ABSTRACT
Evidence suggests that troponin (Tn) elevation during acute exacerbation of chronic obstructive pulmonary disease (AECOPD) may predict an increase in mortality risk. We performed an observational study of 935 patients admitted to hospital for AECOPD from January 2010 to December 2012. Principal clinical and laboratory data were recorded, especially ischemic heart disease (IHD) history, Tn T values and cardiovascular drug prescription. The occurrence of all-cause death, cardiac death (CD), nonfatal myocardial infarction (MI), heart failure and cerebrovascular accident (CVA) was assessed on December 2013. Overall, 694 patients respected inclusion and exclusion criteria. We identified 210 (30%) patients without Tn elevation (negative Tn T group) and 484 (70%) patients with Tn elevation (positive Tn T group). With the exception of CVA, all adverse events were significantly higher in positive Tn T group as compared to negative Tn T group. At multivariable analysis, positive Tn T failed to predict all-cause death. Contrarily, positive Tn T emerged as independent predictors of CD (HR 1.61, 95%CI 1.2–2.2, p = 0.04), nonfatal MI (HR 3.12, 95%CI 1.4–8.1, p = 0.03) and composite endpoint including CD and nonfatal MI (HR 1.73, 95%CI 1.2–2.7, p = 0.03). Of note, positive Tn T stratified prognosis in patients without IHD history, but not in those with IHD history. In conclusion, after hospital admission for AECOPD, we observed a significant increase in the risk of cardiac adverse events in patients with Tn T elevation, especially in those without IHD history.
Introduction
The natural history of chronic obstructive pulmonary disease (COPD) is punctuated by recurrent episodes of exacerbation (Citation1). Ischemic heart disease (IHD) is frequently associated with COPD, and the risk of cardiac adverse events appears to be particularly high after acute exacerbation of COPD (AECOPD) (Citation1–4). Several authors investigated the prognostic role of the most common marker of cardiac injury, the cardiac troponin (Tn), in patients with AECOPD, showing a significant association between Tn elevation and all-cause mortality (Citation5–11). Nevertheless, the relationship between Tn elevation during AECOPD, cardiac adverse events, IHD history and cardiovascular therapy (e.g. antiplatelet agents) remains unclear. The aim of this analysis is to describe the long-term occurrence of cardiac adverse events in patients admitted to hospital for AECOPD and their relationship with IHD history, troponin elevation and cardiovascular treatment.
Materials and Methods
Study population
According to similar studies (Citation8,Citation12), inclusion criteria were: admission to our University Hospital (from January 2010 to December 2012) with a primary diagnosis of AECOPD and prior diagnosis of COPD confirmed by spirometry. Exclusion criteria were: previous diagnosis of sarcoidosis, interstitial lung disease, asthma, bronchiectasis or neuromuscular disease, community-acquired pneumonia, pulmonary embolism, recent myocardial infarction (MI) and/or percutaneous coronary intervention and/or surgical coronary revascularization and/or heart failure (<6 months). Overall, 935 patients were admitted with primary diagnosis of AECOPD (Figure ). Two independent physicians blinded to outcome reviewed all hospital records and source documentation of these patients.
Figure 1. Study flow-chart. COPD: chronic obstructive pulmonary disease. Tn: troponin. MI: myocardial infarction. AECOPD: acute exacerbation of COPD. IHD: ischemic heart disease. CVA: cerebrovascular accident.
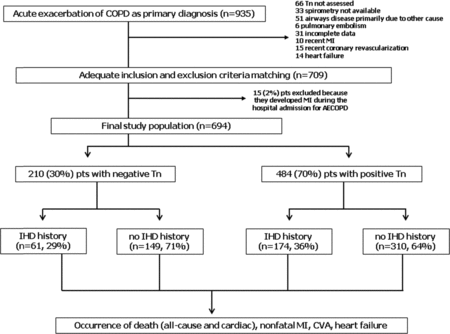
The first reviewer (RP) assessed the appropriateness of inclusion and exclusion criteria, excluding 226 patients (Figure ). Of note, 66 (7%) patients were excluded for the absence of Tn T assessment (Figure ). The second reviewer (SP) evaluated the medical history of IHD (presence vs. absence) and Tn values (below vs. above the upper limit of normality). In 15 (2%) patients symptoms, electrocardiographic signs and Tn values were diagnostic for MI (according the Universal Definition of MI, (Citation13)) concomitant to AECOPD. These patients were excluded (Figure ). This study was conducted in accordance with the amended Declaration of Helsinki. Local institutional review board (Comitato Etico della Provincia di Ferrara) approved the study, and an ordinary written informed consent for data collection was obtained from all patients.
Definition of AECOPD and IHD
According to current guidelines (Citation1), we defined AECOPD as an acute event characterized by a worsening of the patient's respiratory symptoms (acute change of baseline dyspnea, cough, and/or sputum production) that was beyond normal day-today variations and led to hospital admission and a change in medication. The presence of IHD was defined as evidence of documented coronary artery disease. Accordingly, at least one of the following criteria should be respected and documented by reports: previous MI, previous coronary revascularization (percutaneous or surgical), angiographic evidence of at least 50% narrowing of one or more major coronary arteries, positive electrocardiogram, echo, or nuclear stress test suggesting significant myocardial ischemia (Citation14).
Cardiac troponin assessment and positive troponin definition
According to institutional protocol, Tn T assessment in patients with AECOPD was not mandatory, but strongly recommended. We measured high sensitivity cardiac Tn T according to the instructions of the manufacturer (Cobas, Roche Diagnostics, Mannheim, Germany). The upper reference limit (URL) was 14 ng/L. In all patients the first evaluation was performed within 24 hours from hospital admission. A patient was categorized as Tn negative if all available values were below the URL, whereas was considered positive if at least one value was above the URL.
Endpoints of the study and follow-up
The endpoints of the study were all-cause death and cardiac related adverse events. Cardiac death (CD), nonfatal MI, cerebrovascular accident (CVA, ischemic stroke and/or transient ischemic attack) and heart failure (HF) requiring hospitalization were considered cardiac related adverse events. For further analysis, CD and nonfatal MI were considered alone and combined in a composite endpoint. In the composite endpoint, patients with two or more adverse events were censored at the occurrence of first event. To define CD we applied the Academic Research Consortium classification (Citation15). CD is defined as death due to documented cardiac cause, which include but is not limited to deaths resulting from arrhythmias, sudden death (witnessed or unwitnessed), MI, heart failure (HF) or complications of a cardiac procedure (Citation15).
In addition, deaths not clearly attributable to non-cardiac causes were considered CD (Citation15). Specifically, unexpected death even in patients with coexisting and potentially fatal noncardiac disease should be classified as cardiac unless history related to the noncardiac diagnosis suggests death was imminent (Citation15). The endpoints were assessed on December 2013. The median follow-up was 701 days (374-1016). Follow-up was obtained directly and independently from the Programmazione e controllo di gestione, Statistica Sanitaria of our institution (NN, FG) (Citation4,Citation16–19). They retrieved data from hospital discharge records, general practitioner files, medical ward reports, emergency room registrations and mortality registries. As previously reported, this ensures a complete follow-up for 100% of patients (Citation4,Citation16–19).
Finally, this system permits the collection and monitoring of all adverse events (occurring in our and other institutions and at home) (Citation4,Citation16–19). Validity of the data, and its adjudication, was confirmed by manual review of hospital records, mortality registries, source documentation by an independent reviewer blinded to IHD history and Tn elevation (MM). Phone interviews (to patients and/or their relatives) were performed for additional information whenever deemed necessary (MM). The cause of death is reported in the mortality registry (Citation4,Citation16–19). It is established by the physician who ascertains the death. The independent reviewer (MM), after source's analysis, may confirm or not the cause of death. Of note, the 79% of deaths occurred in-hospital. In these cases, all hospital records (emergency room admission, electrocardiogram, laboratory tests, etc) were strictly reviewed to establish the cause of death. Information about medical therapy was obtained via analysis of two computer-based systems (Assistenza Farmaceutica Territoriale and Farmaci a Erogazione Diretta) recording all drug prescriptions to resident patients (NN, FG) (Citation4,Citation18).
Statistical analysis
Continuous data were tested for normal distribution with the Kolmogorov-Smirnov test. Normally distributed values were presented as mean ± SD and were compared by t test and 1-way ANOVA; otherwise median value (interquartile range), the Mann-Whitney U and Kruskal-Wallis tests were used. Categorical variables were summarized in terms of number and percentages and were compared by using two-sided Fisher's exact test. Survival curves were generated by the Kaplan-Meier method, and differences were evaluated using the log-rank test. Patients We applied univariable Cox proportional hazard regression models to evaluate the relation between variables of Table and the incidence of adverse events. Variables with a probability value <0.1 were then entered into a multivariable analysis to identify the independent predictors for adverse events. We performed a Cox regression analysis with interaction testing to determine whether the effect of Tn T elevation (positive vs. negative) on the principal outcome was consistent across patients with IHD history vs. those without IHD history (Citation20). Interaction test was performed with likelihood ratio tests of the null hypothesis that the interaction coefficient was zero (Citation20). All tests were 2- sided and the statistical significance was defined as p < 0.05. All analyses were performed with STATISTICA 8 (Statsoft Inc, Tulsa, Okla, USA).
Table 1. Principal characteristics of study population stratified according to troponin status
Results
The final study population included 694 patients admitted to hospital for AECOPD (Figure ). According to Tn values, we identified 210 (30%) patients without Tn elevation (negative Tn T group) and 484 (70%) patients with Tn elevation (positive Tn T group) (Figure ). Their baseline characteristics are reported in Table .
Troponin T values
Two and three Tn T assessments were available in 597 (86%) and 542 (78%) patients, respectively. In all study population, median values were 40 ng/L (11–54) at the first sample available, 51 ng/L (19–55) at the second, and 50 ng/L (25–55) at the third, respectively. The number of determinations did not influence the classification in positive vs. negative Tn T group (data not shown). Positive Tn T patients showed a flat pattern of Tn T values (Table ). As compared to those with negative Tn T, patients with positive Tn T showed lower hemoglobin levels (12.4 ± 2 vs. 13.2 ± 2 g/dl, p < 0.01, respectively), higher creatinine values (1.3 ± 0.7 vs. 1 ± 0.4 mg/dl, p < 0.01, respectively) and N terminal pro-brain natriuretic peptide (NT-proBNP) values (990 (310–1531) vs. 905 (288–1476) pg/ml, respectively) (Table ). Patients with IHD history, as compared to those without, showed higher Tn T values (1st: 50 (24–54) vs. 30 (10–50) ng/L, p < 0.01; 2nd: 54 (34–56) vs. 35 (12–48) ng/L, p < 0.01; 3rd: 48 (20–55) vs. 32 (15–40) ng/L, p = 0.01).
All-cause mortality
We observed 231 (33%) deaths (Table ). All-cause mortality was significantly higher in positive Tn T group as compared to negative Tn T group (37.3% vs. 24.2%, p = 0.0002) (Table and Figure ). Of all variables listed in Table , age, white blood cells, haemoglobin, creatinine, NT-proBNP, IHD history and positive Tn T were predictors of all-cause mortality at univariate analysis. After multivariable analysis, age (single change unit: HR 1.04, 95%CI 1.01–1.06, p < 0.01), creatinine (single change unit: HR 1.29, 95%CI 1.1–1.6, p < 0.01) and NT-proBNP (single change unit: HR 1.00006, 95%CI 1.00003–1.00008, p < 0.01; above vs. below the median value: HR 1.8, 95%CI 1.4–2.3, p < 0.01) emerged as independent predictors.
Figure 2. Cumulative incidence of adverse events stratified according to Tn T elevation. Solid line: positive Tn T. Dotted line: negative Tn T. A: cumulative incidence of all-cause death. Tn: troponin. B: cumulative incidence of cardiac death and nonfatal MI. Tn: troponin. MI: myocardial infarction.
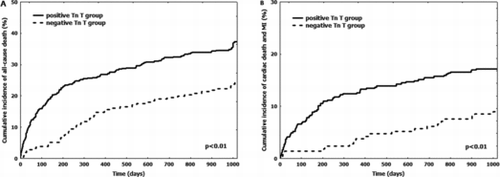
Table 2. Adverse events of study population stratified according troponin status
Cardiac-related adverse events
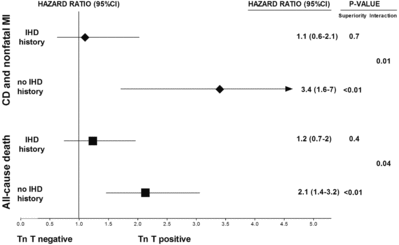
CD occurred in 81 (12%) patients (Table ). It represented the 35% of all deaths. It was significantly higher in positive Tn T group as compared to negative Tn T group (13.7% vs. 7.1%, p = 0.008). Nonfatal MI is observed in 33 (5%) patients (Table ). It was more frequent in positive Tn T group (6% vs. 1.9%, p = 0.01). The cumulative incidence of CD and nonfatal MI was 15% (102 adverse events) (Table ). Its occurrence was significantly higher in positive Tn T group vs. negative Tm T group (17.2% vs. 9%, p = 0.003) (Figure ). Age, haemoglobin, creatinine, NT-proBNP, IHD history, diabetes and positive Tn T were predictors of CD and nonfatal MI at univariate analysis. After multivariable analysis, creatinine (single change unit: HR 1.43, 95%CI 1.1–1.8, p < 0.01), IHD history (HR 1.65, 95%CI 1.1–2.4, p = 0.04) and positive Tn T (HR 1.73, 95%CI 1.2–2.7, p = 0.03) emerged as independent predictors. Of note, positive Tn T emerged also as independent predictor of CD alone (1.61, 95%CI 1.2–2.2, p = 0.04) and nonfatal MI alone (HR 3.12, 95%CI 1.4–8.1, p = 0.03). Finally, hospital admission for HF was more frequent in patients of positive Tn T group, whereas the incidence of CVA was similar between groups (Table ). Of note, positive Tn T emerged as independent predictor of HF after multivariable analysis (HR 2.31, 95%CI 1.5-6.1, p = 0.03).
Interaction between IHD history and troponin elevation on adverse events
Considering patients with IHD history, we observed 68 (39%) all-cause deaths in positive Tn T group vs. 22 (35%) in negative Tn T group (p = 0.4). Similarly, the occurrence of CD and nonfatal MI did not differ (21% in positive Tn T group vs. 19% in negative Tn T group, p = 0.7). Contrarily, in patients without IHD history, we observed an higher occurrence of all-cause mortality in the positive Tn T group (n = 112, 36%) as compared to negative Tn T group (n = 29, 19%) (p = 0.0006). Similarly, the incidence of CD and nonfatal MI was higher in the positive Tn T group (n = 47, 15%) vs. that of negative Tn T group (n = 7, 5%) (p = 0.0005). Cumulative incidence of all-cause death and of cardiac death and nonfatal MI stratified according IHD history and Tn T elevation is shown in Figures and , respectively. Interaction test including Tn T status (positive vs. negative) and IHD history (presence vs. absence) reached the statistical significance (Figure ).
Figure 3. Cumulative incidence of adverse events stratified according to Tn T elevation and IHD history. Solid line: positive Tn T. Dotted line: negative Tn T. Thinner lines: patients with positive IHD history. Larger lines: patients with negative IHD history. A: cumulative incidence of all-cause death. Tn: troponin. IHD: ischemic heart disease. B: cumulative incidence of cardiac death and nonfatal MI. Tn: troponin. IHD: ischemic heart disease. MI: myocardial infarction.
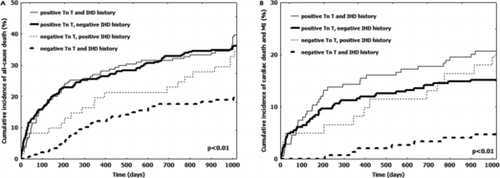
Figure 4. Hazard risk of adverse events according to IHD history and troponin status. Subgroup analysis is shown with hazard ratios and 95% confidence intervals (CI) for adverse events (composite endpoint including cardiac death and nonfatal MI; all-cause death) among subgroups of patients (with IHD history vs. without IHD history) according troponin status (negative vs. positive). The probability value for interaction represents the likelihood of interaction between the variables. IHD: ischemic heart disease.
Cardiovascular therapy
Table reported the prescription of cardiovascular drugs before and after the hospital admission for AECOPD. After discharge, aspirin prescription tended to increase in positive Tn T group (from 55% to 63%, p = 0.09). All-causes death did not differ between patients in aspirin and not in aspirin (32% vs. 36%, p = 0.3). Similar data is observed for CD and nonfatal MI (13% vs.15%, p = 0.5). This is confirmed also analyzing separately positive vs. negative Tn T groups (data not shown). The hospital admission for AECOPD did not change the prescription of other cardiovascular drugs (Table ).
Discussion
Previous studies investigated the prognostic role of cardiac biomarkers in patients with AECOPD with conflicting results (Citation5–11). Some authors agreed in describing a significant increase of the all-cause mortality in patients with AECOPD and Tn elevation (Citation5–9). Contrarily, other prospective studies failed to correlate elevated levels of Tn T with long-term mortality (Citation10,11). The interpretation of these worthy studies and of their conflicting results is limited by different issues such as design, small sample size, short-term follow-up (Citation5–11). Moreover, although Tn T is a marker of myocardial necrosis, the primary outcome was the all-cause mortality and not specific cardiac endpoints (Citation5–11). Finally, at the best of our knowledge, no study investigated the relationship between Tn elevation during AECOPD and MI. As compared to prior studies, we have a bigger sample size (n = 694 patients), a minimally selected study population, and a long-term follow-up (≈2-years, range 1–4 years).
The principal strengths of our work are the collection of cardiac adverse events, their blinded adjudication and the data of cardiovascular therapy (especially antiplatelet agents). As previously described, patients with Tn T elevation showed an higher occurrence of all-cause death (Citation5–9). Nevertheless, positive Tn T did not emerge as independent predictor of all-cause death. This is consistent with previous reports (Citation10,11). Contrarily, we found a strong relationship between Tn T elevation during AECOPD and cardiac adverse events. Positive Tn T emerged as independent predictor of cardiac death alone. Similarly, it predicted nonfatal MI alone. Interestingly, the prognostic role of Tn T elevation is limited to patients without IHD history. IHD history is by itself a marker of heightened cardiovascular risk. In these patients Tn assessment does not help in further stratification of the prognosis.
Oppositely, Tn T elevation during AECOPD in patients without IHD identifies those at higher risk of cardiac adverse events. It is plausible that in these patients was present a silent coronary artery disease. The alterations in inflammation, endothelial function and platelet reactivity associated with AECOPD may contribute to destabilize underlying coronary artery disease (Citation2,3). Tn T elevation during AECOPD could be a marker of this disease activation. This is indirectly confirmed by the higher incidence of cardiac adverse events in the first months after AECOPD. Recently, Harrison et al. reported that thrombocytosis was associated with both 1-year mortality and in-hospital mortality in patients with AECOPD (Citation12). We are not able to confirm this finding.
Several reasons may explain this discrepancy: different risk profile of study population, reduced number of patients with thrombocytosis (5% vs. 11.7%), different clinical endpoints. Interestingly, also in the paper of Harrison et al. cardiovascular hospitalization was not significantly increased in patients with thrombocytosis (Citation12). The authors reported also that aspirin or clopidogrel treatment correlated with a reduction in 1-year mortality, but not with in-hospital mortality, cardiovascular death and hospitalization for cardiac cause (Citation12). Similarly, we described the prescription of antiplatelet agents. Despite the consistent use of antiplatelet agents, the recurrence of cardiac adverse events remains high.
We demonstrated that patients with IHD and COPD have a heightened on-treatment platelet reactivity (Citation21). This finding may help to explain their worst outcome (Citation21). Currently, new strongest antiplatelet agents are available (e.g. prasugrel, ticagrelor). Probably, a strongest platelet inhibition may contribute to reduce cardiac adverse events in COPD patients and future studies are necessary to validate this hypothesis. Simvastatin 40 mg has been evaluated in preventing the exacerbation rates in COPD patients with negative results (Citation22). We are unaware if statins may influence the occurrence of cardiac adverse events after AECOPD. We may not exclude that higher doses or tailored administration in patients with AECOPD and Tn elevation could modulate cardiac adverse events.
Study limitations
First, it is an observational study and therefore prone to the inherent weaknesses of this type of study. In particular, we may not exclude that in some patient the cause of death may be wrong or not adequately addressed. However, this method has been well used in similar studies (Citation4,Citation16–19,Citation23), and independent reviewers controlled all data and adverse events. Second, we did not have information about COPD severity (e.g. GOLD stage). This should be considered a major limitation. Nevertheless, previous studies suggested that, during AECOPD, spirometry values and/or COPD severity did not influence Tn T elevation (Citation24,25). Third, this is a single-center study. Nevertheless, our University Hospital is the hub center for a large Italian population and we therefore feel that the results should be generalizable to other populations. Finally, we could not exclude bias from residual confounding factors that were not considered in our database and/or analysis.
Conclusions
Patients admitted to hospital for AECOPD and concomitant Tn T elevation are at very high risk of cardiac adverse events after discharge. This is particularly observed in patients without prior diagnosis of IHD. Future studies are clearly on demand to clarify how improve their management (e.g. screening for silent IHD) and treatment (e.g. strongest antiplatelet drugs).
Declaration of Interest Section
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) updated 2014.
- Donaldson GC, Hurst JR, Smith CJ, et al. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010; 137:1091–1097.
- Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370:797–799.
- Campo G, Guastaroba P, Marzocchi A, et al. Impact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest. 2013; 144:750–757.
- Baillard C, Boussarsar M, Fosse JP, et al. Cardiac troponin I in patients with severe exacerbation of chronic obstructive pulmonary disease. Intensive Care Med 2003; 29:584–589.
- Fruchter O, Yigla M. Cardiac troponin-I predicts long-term mortality in chronic obstructive pulmonary disease. COPD 2009; 6:155–161.
- Martins CS, Rodrigues MJ, Miranda VP, et al. Prognostic value of cardiac troponin I in patients with COPD acute exacerbation. Neth J Med 2009; 67:341–349.
- Brekke PH, Omland T, Holmedal SH, et al. Troponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbation. Eur Respir J 2008; 31:563–570.
- Hoiseth AD, Neukamm A, Karlsson BD, Omland T, Brekke PH, Soyseth V. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax 2011; 66:775–781.
- Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011; 66:764–748.
- Marcun R, Sustic A, Brguljan PM, Kadivec S, Farkas J, Kosnik M, et al. Cardiac biomarkers predict outcome after hospitalisation for an acute exacerbation of chronic obstructive pulmonary disease. Int J Cardiol 2012; 161:156–159.
- Harrison MT, Short P, Williamson PA, Singanayagam A, Chalmers JD, Schembri S. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: a role for antiplatelet therapy? Thorax 2014; 69:609–15.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012; 33:2551–67.
- EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003; 362:782–788
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007; 115:2344–2351.
- Campo G, Saia F, Guastaroba P, et al. Prognostic impact of hospital readmissions after primary percutaneous coronary intervention. Arch Intern Med 2011; 171:1948–1949.
- Campo G, Napoli N, Serenelli C, Tebaldi M, Ferrari R. Impact of a recent hospitalization on treatment and prognosis of ST-segment elevation myocardial infarction. Int J Cardiol 2013; 167:296–297.
- Saia F, Marino M, Campo G, et al. Incidence and outcome of high on-treatment platelet reactivity in patients with non-ST elevation acute coronary syndromes undergoing percutaneous coronary intervention. Am J Cardiol 2013; 112:792–798.
- Marzocchi A, Saia F, Piovaccari G, et al. Long-term safety and efficacy of drug-eluting stents: two-year results of the REAL (REgistro AngiopLastiche dell’Emilia Romagna) multicenter registry. Circulation 2007; 115:3181–3188.
- Campo G, Tebaldi M, Vranckx P, Biscaglia S, Tumscitz C, Ferrari R, Valgimigli M. Short- versus long-term duration of dual antiplatelet therapy in patients treated for in-stent restenosis: a PRODIGY trial substudy. J Am Coll Cardiol 2014; 63:506–512.
- Campo G, Pavasini R, Pollina A, Tebaldi M, Ferrari R. On-treatment platelet reactivity in patients with chronic obstructive pulmonary disease undergoing percutaneous coronary intervention. Thorax 2014; 69:80–81.
- Criner GJ, Connett JE, Aaron SD, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med 2014; 370:2201–2210.
- Fokkema ML, James SK, Albertsson P, et al. Population trends in percutaneous coronary intervention: 20-year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J Am Coll Cardiol 2013; 61:1222–1230.
- Søyseth V, Bhatnagar R, Holmedahl NH, Neukamm A, Høiseth AD, Hagve TA, Einvik G, Omland T. Acute exacerbation of COPD is associated with fourfold elevation of cardiac troponin T. Heart 2013; 99:122–126.
- Høiseth AD, Omland T, Hagve TA, Brekke PH, Søyseth V. Determinants of high-sensitivity cardiac troponin T during acute exacerbation of chronic obstructive pulmonary disease: a prospective cohort study. BMC Pulm Med 2012; 12:22–29.
