Abstract
Background: Non-invasive positive pressure ventilation (NPPV) in addition to supplemental oxygen improves arterial oxygenation, walking distance and dyspnea when applied during exercise in stable hypercapnic COPD patients. The aim of the current study was to investigate whether NPPV without supplemental oxygen is capable of preventing severe exercise-induced hypoxemia in these patients when applied during walking. Methods and Results: 15 stable hypercapnic COPD patients (FEV1 29.9 ± 15.9%) performed two 6-minute walk tests (6MWT) with a rollator in a randomized cross-over design: using either supplemental oxygen
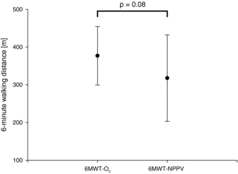
(2.4 ± 0.7 L/min) or NPPV (inspiratory/expiratory positive airway pressure of 28.2 ± 2.8 / 5.5 ± 1.5 mbar) without supplemental oxygen. Results: 10 patients were able to complete both 6MWT. 6MWT with supplemental oxygen resulted in no changes for PO2 (pre: 67.3 ± 11.2 mmHg vs. post: 65.6 ± 12.0 mmHg, p = 0.72) whereas PCO2 increased (pre: 50.9 ± 8.1 mmHg vs. post: 54.3 ± 10.0 mmHg (p < 0.03). During 6MWT with NPPV PO2 significantly decreased from 66.8 ± 7.2 mmHg to 55.5 ± 10.6 mmHg (p < 0.02) whereas no changes occurred in PCO2 (pre: 50.6 ± 7.5 mmHg vs. post: 53.0 ± 7.1 mmHg; p = 0.17). Walking distance tended to be lower in 6MWT with NPPV compared to 6MWT with supplemental oxygen alone (318 ± 160 m vs. 377 ± 108 m; p = 0.08). Conclusion: The use of NPPV during walking without the application of supplemental oxygen does not prevent exercise-induced hypoxemia in patients with stable hypercapnic COPD.
Introduction
Patients with very severe COPD suffer from exercise-induced dyspnea and, as a consequence, from exercise limitation (Citation1). Furthermore, it has been shown that simple daily activities such as walking or stair-climbing are associated with transient oxygen desaturations (Citation2, Citation3). Beside medication there are non-pharmaceutical strategies to improve exercise capacity and oxygenation in these patients. Recently it has been shown that noninvasive positive pressure ventilation (NPPV) applied during walking prevents exercise-induced dyspnea and deoxygenation to a further extent than supplemental oxygen alone (Citation4).
Additionally, patients were able to walk farther when NPPV was applied (Citation4). Although the application of NPPV during walking in COPD patients was investigated in several trials, the underlying mechanisms, especially the fact that exercise induced hypoxemia was prevented in the most recent trials remain unclear (Citation4, Citation5). It has been speculated that NPPV during walking might lead to an improvement in pulmonary hemodynamics and ventilation-perfusion mismatch (Citation4). Additionally, exercise-induced hyperinflation could have been prevented or reduced when NPPV was applied during exercise and thus result in improved oxygenation. This raises the question whether NPPV without supplemental oxygen applied during exercise is capable of preventing severe hypoxemia.
Therefore the aim of the current study is to investigate the effect of NPPV without supplemental oxygen on a 6-minute walk test (6MWT-NPPV) compared to a 6-minute walk test with supplemental oxygen (6MWT-O2). This is suggested to be of clinical importance since carrying a supplemental oxygen device in addition to a ventilator is supposed to reduce exercise capacity due to the additional weight in hypercapnic COPD patients primarily benefitting from ventilator assistance during walking (Citation5).
Materials and Methods
The study received approval from the Institutional Review Board for Human Studies at the University Hospital Freiburg, Germany, and written informed consent was given by each subject before participation. All procedures conformed to the standards set by the Declaration of Helsinki in its latest revision. The trial was registered at the German Clinical Trials Register (Trial registration number: DRKS00005720).
Patients
Fifteen patients with stable hypercapnic COPD (1 female, 14 male) volunteered to participate in the study. All patients reported dyspnoea during mild exertion despite optimal treatment with anti-obstructive and anti-inflammatory medication. Participants were established on long-term oxygen therapy for 24 hours/day according to national guidelines (Citation6). Patients with an acute exacerbation, bronchiectasis, rib cage deformities, neuromuscular disorders and post-tuberculosis sequelae were excluded from the study. All subjects had to avoid stressful physical activity for 24 hours before the measurements.
NPPV
NPPV for home mechanical ventilation was established in all patients at least three months prior to study inclusion. Ventilator settings were chosen to decrease PCO2 levels as close as possible to normocapnic values. Therefore high-intensity NPPV in a pressure assist/control mode was chosen as described previously (Citation7, Citation8). During the study a Vivo 40 (Breas Medical AB, Mölnlycke, Sweden) with a leakage system (Silentflow, Weinmann, Hamburg, Germany) was used for NPPV. Rise time was set short (setting 1 or 2 of the Vivo 40). Patients used their own full-face mask during 6MWT-NPPV.
Study Design
According to international guidelines (Citation9, Citation10, Citation11) pulmonary function was assessed by body-plethysmography (Masterlab-Compact Labor; Jaeger, Hochberg, Germany) with reference values according to (Citation12). A standardized 6-minute walk test (6MWT) was performed according to international guidelines (Citation13). Prior to and immediately after 6MWT, dyspnea assessed by the Borg dyspnea scale (Citation14), blood pressure, heart frequency, arterial saturation assessed by pulse oximetry, vital capacity (VC), inspiratory capacity (IC) and forced expiratory volume in 1 second (FEV1) (ZAN-100â, nSpire health GmbH, Oberthulba, Germany), blood gases (Cobas b221â, Roche, Mannheim, Germany) and blood lactate (Super GLâ, Hitado Diagnostic Systems, Moehnensee, Germany) taken from the arterialized earlobe were registered. All patients were familiarized with 6MWT during former hospital visits. A familiarization with 6MWT and NPPV has not been performed prior to the study.
Patients performed two 6MWT using a rollator (Meyra, Kalletal, Germany) in a randomized cross-over design on two consecutive days: one with supplemental oxygen via a nasal cannula and the other with NPPV without supplemental oxygen. The ventilator and the oxygen tank were placed on the rollator on both study days in order to sustain the same weight level. Ventilator settings as well as flow rate for supplemental oxygen were chosen according to the settings used at home. No changes in these settings were made during the study. Baseline blood gas analyses before 6MWT were performed with supplemental oxygen on both study days.
Statistical analysis
Sigma-Plot 11.2â software (Systat Software, Point Richmond, USA) was used for statistical analysis. Data are presented as mean ± S.D. for normal distribution (Shapiro–Wilk test) and median and interquartile range (IQR) for non-normal distribution. The 95% confidence interval of the mean (C.I.) is given where appropriate. A paired Student's t-test was performed for two-group comparison in normally distributed data. Wilcoxon signed-rank test was used for two-group comparison in non-normally distributed data.
Primary outcome parameter was the difference in partial oxygen pressure (PO2) after 6MWT with supplemental oxygen compared to PO2 after 6MWT with NPPV without supplemental oxygen. Ten subjects were needed following sample size determination (paired Student's t-test; power, 0.8; two-sided type I error, 0.05) with a mean difference in PO2 of at least 10 mmHg and an estimated S.D. of 10 mmHg. These values are based on previous studies (Citation4, Citation5). Due to an expected drop-out rate based on pre-testings and statistical consultation 15 subjects were included in the study.
Results
Fifteen patients with chronic hypercapnic COPD were included in the study. Demographic data, lung function parameters and NPPV settings are illustrated in Table . Patients performed home mechanical ventilation for a mean of 10.6 ± 7.8 months for at least 8 hours every night. Five patients were not able to perform the 6MWT due to dyspnea, mask discomfort or anxiety during walking with NPPV. These patients were excluded from further analysis. Results for both study days are presented in Table . Supplemental oxygen flow rate during 6MWT-O2 was 2.4 ± 0.7 L/min.
Table 1. Demographic data, lung function parameters, NPPV settings (n = 15)
Table 2. Blood gases, cardiac frequency, blood pressure, dyspnea and walking distance before and after the 6-min walking test (6MWT); n = 10
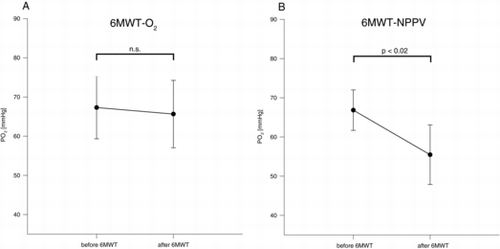
There was no change in PO2 comparing values prior to and after 6MWT-O2 (p = 0.72), whereas PO2 decreased significantly when comparing values prior to and after 6MWT-NPPV (p < 0.02) as illustrated in Figure . PO2 was lower than 55 mmHg in 6 out of 10 patients after 6MWT-NPPV. PCO2 increased comparing values prior to and after 6MWT-O2 (p < 0.03) and did not change in 6MWT-NPPV (p = 0.17). There was no difference in inspiratory capacity (IC) before and immediately after 6MWT in both trials (6MWT-NPPV: 2.0 ± 0.6 L vs. 1.7 ± 0.6 L; p = 0.11; n = 7; 6MWT-O2: 1.8 ± 0.6l vs. 1.7 ± 0.5 L; p = 0.40; n = 7) and no difference in IC when comparing values after 6MWT-NPPV and 6MWT-O2 (p = 0.91). Differences in 6-minute walking distance are illustrated in
Discussion
The study has shown that NPPV without supplemental oxygen applied during walking is neither suitable for preventing exercise-induced hypoxemia nor dyspnea or improving walking distance in patients with stable hypercapnic COPD with hypoxemia. On the contrary, NPPV without supplemental oxygen even resulted in exercise-related deoxygenation and this effect was not found while walking with supplemental oxygen alone.
Recently it has been shown by our group that the application of NPPV in addition to supplemental oxygen markedly improves arterial oxygenation during 6MWT (Citation4), whereas the application of supplemental oxygen alone resulted in significant deoxygenation (Citation4, Citation5). The latter finding is in contrast to the results of the current study where the application of supplemental oxygen alone did not result in a significant deoxygenation. Interestingly PO2 values after 6MWT with supplemental oxygen in the current study are comparable to ones assessed in a former study (65.6 ± 12.0 vs. 61.6 ± 9.9 mmHg) (Citation4), whereas baseline PO2 values were higher (Citation4). However the reason for the differences between the studies regarding a deoxygenation during 6MWT with supplemental oxygen remains unclear.
Additionally, walking distance was improved after 6MWT with NPPV and supplemental oxygen compared to 6MWT with supplemental oxygen alone (Citation4). Another study has shown that increasing supplemental oxygen during walking in this patient group did not improve oxygenation, whereas again only NPPV in addition to supplemental oxygen was capable of preventing exercise-induced hypoxemia (Citation5).
Several mechanisms are discussed to be responsible for the improvement in arterial oxygenation when NPPV and supplemental oxygen are applied during exercise: 1) improvements in ventilation-perfusion mismatch; 2) changes in pulmonary haemodynamics; and, 3) a reduction in hyperinflation achieved by NPPV (Citation4, Citation5, Citation15). In the current study exercise-induced dynamic hyperinflation seems to play only a minor role since changes in inspiratory capacity, an indicator for dynamic hyperinflation (Citation16) did not reach statistical significance after both 6MWT.
However, it is known that the proportion of oxygen consumption of respiratory muscles compared to the oxygen uptake during exercise in COPD patients is four to ten times higher than in healthy subjects during exercise (Citation17, Citation18). By unloading respiratory muscles during exercise, one would expect that more oxygen could be provided for locomotive muscles and hence improve walking distance or arterial oxygenation during exercise in these patients.
In the current study high-intensity NPPV was applied during 6MWT with the aim of reducing PCO2 levels as close as possible to normocapnic values (Citation7) as indicated by high values of inspiratory positive airway pressure and breathing frequency. It has been shown that high-intensity NPPV is capable of decreasing the transdiaphragmatic pressure-time product (PTPdi), an index of diaphragm oxygen expenditure (Citation19). Here, high-intensity NPPV decreased PTPdi by more than 90% compared to spontaneous breathing and 60% of the patients achieved almost complete resting of the diaphragm (Citation19). Given that, potentially more oxygen could be provided to locomotive muscles during exercise when using high-intensity NPPV. This is supported by the fact that despite lower values for PO2 after 6MWT-NPPV blood lactate levels as well as pH were not different compared to 6MWT-O2, indicating that anaerobic metabolism of locomotive muscles was not different in both runs. It has already been proven that in healthy subjects respiratory muscle unloading using a mechanical ventilator is capable of reducing exercise-induced diaphragmatic fatigue and increasing time to exhaustion in constant-load trials (Citation20, Citation21).
However, respiratory muscle unloading in hypercapnic COPD patients without supplemental oxygen is not sufficient to prevent exercise-induced deoxygenation. This is supported by our data showing that although exercise-induced hypercapnia was prevented during walking with NPPV without oxygen, significant deoxygenation was present. PO2 values of several subjects decreased below a critical level of 55mmHg. According to international and national guidelines supplemental oxygen is recommended for PO2 values below 55 mmHg at rest or during exercise (Citation6, Citation22). Therefore the usage of NPPV in the absence of supplemental oxygen during walking cannot be recommended for patients with stable hypercapnic COPD and resting hypoxemia. To achieve positive effects with regard to oxygenation and exercise capacity, the combination of NPPV and supplemental oxygen should be applied during walking in hypercapnic COPD patients as outlined in previous studies (Citation4, Citation5).
Limitations
This study has some limitations that have to be addressed. One point of criticism might be that blood gas analyses prior to 6MWT-NPPV were performed with supplemental oxygen whereas blood gas analyses with NPPV and room air were not performed. Therefore, it is not possible to adequately determine the degree of hypoxaemia that occurred at rest with NPPV alone after the removal of supplemental oxygen and the degree of additional hypoxaemia that occurred during exercise.
Additionally it might be argued that there is a learning effect when performing a 6MWT on two study days. However, all subjects were familiar with the 6MWT and a practice 6MWT was not performed on the same day as testing. No order effect was found which suggests that a learning effect was unlikely.
Another limitation might be the relative high dropout rate (33%). The results may only be generalized to a highly selected population who could tolerate wearing a mask and walking with NPPV.
Conclusion
NPPV applied during walking in hypercapnic COPD patients with hypoxaemia does not prevent exercise-induced deoxygenation when supplemental oxygen is not additionally applied. Therefore, unloading respiratory muscles alone during walking is not capable of preventing exercise-induced oxygen desaturation in patients with stable hypercapnic COPD.
Acknowledgements
The authors thank all subjects for their effort devoted to this study. They are grateful to Franziska Farquharson for the helpful comments on the manuscript.
Funding
David J. Walker received travel funding for national and international research conferences by Vivisol and Sapio Life.
Stephan Walterspacher has received travel grants for attending international and national scientific meetings from Vivisol, Weinmann, Heinen & Löwenstein and Bayer. Research projects have been supported by Philips-Respironics and Weinmann. Speeking fees on topics of respiratory medicine have been granted by Weinmann and Fisher & Pykel.
Emelie Ekkernkamp received travel funding for national and international research conferences by ResMed, Vivisol.
Jan H. Storre received speaking fees from the following companies: Breas Medical AB, Mölnlycke, Sweden; Respironics Inc., Pittsburgh, PA, USA; ResMed Germany Inc., Martinsried, Germany; Heinen und Löwenstein, Germany. Jan H. Storre received also honorarium from Respironics, USA for expertise. JHS received travel funding for national and international research congresses from Breas Medical GmbH Germany, Heinen und Löwenstein, Germany; Respironics International, Vivisol Germany, Weinmann GmbH.
Wolfram Windisch received speaking fees from the following companies: Breas, Sweden; Covedien, France; Linde, Germany; Maquet, Germany; Radiometer, Germany; Respironics, USA; ResMed, Germany; Heinen und Löwenstein, Germany; VitalAire, Germany; Weinmann, Germany.
Michael Dreher received speaking fees from ResMed, Dräger Medical, Weinmann, Breas Medical GmbH, Germany, Heinen und Löwenstein, Linde and Respironics.
The authors alone are responsible for the content and writing of the paper.
References
- Ambrosino N, Strambi S. New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J 2004 Aug 1; 24(2):313–322.
- Dreher M, Walterspacher S, Sonntag F, Prettin S, Kabitz HJ, Windisch W. Exercise in severe COPD: Is walking different from stair-climbing? Respir Med 2008 Jun; 102(6):912–918.
- Schenkel NS, Burdet L, Muralt B, Fitting JW. Oxygen saturation during daily activities in chronic obstructive pulmonary disease. Eur Respir J 1996 Dec 1; 9(12):2584–2589.
- Dreher M, Storre JH, Windisch W. Noninvasive ventilation during walking in patients with severe COPD: a randomised cross-over trial. Eur Respir J 2007 May 1; 29(5):930–936.
- Dreher M, Doncheva E, Schwoerer A, Walterspacher S, Sonntag F, Kabitz HJ et al. Preserving oxygenation during walking in severe chronic obstructive pulmonary disease: noninvasive ventilation versus oxygen therapy. Respiration 2009; 78(2):154–160.
- Magnussen H, Kirsten A-M, Köhler D, Morr H, Sitter H, Worth H. Leitlinien zur Langzeit-Sauerstofftherapie. Pneumologie 2008 Nov 14; 62(12):748–756.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010 Apr 1; 65(4):303–308.
- Dreher M, Ekkernkamp E, Walterspacher S, Walker D, Schmoor C, Storre JH et al. Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest 2011 Oct; 140(4):939–945.
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R et al. General considerations for lung function testing. Eur Respir J 2005 Jul 1; 26(1):153–161.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A et al. Standardisation of spirometry. Eur Respir J 2005 Aug 1; 26(2):319–338.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005 Sep 1; 26(3):511–522.
- Matthys H, Zaiss AW, Theissen JL, Virchow JC, Werner P. Definitionen, Soll- und Meßwerte zur Diagnose obstruktiver, restriktiver sowie gemischter Ventilationsstörungen für die klinische Lungenfunktionsdiagnostik. Atemw-Lungenkrkh 1995; 21:130–138.
- American Thoracic Society. ATS Statement Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med 2002 Jul 1; 166(1):111–117.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14(5):377–381.
- Díaz O, Bégin P, Torrealba B, Jover E, Lisboa C. Effects of noninvasive ventilation on lung hyperinflation in stable hypercapnic COPD. Eur Respir J 2002 Dec 1; 20(6):1490–1498.
- Calverley PMA. Dynamic hyperinflation: is it worth measuring? Proc Am Thorac Soc 2006 May; 3(3):239–244.
- Donahoe M, Rogers RM, Wilson DO, Pennock BE. Oxygen consumption of the respiratory muscles in normal and in malnourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989 Aug; 140(2):385–391.
- Mannix ET, Manfredi F, Palange P, Dowdeswell IR, Farber MO. OXygen may lower the o2 cost of ventilation in chronic obstructive lung disease. Chest 1992 Apr 1; 101(4):910–915.
- Lukácsovits J, Carlucci A, Hill N, Ceriana P, Pisani L, Schreiber A et al. Physiological changes during low- and high-intensity noninvasive ventilation. Eur Respir J 2012 Apr 1; 39(4):869–875.
- Babcock MA, Johnson BD, Pegelow DF, Suman OE, Griffin D, Dempsey JA. Hypoxic effects on exercise-induced diaphragmatic fatigue in normal healthy humans. J Appl Physiol 1995 Jan 1; 78(1):82–92.
- Harms CA, Wetter TJ, Croix CMS, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol 2000 Jul 1; 89(1):131–138.
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T et al. Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011 Aug 2; 155(3):179–191.