Abstract
Diaphragmatic weakness in chronic obstructive pulmonary disease (COPD) is ascribed to hyperinflation-induced diaphragm shortening as well as impairment in cellular and subcellular structures. Although phrenic neuropathy is known to cause diaphragmatic weakness, phrenic neuropathy is rarely considered in COPD. This work aimed at assessing phrenic nerve conduction in COPD and its relation to radiographic hyperinflation and pulmonary function. Patients and methods: Forty COPD patients were evaluated. Radiographic parameters of lung hyperinflation were measured on postero-anterior and lateral chest x-ray films. Flow volume loop parameters were obtained from all patients. Motor conduction study of the phrenic nerves was performed and potentials were recorded over the xiphoid process and the ipsilateral 7th intercostal space. Twenty-seven healthy subjects were enrolled as controls. Results: Parameters of phrenic nerve conduction differed significantly in patients compared to controls. Phrenic nerve abnormalities were detected in 17 patients (42.5%). Electrophysiological measures correlated with diaphragmatic angle of depression on lateral view films and with lung height on postero-anterior films. They did not correlate with the flow volume loop data or disease severity score. Conclusion: Phrenic nerve conduction abnormality is an appreciated finding in COPD. Nerve stretching associated with diaphragmatic descent can be a suggested mechanism for nerve lesion. The presence of phrenic neuropathy may be an additional contributing factor to diaphragmatic dysfunction in COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) has been predicted to become the third leading cause of death and the fifth commonest cause of disability in the world by 2020 (Citation1). Hypercapnic respiratory failure is the most important cause of death in COPD (Citation2) and is associated with morbidity in those patients (Citation3). Structural and functional diaphragmatic weakness in the presence of a high mechanical load on the respiratory muscle pump, i.e. load-capacity imbalance, is significant in the pathophysiology of respiratory failure in COPD (Citation4).
The diaphragm is the chief muscle of inspiration (Citation5). Diaphragmatic weakness and dysfunction in COPD is ascribed to hyperinflation-induced diaphragm shortening, which places the diaphragm at a mechanical disadvantage. Impairment in cellular and subcellular structures has been more recently implicated (Citation6). These impairments are related to changes in fibre size, sarcomere length, muscle mass and muscle metabolism (Citation7). The functional impact of these mechanical and structural changes is reduced force-generating capacity of the diaphragm. This was clearly reflected by reduced transdiaphragmatic pressure measured in patients with COPD (Citation8). Force-generating capacity of the diaphragm is however influenced by other factors such as phrenic nerve conductance (Citation6). Phrenic neuropathy is known to cause diaphragmatic weakness (Citation9). Phrenic nerve involvement is however rarely considered in COPD patients. This work aimed at assessing phrenic nerve conduction in COPD patients and its relation to radiographic measures of lung hyperinflation and pulmonary function.
Materials and Methods
Forty patients were enrolled in the study. Patients were recruited from those attending the Chest Department, Faculty of Medicine, Alexandria University in Egypt. The diagnosis of COPD was made according to “the Global Initiative for Chronic Obstructive Lung Disease” (GOLD) guidelines based on clinical evaluation and a post-bronchodilator FEV1/FVC of < 0.70. The severity of airflow limitation was established as mild: FEV1 > 80%, moderate: FEV1 < 80% and >50%, severe: FEV1 < 50% and > 30%, and very severe: FEV1 < 30% of predicted value (Citation10). To be included, patients had to be in a stable condition, free from exacerbations for at least 2 months. Exclusion criteria included the presence of a secondary diagnosis of any type of pulmonary disease as well as any process that can obscure visualization of the costophrenic or sternophrenic junctions such as effusion or congestive heart failure. In addition, patients with neurological disorders, diabetes mellitus, or any systemic or endocrinal disease affecting the nervous system were excluded.
Patients consisted of 40 men with a mean age of 62.2 ± 8.3 years (range: 42–74 years), a mean height of 170.5 ± 5.9 cm, and a mean body mass index of 24.6 ± 6.7. Twenty-seven healthy men with matched age, height, and body mass index were enrolled as controls. Their mean age was 63.7 ± 7.7 years (range: 44–72 years), their height was 171.8 ± 5 cm, and their body mass index was 25.3 ± 6.4. There were no significant differences between patients and controls regarding their age (P = 0.721), height (P = 0.381), or body mass index (P = 0.67). The research was conducted in accordance with the amended Declaration of Helsinki and the protocol was approved by the Ethics Committee of Faculty of Medicine. An informed consent was obtained from all patients. Patients were assessed physiologically, radiologically, and electrophysiologically. The patients’ breathlessness was assessed using Medical Research Council (MRC) dyspnea scale (Citation11). PaO2 level (partial pressure of oxygen in arterial blood) was obtained from hospital data at time of the study.
Spirometry
Spirometry was performed in the pulmonary function laboratory according to the recommendations of the American Thoracic Society (Citation12, 13). Patients were tested in the sitting position. Each patient performed at least three acceptable forced expiratory maneuvers, which fulfilled the criteria of repeatability (Citation13). Measurements included forced expiratory flow rate in the first second (FEV1), forced vital capacity (FVC), Ratio of FEV1 to FVC (FEV1/FVC), forced expiratory time (FET), peak expiratory flow rate (PEFR), and forced expiratory flow during the middle portion of forced expiration (FEF 25–75%).
Radiologic evaluation
Postero-anterior (PA) and lateral chest X-ray films were obtained at maximal inspiration. The radiographs were acquired by a trained radiographer and were independently read by the chest physician who was blind of the electrophysiological results. Measurements included: lung height, retrosternal air space width, lung width, transverse cardiac diameter, diaphragmatic level, and the diaphragmatic angle of depression (DAOD) measured on PA and lateral chest films (Citation14). Lung height is the vertical distance from the tubercle of the first rib to the apex of the diaphragm on PA film.
Retrosternal air space width is the horizontal distance from the sternum to the aorta at the closest point on lateral film. Lung width is the horizontal distance between the interior aspect of the ribs on the right and left sides at the level of the apex of the right diaphragm on PA film. Transverse cardiac diameter is the transverse diameter of the heart at its widest point on PA film. Diaphragmatic level is measured by the number of visible ribs posteriorly to the nearest half-rib on PA film. The DAOD on the PA film is the angle between a line drawn from the costophrenic junction to the vertebrophrenic junction and another line from the costophrenic junction to the diaphragmatic apex. The DAOD on lateral film is the angle between a line drawn from the sternophrenic angle to posterior costophrenic junction and another one from the posterior costophrenic junction to the diaphragmatic apex.
Electrophysiological evaluation
Electrophysiological studies were conducted on a NIHON KOHDEN Neuropack MEB-7102 mobile unit with a two-channel evoked potential/electromyography (EMG) measuring system (Nihon Kohden Corp., Tokyo, Japan). Evaluation included motor nerve-conduction study of the phrenic nerve on both sides. Electrophysiological work-up for exclusion of peripheral neuropathy was carried out and included sensory conduction study of sural nerve, motor conduction studies of posterior tibial and deep peroneal nerves, and EMG of the tibialis anterior and soleus muscles.
Motor nerve-conduction study of the phrenic nerve
Patients were examined while lying supine with the head slightly elevated and rotated to the side opposite to the nerve under stimulation. The phrenic nerve was supramaximally stimulated at the posterior border of the sternomastoid muscle at the level of the cricoid cartilage with a bipolar surface stimulating electrode (Citation15). The anode was placed proximal to the cathode. Rectangular pulses of 0.2–0.5ms duration were used. Diaphragmatic potentials were first recorded by placing the active recording electrode 5cm superior to the tip of the xiphoid process and the reference electrode over the ipsilateral costal margin 16 cm from the active electrode (xiphoid process recording) (Citation16). Diaphragmatic potentials were also recorded by placing the active electrode over the 7th intercostal space at the anterior axillary line and the reference electrode at the 8th intercostal space (intercostal space recording) (Citation17).
A ground disc electrode was located between the stimulating and the recording points. The responses were recorded with a filter bandwidth of 5 Hz–2 kHz. The gain was set to 200–2000 μV and the sweep speed to 50 ms. Measurements included the latency and the amplitude of the response. Due to the variation in the recorded CMAP between inspiration (higher amplitude) and expiration, recordings were made during normal inspiration. Two responses were recorded and the average values were calculated. Abnormality was defined as absent or delayed potentials exceeding cut-off point obtained from the control group (mean+3 standard deviations (SD)), side-to-side latency difference exceeding the cut-off point obtained from the control group, amplitude reduction below the cut-off value of the controls, and/or amplitude reduction by >50% that of the opposite side. Definition of normality as mean ± 3SD was chosen so that normal persons are not inadvertently labeled as having an abnormality.
Statistical analysis
The statistical Package of Social Science (SPSS version 17) software was used to analyze data. Analysis of variance (ANOVA) and post hoc tests were used for comparison of means. The unpaired Student's t-test was used for side-to-side latency comparison between patients and controls. Pearson's correlation test was used to assess the relationship between electrophysiological measures and parametric measures whereas; Spearman's correlation was used to assess the relationship between electrophysiological measures and non-parametric measures. A level of P ≤ 0.05 was considered significant.
Results
The mean disease duration was 13.4 ± 8.6 years (ranging from 2–40 years). The rating of MRC dyspnea ranged from 1 to 4 with a mean of 3.15 ± 0.84. Mean flow volume loop parameters and PaO2 level in the studied patients are presented in Table . Three patients had mild COPD, 8 had moderate COPD, 16 had severe COPD, and 13 had very severe COPD.
Table 1. Flow volume loop parameters and PaO2 level in the studied COPD patients
Radiographic study
Radiographic measures of lung hyperinflation are presented in Table . Lung height, retrosternal air space width, and diaphragmatic level were significantly increased in patients compared to controls, whereas DAOD on PA and lateral view films was significantly decreased in patients compared to controls.
Table 2. Radiographic measures of lung hyperinflation in COPD patients and controls
Electrophysiological study
Electrophysiological findings recorded from patients and controls are presented in Table . The mean values of the controls represent the pooled data from the right and left sides since no significant differences were found between both sides. Responses from the 7th intercostal space were significantly delayed with significantly increased interside latency difference in patients compared to controls (Table ). In addition, mean compound muscle action potential (CMAP) amplitudes were significantly reduced in patients compared to controls.
Table 3. Phrenic nerve conduction studies in COPD patients and controls
Table 4. Side-to-side latency difference (ms) of the recorded phrenic nerve potentials in COPD patients and controls
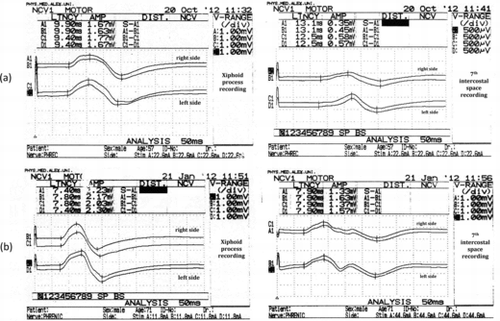
At individual level, the phrenic nerve potentials were easily elicited in all patients and controls and were reproducible. Twenty-three patients (57.5%) had normal conduction study of their phrenic nerves. Abnormalities were detected in 17 patients (42.5%) with prolongation of the terminal latency of the responses recorded from the 7th intercostal space on one or both sides (8 and 9 patients respectively) and reduction of the amplitude in only 5 of them. Prolongation of the responses recorded from the xiphoid process were detected in only 4 of those 17 patients (unilateral in 3 and bilateral in 1) with reduced CMAP amplitude in only 1 of them. In 6 patients, high amplitude values (>2mV) were recorded from the xyphoid process. None of these patients had prolongation of the recorded potentials. Figure presents phrenic nerve conduction studies in a healthy subject and in a patient with COPD.
Figure 1. Diaphragmatic compound muscle action potentials recorded in a patient with COPD (a) showing prolongation of the distal latency of the potentials recorded over the xyphoid process on the right side and the 7th intercostal space on both sides with normal amplitude. Diaphragmatic potentials recorded in a healthy subject are also shown (b).
Correlation analyses
Table presents the correlation analyses between electrophysiological parameters, dyspnea score, flow volume loop parameters, PaO2, and radiographic measures of lung hyper-inflation. Electrophysiological parameters did not correlate with MRC dyspnea score, PaO2 level, or any of the flow volume loop parameters, whereas the latency of the recorded responses correlated with two of the radiographic measures of lung hyperinflation. The latency of the responses recorded from the xiphoid process correlated positively with lung height on both sides. In addition, the latency of the responses recorded from the xiphoid process and from the 7th intercostal space correlated negatively with DAOD on lateral view films on both sides.
Table 5. Correlation analyses between electrophysiological parameters, dyspnea score, flow volume loop parameters, PaO2, and radiographic measures of lung hyper-inflation
Discussion
In the current study, the mean latency of the phrenic nerve potentials and the mean amplitudes recorded from the branches to the costal diaphragm were significantly different in patients compared to controls. In addition, 17/40 COPD patients (42.5%) had evidence of phrenic nerve affection. Only three studies have assessed the phrenic nerve conduction in patients with COPD. Lu et al. (Citation18) found reduced CMAP amplitude of the phrenic nerve in a small number of patients with COPD. Hopkinson and colleagues (Citation19) found significantly longer mean phrenic nerve latency in COPD patients compared to healthy controls with no difference in CMAP amplitudes. Podnar and Harlander (Citation20) found significantly larger CMAP amplitude with significantly prolonged terminal latencies in COPD patients. Hopkinson et al. as well as Podnar and Harlander related the delayed responses of the phrenic nerves to polyneuropathy reported in a portion of COPD patients. The possible relation between phrenic neuropathy and diaphragmatic descent was not considered in any of these studies.
In the current study, phrenic nerve abnormality is unlikely part of polyneuropathy reported in patients with COPD (Citation21, 22) since the conduction studies along the peripheral lower limb nerves and EMG of the tibials anterior and soleus were all normal. Moreover, hypoxemia (as assessed by PaO2), which is the most important factor for neuropathy in COPD (Citation23) did not correlate with phrenic nerve conduction study parameters. The presence of negative correlation between terminal latency of the phrenic nerve and the DAOD on lateral view films and positive correlation between terminal latency of the phrenic nerve and lung height on PA view films suggests that a probable mechanism for phrenic nerve abnormality is stretch of the nerve (traction neuropathy) secondary to diaphragmatic descent associated with lung hyperinflation. This mechanism of phrenic nerve stretch is analogous to pudendal nerve stretching induced by perineal descent in association with chronic straining during defecation (Citation24, 25). Stretch neuropathy of the phrenic nerve was not previously considered in patients with COPD.
The possibility of phrenic nerve stretching in COPD can be considered in view of the intimate relation of the phrenic nerve to the diaphragm. The phrenic nerves which arise from C3-5 roots descend lateral to the inferior vena cava on the right side and over the antero-lateral angle of the pericardial base to reach the diaphragm on the left side. Both nerves divide into an average of 3-5 branches just above the diaphragm (Citation26). Main branches include the sternal, antero-lateral, postero-lateral, and crural branches (Citation27). The branches diverge, enter the muscle or central tendon, and run obliquely for a varying distance in its substance. They then appear on the under surface of the diaphragm and radiate deep to the subphrenic fascia, whence numerous filaments innervate the muscle fibres (Citation26).
The consequence of this anatomical relationship is that diaphragmatic descent is expected to cause traction on the phrenic nerve branches as they penetrate the diaphragm to reach its under surface. The extent of nerve strain is expected to parallel the extent of diaphragmatic descent. The involvement of the branch to the lateral costal diaphragm in all cases with phrenic nerve abnormality suggests that this branch is more strained by diaphragmatic descent than the branch to the sternal diaphragm. This can be related to the variation in the direction and the location of the branches innervating the diaphragm with respect to the stretching force and the subsequent nerve tension in each of these branches. In addition, the descent of the diaphragm is expected to be less manifest at the sternal part where the lung tissue and subsequently the degree of diaphragmatic descent are least.
Generally, factors determining the responses of nerves to stretch include nerve elongation rate; elongation speed; nerve tension; and the period of elongation (Citation28). A nerve can stretch about 6% of its length without changing its activity. But a stretch of more than 15% of its length will cause irreversible damage (Citation29, 30). In the current study, none of these factors was determined and the exact percentage of nerve elongation over its original length was not known. It can, however, be assumed that phrenic nerve lesion in COPD represents slow gradual nerve elongation which has been shown to cause histological changes with modifications in myelin, axon degeneration and regeneration, and deposition of endoneurial collagen (Citation31).
Electrophysiological changes in association with nerve stretch have been assessed in different basic scientific and clinical studies (Citation24, Citation28, Citation32, 33). Basically, prolongation of the latency, as detected in most of our cases, correlates with demyelination. The range of latencies of the recorded potentials varied among the studied patients and this would reflect different grades of severity. The presence of two cases where the terminal latency of the recorded potentials exceeded 20 ms should indicate more severe degree of neuropathy corresponding to severe stretch injury. Mild delay of the recorded potential can be encountered in association with axonal degeneration due to loss of fast conducting fibers.
The recorded CMAP amplitude from the 7th intercostal space was significantly reduced in our studied patients compared to controls. Five patients had amplitude reduction below the cut-off point of the controls. All 5 patients had prolonged latency suggesting that amplitude reduction is probably related to secondary axonal degeneration. Still, the reduced amplitude can be related to reduction in diaphragm muscle mass (loss of muscle bulk) related to impairments in cellular and subcellular structures. Different geometric relationships between recording electrodes and the diaphragm in COPD patients may also account in part for the amplitude difference between patients and controls.
Still an explanation to high CMAP amplitude recorded from the xyphoid process in 6 patients who do not have any prolongation of the response latency can be suggested. Podnar and Harlander (Citation20) found significantly increased diaphragmatic CMAP amplitude in COPD patients and attributed this finding to flattening of the diaphragm with shortening and thickening of the muscle fibers or to increased inspiratory muscle effort leading to compensatory increased diaphragm muscle mass. It is unlikely that flattening of the diaphragm contributed to increased diaphragmatic CMAP amplitude in our patients given that diaphragmatic level and DAOD were not correlated with the CMAP amplitude. Increased diaphragmatic muscle mass secondary to increased inspiratory muscle effort is a likely explanation and is proposed to overweigh the impairments in cellular and subcellular structures. Enrollment in inspiratory muscle training programs can also strength train the diaphragm (Citation34, 35) with associated increase in diaphragm thickness (Citation36) which can be reflected on increased diaphragmatic CMAP amplitude.
Parameters of phrenic nerve conduction did not correlate with flow volume loop parameters or disease severity score. This finding can be explained considering the point that flow volume loop parameters and disease severity both reflect the current degree of airway obstruction rather than the extent of lung hyperinflation and associated diaphragmatic descent. Parameters of nerve conduction did not correlate with dyspnea score as well. Absence of such correlation can be seen in view of the multiplicity and complexity of factors contributing to dyspnea in COPD. Moreover, dyspnea in COPD was found to correlate with diaphragmatic dysfunction (Citation37). This dysfunction can be attributed not only to a phrenic nerve lesion but also to hyperinflation-induced diaphragm shortening, which places the diaphragm at a mechanical disadvantage as well as impairment in cellular and subcellular structures. Further studies are needed to determine the exact contribution of phrenic nerve lesion to the disturbed diaphragmatic function independent of other factors.
In the current study, measures of lung hyperinflation differed significantly between patients and controls. Of these measures, lung height and the degree of diaphragmatic depression represent the most valuable two measures of lung hyperinflation and diaphragmatic descent (Citation14). The DAOD is of particular significance considering the fact that this measure is independent of patients’ dimensions and that it provides a continuous scale of measurements (Citation14). Absence of any correlation between the electrophysiological parameters and the diaphragmatic level is consistent with the fact that alteration in diaphragmatic position in patients with COPD is considered a less accurate measure for determination of lung hyperinflation than flattening of the diaphragm (Citation38).
Technical aspects
The technique of phrenic nerve conduction was easy to perform and the response was reproducible. Sometimes, stimulation of the nerve was more easily achieved with the head in midline. Main technical difficulties were related to co-stimulation of the brachial plexus as indicated by muscle contractions and arm movement. In such case, the cathode was repositioned to preferentially stimulate the phrenic nerve. An alternative to surface recording whereby contamination by action potentials arising in extradiaphragmatic muscles is extremely remote is the use of the oesophageal diaphragm recording technology. However, with esophageal recording, the crural diaphragm is preferentially sampled, as opposed to the sternal and costal diaphragm with surface electrodes. Assessment of conduction along the costal branch was important in the current study and most of the abnormalities were detected along the branch to the lateral costal diaphragm.
For intercostal space recording, 7th/8th intercostal space montage was preferred over 8th/9th montage. First, we measured the CMAP amplitude for both montages in our COPD patients and no difference was found. Second, and more importantly, at the level of the 7th intercostal space, the differences in anthropometric measures between COPD patients and controls are kept to their minimum (as compared to 8th and 9th intercostal spaces). This was of particular significance as diaphragmatic CMAP amplitude was found to correlate with chest circumference and lung volume (Citation16).
Study limitations
Needle EMG would be needed to confirm the presence of axonal degeneration and to rule out myopathy of the diaphragm. Needle EMG was not however performed to avoid pneumothorax (Citation39), especially in those high-risk patients. In such case, the exact number of patients with axonal degeneration was not determined. This however can minimally affect the frequency of patients with phrenic nerve abnormalities in this study given that the main electrophysiological abnormality was related to the presence of demyelination.
The average values of two responses were calculated. This can be considered a methodological limitation where the average of 3 or more responses is considered in other studies. It is unlikely that this limitation would influence the results of the study given that the nerve was supramaximally stimulated which insures maximum amplitude for all recordings.
Conclusion
Phrenic nerve conduction abnormality is an appreciated finding in COPD. Possible nerve stretching associated with diaphragmatic descent can be a suggested mechanism for nerve lesion. In COPD, phrenic nerve involvement may be an additional contributing factor to diaphragmatic dysfunction in those patients. The force-generating capacity of the diaphragm can be influenced and the ability of the diaphragm to maintain alveolar ventilation can be compromised. Further studies are needed to determine the exact contribution of phrenic nerve lesion to the disturbed diaphragmatic function independent of other factors.
Declaration of Interest Statement
We certify that there aren't any financial or personal relationships with other people or organizations that could inappropriately influence (bias) our work. We also certify that there isn't any affiliation with any organization with a financial interest, direct or indirect, in the subject matter or materials discussed in the manuscript that may affect the conduct or reporting of the work submitted.
The authors alone are responsible for the content and writing of the paper.
References
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med 1998; 4:1241–3.
- Zielinski J, MacNee W, Wedzicha J, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis 1997; 52:43–47.
- Decramer M, Gosselink R, Troosters T, et al. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J 1997; 10:417–423.
- Orozco-Levi M. Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J 2003; 22(Suppl 46):41s–51s.
- Khanorkar SV, ed. Mechanics of respiration. In: Insights in physiology. 1st ed. Chapter 33. New Delhi/Banama City/London: Jaypee Brothers Medical Publishers (P) Ltd., 2012:202–208.
- Ottenheijm CAC, Heunks LMA, Dekhuijzen RPN. Diaphragm adaptations in patients with COPD. Respir Res (Internet). 2008; 9(1):12. Available from: <http://respiratory-research.com/content/9/1/12>. Last accessed March 29, 2014.
- Levine S, Nguyen T, Kaiser LR, et al. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med 2003; 168:706–713.
- Newell SZ, McKenzie DK, Gandevia SC. Inspiratory and skeletal muscle strength and endurance and diaphragmatic activation in patients with chronic airflow limitation. Thorax 1989; 44:903–912.
- Wilcox PG, Pardy RL. Diaphragmatic weakness and paralysis. Lung 1989; 167(6):323–341.
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD Executive Summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Fletcher CM, Elmes PC, Fairbairn AS, et al. Significance of Respiratory Symptoms and the Diagnosis of Chronic Bronchitis in a Working Population. Br Med J 1959; 2(5147):257–266.
- Miller MR, Crapo R, Hankinson J, et al. ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J 2005; 26:153–161.
- Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Rothpearl A, Varma AO, Goodman K. Radiographic measures of hyperinflation in clinical emphysema. Discrimination of patients from controls and relationship to physiologic and mechanical lung function. Chest 1988; 94:907–913.
- Luo YM, Polkey MI, Lyall RA, et al. Effect of brachial plexus co-activation on phrenic nerve conduction time. Thorax 1999; 54:765–770.
- Chen R, Collins S, Remtulla H, et al. Phrenic nerve conduction study in normal subjects. Muscle Nerve 1995; 18(3):330–335.
- Canbaz S, Turgut N, Halici U, et al. Electrophysiological evaluation of phrenic nerve injury during cardiac surgery – a prospective, controlled, clinical study. BMC Surgery (Internet). 2004; 4:2. Available from: <http://biomedcentral.com/1471-2482/4/2>. Last accessed March 29, 2014.
- Lu Z, Tang X, Huang X. Phrenic nerve conduction and diaphragmatic motor evoked potentials: evaluation of respiratory dysfunction. Chin Med J 1998; 111:496–499.
- Hopkinson NS, Sharshar T, Ross ET, et al. Corticospinal control of respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol 2004; 141:1–12.
- Podnar S, Harlander M. Phrenic nerve conduction studies in patients with chronic obstructive pulmonary disease. Muscle Nerve 2013; 47(4):504–509.
- Gupta PP, Agarwal D. Chronic obstructive pulmonary disease and peripheral neuropathy. Lung India 2006; 23:25–33.
- Oncel C, Baser S, Cam M, et al. Peripheral neuropathy in chronic obstructive pulmonary disease. COPD. 2010;7(1):11–6.
- Deluca C, Buffone E, Minguzzi E. Chronic obstructive pulmonary disease (COPD): Neuromuscular implications. In: Dal Negro RW, Hodder R, eds. Long Term Oxygen Therapy New Insights and Perspectives. Springer-Verlag: Italia, Milan Dordrecht Heidelberg London New York, 2012; 57–66.
- Snooks SJ, Barnes PRH, Swash M. Damage to the innervation of the voluntary anal and periurethral sphincter musculature in incontinence: an electrophysiological study. J Neurol Neurosurg Psych 1984; 47:1269–1273.
- Swash M. New concepts in incontinence. Br Med J 1985; 290:4–5.
- Muller Botha GS. The anatomy of phrenic nerve termination and the motor innervation of the diaphragm. Thorax 1957; 12(1):50–56.
- King JE, Rajesh PB. Benign disease of the diaphragm. In: Fielding JWL, Hallissey MT, eds. Upper gastrointestinal surgery. Chapter 9. Springer-Verlag: London/Berlin/Heidelberg, 2005; 117–127.
- Shibukawa M, Shirai Y. Experimental study on slow-speed elongation injury of the peripheral nerve: electrophysiological and histological changes. J Orthop Sci 2001; 6:262–268.
- Bora FW, Pleasure DE, Didizian NA. A study of nerve regeneration and neuroma formation after nerve suture by various techniques. J Hand Surg 1976; 1:138–143.
- Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve 1990; 13:771–784.
- Topp KS, Boyd BS. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys Ther 2006; 86:92–109.
- Skoulis TG, Vekris MD, Terzis JK. Effect of distraction osteogenesis on the peripheral nerve: experimental study in the rat. J Reconstr Microsurg 1998; 14:565–574.
- Lajtai G, Wieser K, Ofner M, et al. Electromyography and nerve conduction velocity for the evaluation of the infraspinatus muscle and the suprascapular nerve in professional beach volleyball players. Am J Sports Med 2012; 40(10):2303–2308.
- Lőtters F, van Tol B, Kwakkel G, et al. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J 2002; 20:570–576.
- Serón P, Riedemann P, Muñoz S, et al. Effect of inspiratory muscle training on muscle strength and quality of life in patients with chronic airflow limitation: a randomized controlled trial. Arch Bronconeumol 2005; 41(11):601–606.
- Enright SJ, Unnithan VB, Heward C, et al. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys Ther 2006; 86:345–354.
- Paulin E, Yamaguti WP, Chammas MC, et al. Influence of diaphragmatic mobility on exercise tolerance and dyspnea in patients with COPD. Respir Med 2007; 101(10):2113–2118.
- O’Driscoll CM, Chan ED, Fernandez E. Imaging of emphysema. In: Lynch DA, Newell JD, Lee JS, eds. Imaging of Diffuse Lung Disease. Chapter 7. BC Decker: Hamilton/Ontario/Canada, 2000; 199–233.
- Saadeh PB, Crisafulli C, Garofalo M. Needle EMG of the diaphragm. Muscle Nerve 1993; 16:323–325.
