilization of a standardized and predefined approach to cause of death determination by an independent committee blinded to treatment allocation was considered to be important. This approach also allowed assignment of a unique cause of death per patient, rather than potentially several AEs leading to death, as assigned by the site investigator.
Although the overall incidence of neoplasms was within the range of that typically reported for a COPD population (Citation25,Citation28), there were few cases with placebo and more with olodaterol 10 μg and formoterol than with olodaterol 5 μg. An association of lung cancer with LABA treatment has not been reported in the literature and preclinical studies of olodaterol suggested no evidence for carcinogenic potential.
Throughout its development program, olodaterol was administered via the Respimat® inhaler, a propellant-free inhaler that uses mechanical force to provide a metered dose of medication as a Soft Mist™ (Citation29). Thus, olodaterol delivered via the Respimat® inhaler provides an additional element of choice for physicians prescribing bronchodilators, potentially allowing optimization of treatment for individual patients. Recently, the safety of tiotropium delivered via Respimat® has been compared to delivery through a dry powder inhaler in a large trial of 17 135 patients (TIOSPIR). In this study, tiotropium 5 μg delivered via Respimat® and tiotropium 18 μg delivered via HandiHaler® had comparable risks of death and first exacerbation.
The present analysis represents a valuable addition to the growing evidence base for the safety of LABAs in COPD, reporting no clinically relevant, dose-related adverse safety signals or patterns in an extensive array of different parameters during ECG and Holter monitoring associated with olodaterol, along with similar incidences of cardiovascular AEs when compared to placebo and formoterol in the general trial population. The frequency of cardiovascular AEs, including MACE, across treatments was similar in subgroups of patients with a history of cardiac disease and in those who were taking β-blockers.
Conclusions
These data provide robust, comprehensive evidence for the long-term safety and tolerability of once-daily olodaterol 5 and 10 μg delivered via Respimat® in a large population of patients with moderate to very severe COPD, including those with a history of cardiovascular disease or β-blocker use and patients with very severe COPD on multiple concomitant medications. Although the risk:benefit ratio was considered positive for both 5 and 10 μg olodaterol, 5 μg was selected as the dose for submission and has been registered in more than 30 countries worldwide, including, more recently, the United States.
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. They take full responsibility for the scope, direction, content of, and editorial decisions relating to, the manuscript, were involved at all stages of development, and have approved the submitted manuscript. The authors received no compensation related to the development of the manuscript.
Declaration of Interest Statement
L. McGarvey has served as a committee member for Applied Clinical Intelligence, received grants from Asthma UK, Northern Ireland Chest Heart & Stroke, NC3Rs, British Heart Foundation, and Chiesi, and performed consultancy for Almirall, Napp Pharmaceuticals, GlaxoSmithKline, Chiesi, and Boehringer Ingelheim. D. Niewoehner has served on advisory boards and adjudication committees for GlaxoSmithKline, Boehringer Ingelheim, Forest, Gilead, Novartis, and AstraZeneca. S. Magder has received personal fees from Boehringer Ingelheim and GlaxoSmithKline. P. Sachs has served on advisory boards for Boehringer Ingelheim. K. Tetzlaff, A. Hamilton, U. Bothner, and L. Korducki are employees of Boehringer Ingelheim. C. Vogelmeier has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, Janssen, Mundipharma, Novartis, and Takeda, and a grant from Grifols. A. Koch has received a grant from Actelion Pharmaceuticals and has taken part in congresses for Boehringer Ingelheim, Almirall, Bayer, and Novartis. G.T. Ferguson has received grants from Boehringer Ingelheim, Novartis, AstraZeneca, Pearl Therapeutics, and Forest, personal fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Pearl Therapeutics, Sunovion, and Forest, and non-financial support from Boehringer Ingelheim, GlaxoSmithKline, and Forest.
L. McGarvey, D. Niewoehner, S. Magder, and C. Vogelmeier formed the Mortality Adjudication Committee, providing analysis of the data. G.T. Ferguson, P. Sachs, and A. Koch were the Principal Investigators on the 1222.11 (P. Sachs), 1222.12 (G.T. Ferguson), 1222.13 (A. Koch), and 1222.14 (A. Koch) trials, and contributed to the study conception and design, provided oversight of the studies, and analysis of the data. K. Tetzlaff, A. Hamilton, U. Bothner and L. Korducki contributed to the study conception and design, provided oversight of the studies, and analysis of the data.
This work was supported by Boehringer Ingelheim Pharma GmbH & Co. KG. Medical writing assistance was provided by Caitlin Watson, PhD, of Complete HealthVizion, which was contracted and compensated by Boehringer Ingelheim Pharma GmbH & Co. KG.
Supplementary Material
Download MS Word (486.5 KB)Supplementary material for this article is available and can be accessed at http://dx.doi.org/10.3109/15412555.2014.991864
References
- Bouyssou T, Casarosa P, Naline E, Pestel S, Konetzki I, Devillier P, Schnapp A. Pharmacological characterization of olodaterol, a novel inhaled β2-adrenoceptor agonist exerting a 24-hour-long duration of action in preclinical models. J Pharmacol Exp Ther 2010; 334(1):53–62.
- Casarosa P, Kollak I, Kiechle T, Ostermann A, Schnapp A, Kiesling R, Pieper M, Sieger P, Gantner F. Functional and biochemical rationales for the 24-hour-long duration of action of olodaterol. J Pharmacol Exp Ther 2011; 337(3):600-609.
- van Noord JA, Smeets JJ, Drenth BM, Rascher J, Pivovarova A, Hamilton AL, Cornelissen PJG. 24-hour bronchodilation following a single dose of the novel β2-agonist olodaterol in COPD. Pulm Pharmacol Ther 2011; 24(6):666–672.
- van Noord JA, Korducki L, Hamilton A, Koker P. Four weeks once daily treatment with BI 1744 CL, a novel long-acting β2-agonist, is effective in COPD patients. Am J Respir Crit Care Med. 2009; 179, A6183 (abstract).
- Joos G, Aumann JL, Coeck C, Korducki L, Hamilton AL, van Noord J. Comparison of 24-hour FEV1 profile for once-daily versus twice-daily treatment with olodaterol, a novel long-acting B2-agonist, in patients with COPD. Am J Respir Crit Care Med 2012; 185, A2930 (abstract).
- Ferguson G, Feldman G, Hofbauer P, Hamilton A, Allen L, Korducki L, Sachs P. Lung function efficacy of olodaterol QD delivered via Respimat® in COPD patients: Results from two 48-week studies. Eur Respir J 2013; 42 (Suppl 57):5s, 187 (abstract).
- Koch A, Pizzichini E, Hamilton A, Hart L, Korducki L, De Salvo MC, Paggiaro P. Lung function efficacy of olodaterol QD delivered via Respimat® vs placebo and formoterol BID in patients with COPD: Two 48-week studies. Eur Respir J 2013; 42 (Suppl 57):146s, P764 (abstract).
- Koch A, Paggiaro P, Hamilton A, Hart L, Korducki L, De Salvo MC, Pizzichini E. Symptomatic benefit of olodaterol QD delivered via Respimat® vs placebo and formoterol BID in patients with COPD: Combined analysis from two 48-week studies. Eur Respir J 2013; 42 (Suppl 57):145s, P763 (abstract).
- Lange P, Aumann J-L, Derom E, Hamilton A, Tetzlaff K, Ting N, van Noord JA. The 24-h FEV1 time profile of olodaterol QD delivered via Respimat ® in COPD: Results from two 6-week studies. Eur Respir J 2013; 42 (Suppl 57):982s, 4635 (abstract).
- Feldman G, Bernstein JA, Hamilton A, Nivens C, LaForce C. The 24-hour FEV1 time profile of olodaterol once daily (QD) via Respimat® and formoterol twice daily (BID) via Aerolizer® in patients with COPD: results from two 6-week studies. Chest 2013; 144 (No. 4 Meeting Abstracts), 749A (abstract).
- Maltais F, Kirsten A-M, Hamilton A, De Sousa D, Wang F, Decramer M. Evaluation of the effects of olodaterol on exercise endurance in patients with COPD: results from two 6-week studies. Chest 2013; 144 (No. 4 Meeting Abstracts), 748A (abstract).
- US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry. Chronic obstructive pulmonary disease: developing drugs for treatment. Draft guidance [Internet]. [updated 2007 Nov 1; cited 2012 Apr 26]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071575.pdf.
- European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD) [Internet]. [updated 2012 Jul 21; cited 2013 Aug 7]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/08/WC500130880.pdf.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013 [Internet]. [updated 2013; cited 2014 Mar 5]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf.
- McGarvey LP, Magder S, Burkhart D, Kesten S, Liu D, Manuel RC, Niewoehner DE. Cause-specific mortality adjudication in the UPLIFT® COPD trial: findings and recommendations. Respir Med 2012; 106(4):515–521.
- Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B, on behalf of the INDORSE Study Investigators. Long-term safety and efficacy of indacaterol, a long-acting β2-agonist, in subjects with COPD. A randomized, placebo-controlled study. Chest 2011; 140(1):68–75.
- Kerwin E, Hébert J, Gallagher N, Martin C, Overend T, Alagappan VKT, Lu Y, Banerji D. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J 2012; 40(5):1106–1114.
- Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res 2010; 11:149.
- Chowdhury BA, Dal Pan G. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N Engl J Med 2010; 362(13):1169–1171.
- Mapel DW, Dalal AA, Blanchette CM, Petersen H, Ferguson GT. Severity of COPD at initial spirometry-confirmed diagnosis: data from medical charts and administrative claims. Int J Chron Obstruct Pulmon Dis 2011; 6:573–581.
- Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J 2014; 43(4):993–1002.
- Bateman E, Singh D, Smith D, Disse B, Towse L, Massey D, Blatchford J, Pavia D, Hodder R. Efficacy and safety of tiotropium Respimat® SMI in COPD in two 1-year randomized studies. Int J Chron Obstruct Pulmon Dis 2010; 5:197–208.
- Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, Dusser D, Joseph E, Kattenbeck S, Koenen-Bergmann M, Pledger G, Calverley P, for the TIOSPIR Investigators. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013; 369:1491–1501.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M, for the UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359(15):1543–1554.
- Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, for the TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356(8):775–789.
- Drummond MB, Wise RA, John M, Zvarich MT, McGarvey LP. Accuracy of death certificates in COPD: analysis from the TORCH trial. COPD 2010; 7(3):179–185.
- McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007; 62(5):411–415.
- de Torres JP, Marín JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, Baz-Dávila R, Zulueta JJ, Aguirre-Jaime A, Saetta M, Cosio MG, Celli BR. Lung cancer in patients with chronic obstructive pulmonary disease. Incidence and predicting factors. Am J Respir Crit Care Med 2011; 184(8):913–919.
- Dalby R, Spallek M, Voshaar T. A review of the development of Respimat® Soft Mist™ Inhaler. Int J Pharm 2004; 283(1–2):1–9.