Abstract
Background: There is little evidence that the guideline-recommended oxygen saturation of 92% is the best cut-off point for detecting hypoxemia in COPD exacerbations. Objective: To detect and validate pulse oximetry oxygen saturation cut-off values likely to detect hypoxemia in patients with aeCOPD, to explore the correlation between oxygen saturation measured by pulse oximetry and hypoxemia or hypercapnic respiratory failure. Methodology: Cross-sectional study nested in the IRYSS-COPD study with 2,181 episodes of aeCOPD recruited between 2008 and 2010 in 16 hospitals belonging to the Spanish Public Health System. Data collected include determination of oxygen saturation by pulse oximetry upon arrival in the emergency department (ED), first arterial blood gasometry values, sociodemographic information, background medical history and clinical variables upon ED arrival. Logistic regression models were performed using as the dependent variables hypoxemia (PaO2 < 60 mmHg) and hypercapnic respiratory failure (PaO2 < 60 mmHg and PaCO2 > 45). Optimal cut-off points were calculated. Results: The correlation coefficient between oxygen saturation and pO2 measured by arterial blood gasometry was 0.89. The area under the curve (AUC) for the hypoxemia model was 0.97 (0.96–0.98) and the optimal cut-off point for hypoxemia was an oxygen saturation of 90%. The AUC for hypercapnic respiratory failure was 0.90 (0.87–0.92) and the optimal cut-off point was an oxygen saturation of 88%. Conclusions: Our results support current recommendations for ordering blood gasometry based on pulse oximetry oxygen saturation cut-offs for hypoxemia. We also provide easy to use formulae to calculate pO2 from oxygen saturation measured by pulse oximetry.
Background
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend performing arterial blood gasometry when clinical signs of respiratory failure or right heart failure are observed in patients with stable severe COPD. Arterial blood gasometry is also recommended for tracking or managing oxygen therapy in patients experiencing acute exacerbations of COPD, and when respiratory failure is suspected in order to initiate mechanical ventilation (Citation1,2).
Until the results of blood gasometry are available, pulse oximetry is the screening tool used by physicians to make decisions about acute exacerbations of COPD (aeCOPD). The cut-off values for oxygen saturation as measured by pulse oximetry to detect hypoxemia ranges in different studies from 88% to 92%, depending on the amount of carboxyhemoglobin measured in peripheral blood samples (Citation3). Relatively little is known about the range of oxygen saturation measured by pulse oximetry more related to hypoxemia in patients with aeCOPD. Yet, recommendations for oxygen treatment have been made based on low levels of evidence (Citation4) or articles with small sample sizes (Citation1,Citation5). Although guidelines tend not to replace arterial blood gasometry for detecting hypoxemia, pulse oximetry is recommended as a useful screening tool : guidelines for the management and treatment of acute exacerbations of COPD have set recommendations for oxygen therapy based on values of oxygen saturation measured by arterial blood gasometry.
Pulse oximetry has been, therefore, used in emergency departments and primary care offices to diagnose hypoxemia as well as to manage and monitor patients with chronic obstructive pulmonary disease (COPD) and other respiratory conditions when stable or when experiencing acute exacerbations since this tool assesses oxygenation (Citation1,Citation4,Citation6). On the other hand, although capnography has been developed to assess ventilation in emergency settings, this tool is generally not used in pre-hospital settings or primary care, and is not even available in all emergency departments.
By this reason, decisions about the initial treatment of hypoxemia before the results of arterial blood gasometry are available are based almost exclusively on pulse oximetry even though one of its key limitations is its inability to assess ventilation in the presence of oxygen therapy, which is used by many people with chronic respiratory conditions (Citation6,7). It is known that the use of oxygen therapy in situations of ventilatory failure in acute excaerbations of COPD (aeCOPD) many times contributes to admission to the intensive care unit and increased length of stay (Citation8).
In this context, the aims of this study were to 1) detect or validate cut-off values of oxygen saturation determined by pulse oximetry that are more likely to detect hypoxemia, 2) determine if oxygen saturation measured by pulse oximetry is correlated with hypoxemia or respiratory failure, and 3) identify possible confounders in this relationship.
Methods
We conducted a cross-sectional cohort study nested in the Investigacion en Resultados y Servicios Sanitarios-Chronic Obstructive Pulmonary Disease (IRYSS-COPD) appropriateness study, which has been described elsewhere (Citation9). Patients for this cohort study were recruited from 16 hospitals belonging to the Spanish National Health Service. Patients with symptoms of COPD exacerbation who attended the EDs of these hospitals between June 2008 and September 2010 were invited to participate in the study. The Institutional Review Board of each hospital approved the study. All patients or caregivers had to provide informed consent to participate in the study.
Patients were candidates for the study if they presented to the ED of any of the participating hospitals with symptoms consistent of an exacerbation of COPD. Exacerbation was defined as an event in the natural course of the disease characterized by a change in the patient's baseline dyspnea, cough, and/or sputum that was beyond normal day-to-day variations, was acute in onset, and may have warranted a change in regular medication in a patient with underlying COPD (Citation1).
Patients were considered to have been previously diagnosed with COPD if they had a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) quotient <70%. Patients not previously diagnosed with COPD but in whom the disease was suspected were also eligible for inclusion in the study. This included smokers or former smokers of more than 15 packs per year with dyspnea, cough, or expectoration for more than 3 months per year, and who were experiencing symptoms resembling aeCOPD. The diagnosis had to be confirmed by spirometry within 60 days after the index episode at a time when the patient was stable, i.e., the absence of any increase in symptoms or changes in background therapy. If a diagnosis of COPD was not confirmed, the patient was excluded from the study.
Patients were excluded from the study if they had COPD complicated by a comorbidity such as pneumonia, pneumothorax, or pulmonary embolism; lung cancer; or left cardiac insufficiency. Other exclusion criteria included a diagnosis of asthma, extensive bronchiectasis, sequelae of tuberculosis, pleural thickening, or restrictive diseases. Patients who did not wish to participate were also excluded.
We collected information about patients upon their arrival in the ED (ED arrival). This information included the severity of the exacerbation (gauged by level of consciousness, respiratory rate, heart rate, and blood gasometry)(Citation10); baseline severity of COPD (measured by the FEV1)(Citation1); the presence of comorbidities that affect the exacerbation of COPD, such as diabetes and cardiovascular disease; the number of hospital admissions for COPD exacerbations during the previous 12 months; the patient's ability to carry out necessary treatments at home; response to previous treatments; previous use of home oxygen therapy; and demographic variables such as age and sex. We also collected information about comorbidities included in the Charlson Co-morbidity Index. “Low perfusion” was created as a variable composed by heart rate, blood pressure and presence of arrhythmia: if systolic blood pressure lower than 90 mm Hg and heart rate lower or equal to 60 or greater or equal than 100 and/or presence of arrhythmia, then low perfusion is assumed; otherwise, low perfusion is not assumed.
We defined hypoxemia as pO2 < 60 mm Hg (7.99 kPa). Type II hypercapnic respiratory failure was defined as pO2 < 60 mm Hg (7.99 kPa) and pCO2 > 50 mm Hg (6.66 kPa), while type I respiratory failure was defined as pO2 < 60 mm Hg (7.99 kPa) and pCO2 ≤ 50 mm Hg (6.66 kPa). A pCO2 > 50 mm Hg (6.66 kPa) was defined as hypercapnia.
Statistical analysis
Only aeCOPD episodes in which the oxygen level was measured with both pulse oximetry and arterial blood gasometry were included in the analysis. We excluded aeCOPD episodes in which oxygen saturation upon ED arrival were >92%, for which gasometry is not always required (Citation1). Patients who presented with highly discordant values, such as arterial pO2 < 60 mm Hg and oxygen saturation measured by pulse oximetry > 90% or those with pO2 > 100 mmHg were also excluded.
Descriptive statistics include frequencies and percentages for categorical variables and mean and standard deviation (SD) for continuous variables. The sample was randomly divided into two equal-sized subsamples: a derivation sample, composed of 1090 episodes of COPD exacerbations, and a validation sample, composed of 1091 episodes.
The derivation sample was used to develop a model that describes the relationship between oxygen saturation measured by pulse oximetry and pO2 measured by blood gasometry. The Spearman correlation coefficient between both measures was calculated. The relationship between the two was shown in a scatter plot. As the relationship was not linear, the x axis (pulse oximetry) was divided into sections of the same length and the functional relationship was adjusted using locally weighted polynomial regression.
We compared the adjacent slopes of the polynomial pieces, looking for the point where the maximum slope change occurred. Bootstrap resampling techniques were used to test statistical significance of the slope change at different cut-off points to validate the selected point at which the maximum slope change occurs. Two different regression lines were then calculated, using that point as the cut-off, by performing a multilevel analysis adjusted by hospitals. Finally, both regression models were combined into a prediction rule for pO2 detected by blood gasometry using as a predictor O2 saturation by pulse oximetry. The predictive rule from the derivation cohort was then applied to the validation sample and the value of R2 was calculated as a measure of variability explained by the models.
Logistic regression models were created to explore the probability of detecting hypoxemia and hypercapnic respiratory failure. In addition to oxygen saturation measured by pulse oximetry, other independent variables considered were baseline severity of COPD, age, gender, presence of arrhythmia, blood pressure, smoking habit, hospital admissions for aeCOPD in the previous year, and heart rate and respiratory rate upon ED arrival.
The predictive validity of each model was determined by calculating the area under the receiver operating characteristic (ROC) curve (AUC). Characteristics of the test were evaluated by sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), as well as accuracy. For each model, the point that maximized the sum of sensitivity and specificity was considered the optimal cut-off point.
These models were then applied to the validation sample to compare predictive accuracy by means of comparisons of AUCs, and the cut-off points were validated by analysing sensitivity and specificity (Citation11).
All effects were considered significant at P < 0.05 unless otherwise stated. All statistical analyses were performed using SAS for Windows statistical software, version 9.2 (SAS Institute, Inc., Carey, NC) and R© software version 2.13.0.
Results
We recruited 2877 episodes of COPD exacerbations. We excluded 433 by the reason of missing data in pO2 or in Sat O2 and 181 episodes because presenting discordant values between pulse oxymerey and gasometry, that is, SatO2 > 90 and, at the same time, pO2 < 60. We also excluded 82 patients who presented pO2 > 100. We have excluded by all these reasons 696 episodes of COPD exacerbations.
The analysis includes 2,181 episodes of COPD exacerbation. Of these, 604 episodes (27.71%) presented with type 1 respiratory failure, 472 (21.65%) with type 2, and 693 (31.79) with hypercapnia. The mean oxygen saturation measured by pulse oximetry was 87.28% (±9.33)and pO2 measured by arterial blood gasometry was 59.3 mm Hg (±14.11). Nearly half of the patients (1077 [49.38%]) had significant systemic hypoxemia. Complete characteristics of the derivation and validation cohorts are presented in Table .
Table 1. Descriptive analysis
The correlation coefficient between oxygen saturation measured by pulse oximetry and pO2 determined from arterial blood gasometry was 0.89. The relationship the two measured is shown in Figure . The greater change in slope was detected where O2 saturation is >90%. The resulted equations were:
if SatO2 ≥ 90 then pO2 = (SatO2 × 0.7712) − 13.41
if SatO2 > 90 then pO2 = (SatO2 × 3.3235) − 241.65
Figure 1. Relationship between oxygen saturation by pulse oximetry and calculated from blood gas analysis.
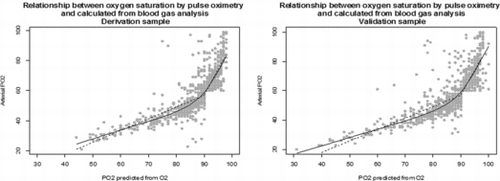
This model explained 74% of the variability in pO2.
The ROC curve for determining the oxygen saturation screening cut-off for systemic hypoxemia by pulse oximetry is shown in Figure . The area under the curve for the model is 0.97 (95% confidence interval [95% CI], 0.96–0.98). The optimal cut-off suggested by this curve is at a pulse oximetry oxygen saturation of 90%. That corresponds with a sensitivity of 100% and a specificity of 84%. Candidate variables included in the model that were significantly associated with oxygen saturation were baseline severity of COPD and dyspnea at rest upon ED arrival. Age, sex, number of hospital admissions for a COPD exacerbation in the previous year, and respiratory rate upon ED arrival were not significantly associated with oxygen saturation. The performance of different screening cut-offs is shown in Table .
Figure 2. ROC curve for ability of oxygen saturation by pulse oximetry predicting systemic hypoxemia.
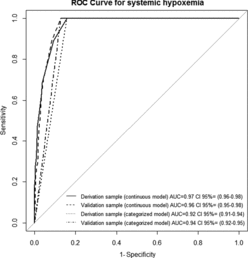
Table 2. Performance of different levels of screening cut-off for the detection of hypoxia by pulse oximetry in derivation sample
The ROC curve for determining the oxygen saturation screening cut-off for hypercapnic respiratory failure by pulse oximetry is shown in Figure . The area under the curve is 0.90 (95% CI, 0.87–0.92). The optimal cut-off suggested by this curve is at pulse oximetry oxygen saturation of ≤88%. That corresponds with a sensitivity of 91% and specificity of 72%. The performance of different screening cut-offs is shown in Table .
Figure 3. ROC curve for the performance of oxygen saturation by pulse oximetry for predicting hypercapnic respiratory failure.
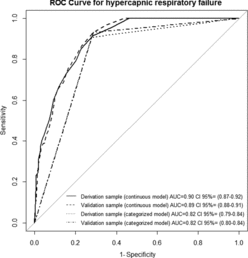
Table 3. Performance of different levels of screening cut-off for the detection of hypercapnic respiratory failure by pulse oximetry
Discussion
In our sample of 2,181 episodes of COPD exacerbations evaluated in 16 different hospitals, the optimal pulse oximetry cut-off point for detecting hypoxemia was an oxygen saturation of 90%, while the optimal cut-off to detect hypercapnic respiratory failure was 88%. These findings support in part GOLD recommendations to assess arterial blood gases when oxygen saturation measured by pulse oximetry is below 92%, and provide information to clinicians about the probability of hypercapnic respiratory failure when oxygen saturation is below 88%.
The GOLD guidelines are widely used in Spain and other countries. However, it must be noted that the GOLD recommendation about the use of gasometry in aeCOPD is based on just one article, which was published in Respiratory Medicine in 2001 (Citation5,Citation7), when the first version of the guideline was published. That article reports results from just 64 sample pairs of oxygen saturation determined by pulse oximetry and pO2 measured by blood gasometry. In 2006, Guryay et al. examined the capability of pulse oximetry to detect hypoxemia and also measured its ability to detect hypercapnia in a sample of 76 patients with exacerbations of COPD (Citation12). They proposed a cut-off point of 88.5% as an indication for hypoxemia, which is clearly lower than cut-off of 90% that we propose. The small sample size of Guryay et al. limits their recommendation. In addition, the sensitivity of their test to detect hypercapnia (73%) was lower than sensitivity we observed (91%).
To our knowledge, no other publications to date have measured the accuracy of pulse oximetry to predict hypoxemia and/or respiratory failure, even though pulse oximetry is widely used to assess the severity of COPD exacerbations and managing diagnosis, oxygen therapy, and ventilatory support (Citation13–16).
Since our study is observational in nature, its results cannot definitively determine that an oxygen saturation below 92% is warranted to order blood gasometry (Citation1). Nevertheless, our findings raise two important points for clinicians:
The relationship between oxygen saturation measured by pulse oxymetry and pO2 measured by arterial blood gasometry is not linear. This could be important to take into account for patients experiencing acute exacerbations of COPD. As oxygen saturation increases, the probability of increasing pO2 increases exponentially .The British Thoracic Society guideline for the use of oxygen therapy in adults at risk of respiratory failure establishes a target oxygen saturation range of 88% to 92% (Citation4). Our results provide easily used formulas to estimate pO2 based on oxygen saturation measured by pulse oximetry.
We encountered different cut-off points for detecting hypoxemia and hypercapnia in our sample. This finding, if confirmed in other studies, could change the decision to delay oxygen therapy until blood gases have been evaluated and to offer individualized doses of oxygen therapy. It is well known that intensive oxygen therapy in patients experiencing COPD exacerbations who are at risk of hypercapnic respiratory can be hazardous, with the use of high-flow oxygen contributing to admission to the intensive care unit (Citation8) and increased length of stay. For that reason we believe that a change in the hypercapnia detection cut-off point to an oxygen saturation determined by pulse oximetry of 88% could delay or decrease the use of oxygen therapy in these patients. As Witting et al. related in 2001 (Citation6), pulse oximetry could detect moderate hypercapnia among patients breathing normal air.
Authors have given different cut off points in order to help decision over these patients. We calculated optimal cut off point for detection of hypercapnic respiratory failure as the point which maximized the sum of sensitivity and specificity. The decision maker, however, can choose the cut-off closest to her/his interest in each specific patient or situation.
The large number of patients and episodes of COPD exacerbations we recruited for this study is an important strength. Sample sizes of similar prior studies were under 100; our sample size was 20-fold higher. In addition, this was a real-life study in which no efforts were made to influence how clinicians treated their patients. This makes the results more generalizable. We also provide easy-to-use formulas for calculating pO2 at the bedside based on oxygen saturation determined by pulse oximetry, which could serve to provide individualized doses of oxygen therapy to patients experiencing COPD exacerbations.
One limitation of our study is the absence of variables related to the probability of respiratory failure, such as acute congestive heart failure, chest wall deformities, carboxyhemoglobin intoxications, etc. Thus we were not able to measure their influence on oxygen saturation. Nevertheless, an intermediate variable of low perfusion (a key reason why pulse oximetry may fail to detect hypoxemia) has been explored in multivariate analysis. We defined this variable depending on blood pressure, cardiac rate and presence of arrhythmia. We also explored whether variables such as baseline severity of COPD, age, gender, and smoking habit significantly affected oxygen saturation cut-offs when stratified analysis was performed. Lee et al. recommended caution when using pulse oximetry as screening tool to detect hypoxemia in patients likely to have carboxyhemoglobin ≥2% (Citation3). Other authors have found no influence of anemia, acidosis, hyperlactatemia, or vasoactive drugs on results of pulse oximetry (Citation17,18). Our findings indicate that pulse oximetry provides a useful way to detect hypoxemia, although future studies must evaluate how well it works in different subgroups of patients and in different situations.
Conclusions
Our results confirm pulse oximetry oxygen saturation cut-off points recommended by the GOLD guidelines for ordering arterial blood gas measurements in patients experiencing acute exacerbations of COPD. Because our study was observational, it should not be used to direct clinical decision making. Yet, the results should serve as the basis for developing future studies in controlled conditions to validate our cut-offs and compare the results obtained by pulse oximetry and capnography. Further work will be required to confirm the oxygen saturation cut-off we recommend for detecting hypercapnia in patients experiencing acute exacerbations of COPD.
Declaration of Interests
This work was supported in part by grants from the Fondo de Investigación Sanitaria (PI 061010, PI061017, PI06714, PI060326, PI060664); Department of Health of the Basque Country; and the thematic networks- Red IRYSS (Investigacion en Resultados y Servicios Sanitarios)- of the Instituto de Salud Carlos III (G03/220), and the European Regional Development Fund (ERDF).
No possible conflicts of interest (e.g., funding sources for consultancies or studies of products) exist in this study. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
We acknowledge editorial assistance of Patrick Skerret. The IRYSS- COPD group included the following co-investigators: Dr. Jesús Martínez-Tapias (Hospital Virgen de las Nieves, Granada); Alba Ruiz (Hospital de Motril, Granada); Dr. Eduardo Briones (Unidad de Epidemiología. Distrito Sanitario Sevilla); Dra. Silvia Vidal (Unidad de Investigación, Hospital Costa del Sol, Marbella); Dr. Emilio Perea-Milla, Francisco Rivas (Servicio de Epidemiología, Hospital Costa del Sol, Málaga – REDISSEC); Dr. Maximino Redondo (Servicio de Laboratorio, Hospital Costa del Sol, Málaga-REDISSEC); Javier Rodríguez Ruiz (Responsable de Enfermería del Área de Urgencias, Hospital Costa del Sol, Málaga); Dra. Marisa Baré (Epidemiología y Evaluación, Corporació Sanitaria Parc Taulí-CSPT, Sabadell REDISSEC), Dr. Manel Lujan, Dra. Concepción Montón (Servicio de Neumología, CSPT/REDISSEC); Dra. Amalia Moreno, Dra. Josune Ormaza, Dr. Javier Pomares (Servicio de Neumología, CSPT); Dr. Juli Font (Medicina, Servicio de Urgencias; CSPT), Dra. Cristina Estirado, Dr. Joaquín Gea (Servicio de Neumología, Hospital del Mar/CIBERES, Barcelona); Dra. Elena Andradas (subdirectora de Promoción de la Salud y Epidemiología del Ministerio de Sanidad, Servicios Sociales e Igualdad), Dr. Juan Antonio Blasco (Unidad de Evaluación de Tecnologías Sanitarias, Agencia Laín Entralgo, Madrid), Dra. Nerea Fernández de Larrea (Subdirección General de Tecnología e Innovación Sanitarias. Consejería de Sanidad de la Comunidad de Madrid/REDISSEC); Rosa Girón (Hospital de La Princesa, Madrid), María del Puerto Cano Aguirre (Hospital de Torrejón, Madrid); Dr. Jose Luis Lobo (Servicio de Neumología, Hospital Txagorritxu, Araba); Dra. Esther Pulido (Servicio de Urgencias, Hospital Galdakao-Usansolo, Bizkaia); Dr. Mikel Sánchez (Servicio de Urgencias, Hospital Galdakao-Usansolo); Dr. Luis Alberto Ruiz (Servicio de Respiratorio, Hospital de Cruces, Bizkaia); Dra. Ane Miren Gastaminza (Hospital San Eloy, Bizkaia); Dra. Eva Tabernero (Servicio de Neumología, Hospital de Santa Marina), Carmen Haro (Servicio de Urgencias, Hospital de Santa Marina, Bizkaia); Dr. Ramon Agüero (Servicio de Neumología, Hospital Marques de Valdecilla, Santander); Dr. Gabriel Gutiérrez (Servicio de Urgencias, Hospital Cruces, Bizkaia); Dra. Belén Elizalde (Dirección Territorial de Gipuzkoa); Dr. Felipe Aizpuru (Unidad de Investigación, Hospital Txagorritxu, Álava/REDISSEC); Dra. Inmaculada Arostegui, Irantzu Barrio (Departamento de Matemática Aplicada, Estadística e Investigación Operativa, UPV/EHU- REDISSEC; Amaia Bilbao (Hospital Universitario Basurto/REDISSEC); Dr. Cristóbal Esteban (Servicio de Neumología, Hospital Galdakao-Usansolo, Bizkaia/REDISSEC); Dra. Nerea González, Susana Garcia, Iratxe Lafuente, Urko Aguirre; Miren Orive, Ane Anton, Dr. Jose M. Quintana (Unidad de Investigación, Hospital Galdakao-Usansolo, Bizkaia/REDISSEC).
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD 2014. Available from: http://www.goldcopd.org./ www goldcopd org/ [2014 Available from: URL:www.goldcopd.org/.
- BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax 1997; 52 Suppl 5:S1–28.
- Lee WW, Mayberry K, Crapo R, Jensen RL. The accuracy of pulse oximetry in the emergency department. Am J Emerg Med 2000; 18(4):427–431.
- O’Driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax 2008; 63 Suppl 6:vi1–68.
- Kelly AM, McAlpine R, Kyle E. How accurate are pulse oximeters in patients with acute exacerbations of chronic obstructive airways disease? Respir Med 2001; 95(5):336–340.
- Witting MD, Lueck CH. The ability of pulse oximetry to screen for hypoxemia and hypercapnia in patients breathing room air. J Emerg Med 2001; 20(4):341–348.
- Witting MD, Hsu S, Granja CA. The sensitivity of room-air pulse oximetry in the detection of hypercapnia. Am J Emerg Med 2005; 23(4):497–500.
- Joosten SA, Koh MS, Bu X, Smallwood D, Irving LB. The effects of oxygen therapy in patients presenting to an emergency department with exacerbation of chronic obstructive pulmonary disease. Med J Aust 2007; 186(5):235–238.
- Quintana JM, Esteban C, Barrio I, Garcia-Gutierrez S, Gonzalez N, Arostegui I et al. The IRYSS-COPD appropriateness study: objectives, methodology, and description of the prospective cohort. BMC Health Serv Res 2011; 11:322.
- Garcia-Gutierrez S, Quintana JM, Aguirre U, Esteban C, Bilbao A, Escobar A et al. Explicit criteria for hospital admission in exacerbations of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2011; 15(5):680–686.
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12:77.
- Guryay MS, Ceylan E, Gunay T, Karaduman S, Bengi F, Parlak I et al. Can spirometry, pulse oximetry and dyspnea scoring reflect respiratory failure in patients with chronic obstructive pulmonary disease exacerbation? Med Princ Pract 2007; 16(5):378–383.
- Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Patel AR, Wedzicha JA. Domiciliary pulse-oximetry at exacerbation of chronic obstructive pulmonary disease: prospective pilot study. BMC Pulm Med 2010; 10:52. doi: 10.1186/1471-2466-10-52.:52-10.
- Mower WR, Sachs C, Nicklin EL, Safa P, Baraff LJ. Effect of routine emergency department triage pulse oximetry screening on medical management. Chest 1995; 108(5):1297–1302.
- Mower WR, Myers G, Nicklin EL, Kearin KT, Baraff LJ, Sachs C. Pulse oximetry as a fifth vital sign in emergency geriatric assessment. Acad Emerg Med 1998; 5(9):858–865.
- Schermer T, Leenders J, in ‘t Veen H van den Bosch W, Wissink A, Smeele I et al. Pulse oximetry in family practice: indications and clinical observations in patients with COPD. Fam Pract 2009; 26(6):524–531.
- Wilson BJ, Cowan HJ, Lord JA, Zuege DJ, Zygun DA. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: a retrospective cohort study. BMC Emerg Med 2010; 10:9. doi: 10.1186/1471-227X-10-9.:9-10.
- Perkins GD, McAuley DF, Giles S, Routledge H, Gao F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care 2003; 7(4):R67.
