Abstract
Background: Whole body vibration training (WBVT) improves muscle force in healthy subjects. Resistance training (RT) is an important component of a pulmonary program. Aim: To investigate the effects of either 12 weeks WBVT or RT, both provided after 15 min of aerobic training as warming up. Methods: COPD patients, referred for pulmonary rehabilitation, were randomized to either a WBVT or a conventional RT group. Primary outcome was the change in 6 Minute Walking Distance (6MWD) after 12 weeks. Maximum exercise capacity (Wmax), quadriceps force (QF), quality of life (QoL) and number of responders, defined as the percentage of patients reaching the minimally clinically important difference (MCID) for the aforementioned outcome measurements were the secondary outcomes. Data are expressed as medians (interquartile range). Results: 62 patients with COPD were included. After WBVT, 6MWD improved by 35 (-14-76) m (p = 0.003), Wmax by 7 (2-23) Watt (p = 0.001), QoL by 13 (4-25) points (p = 0.002) and QF by 9 (-16-29) Nm (NS). In the RT-group, 6MWD, Wmax, QoL and QF increased significantly, with 60 (-13-96) m (p < 0.001), 12 (8-18) Watt (p < 0.001), 11 (3-16) points (p = 0.002) and 12 (-3-44) Nm (p = 0.009), respectively. The MCID for 6MWD (54 m) was reached by 8/26 patients in the WBVT-group and by 16/25 patients in RT-group (p = 0.05). No significant differences between groups were observed for the primary and secondary outcomes. Conclusions: WBVT after 15 min aerobic training enhances 6MWD, Wmax and QoL in COPD patients; however only 30% of patients reached the MCID for 6MWD.
Introduction
Guidelines on treatment of chronic obstructive pulmonary disease (COPD) recommend pulmonary rehabilitation in patients who remain symptomatic, despite an appropriate medical treatment (Citation1). Pulmonary rehabilitation programs generally include endurance and resistance training for a minimum period of 8 weeks, in order to improve exercise capacity, dyspnea, skeletal muscle function and quality of life (QoL) (Citation1, Citation2). Improving skeletal muscle function is essential in COPD patients, since skeletal muscle dysfunction is one of the most frequent factors limiting maximal exercise capacity (Citation3) and has a negative impact on patient survival (Citation4). Resistance training enhances muscle mass and force in patients with COPD, an effect that is often transferred into an improvement in maximal and submaximal exercise capacity (Citation5, Citation6).
Recently, Whole Body Vibration Training (WBVT) has been introduced as an efficient training modality for healthy subjects (Citation7, Citation8). WBVT consists of static and dynamic exercises, performed on a platform that generates vertical sinusoidal vibrations, which can be side-alternating or synchronous, depending on the type of the vibrating platform(9). Small studies in post-menopausal women (Citation10), patients with diabetes (Citation11), multiple sclerosis (Citation12), and stroke (Citation13) have shown that WBVT increases muscle force. Although conclusive evidence is lacking, the most commonly cited mechanism to explain WBVT-induced reflex muscular activity is the tonic vibration reflex (Citation14).
Not all COPD patients respond well to a “conventional” pulmonary rehabilitation program (Citation15). Fear for dyspnea, feelings of exhaustion, muscle soreness and lack of attractiveness can affect patient compliance and the ability to perform exercises (Citation3, Citation16). Interestingly, the effects of WBVT in COPD patients have only been investigated in a few studies. Gloeckl et al. (Citation7) proposed WBVT on top of an existing program and Pleguezelos
et al. (Citation17) compared WBVT with no intervention.
A comparison with a conventional training program, consisting of resistance training (RT), has not been performed so far. If WBVT appeared to be a better-tolerated mode of exercise training than conventional resistance training (RT), it might beneficially impact on compliance, outcome and replace resistance training currently included in most conventional rehabilitation programs (Citation7, Citation16).
The primary aim of this study was to investigate whether WBVT could be proposed as alternative for RT. We therefore specifically wanted to explore the effects of either a 12-week WBVT or conventional RT in COPD patients, both provided after 15 min of aerobic training as warming up. We hypothesized that WBVT would lead to a significant increase in 6MWD in at least 50% of patients. Maximum exercise capacity (Wmax), quadriceps force (QF), quality of life (QoL) and number of responders were secondary outcomes.
Methods
Patient characteristics
We recruited patients with COPD, referred for pulmonary rehabilitation to the Ghent University Hospital. The inclusion criteria combined alterations in pulmonary function with features of disability. Thus, patients were admitted, if either the forced expiratory volume in one second (FEV1) <50% pred. or diffusion capacity (DL,CO) <50% pred. and at least two of the following criteria were fulfilled: a maximal workload (Wmax) <90 Watt, a 6MWD <70% pred., a QoL <100 points on the Chronic Respiratory Disease Questionnaire (CRDQ) or <20 points on the domain dyspnea of the CRDQ, a quadriceps force (QF) <70% pred. or respiratory muscle force <70% pred. (Citation18–Citation20). Patients, who did not comply with those criteria were excluded. Other exclusion criteria were mainly based on currently accepted contraindications for rehabilitation (Citation21) and contraindications for WBVT such as: endoprothesis of hip or knee, joint fusion with metal implants, osteoporosis with vertebral fractures, pacemaker, epilepsy, deep brain and spinal cord stimulation and malignant tumors (Citation22).
Intervention and study design
Patients were randomly allocated by sealed envelopes to either WBVT or conventional resistance training (RT). The outpatient pulmonary rehabilitation program was strictly supervised. Patients trained three times a week for 12 weeks. Oxygen was prescribed to patients who desaturated <88% during exercise testing at intake. Each session in both arms started with 15 min endurance training. Patients allocated to the WBVT-group performed their exercises on a vertical vibration platform, which provided synchronous vibrations (FITVIBE, Gymna, Belgium). The applied frequency was 27 Hz (Citation23) and the peak-to-peak amplitude was 2 mm. Neither the frequency nor the amplitude were changed during the training sessions.
Patients allocated to WBVT received four lower body exercises: a high squat (knee angle between 120° and 130°), a deep squat (knee angle 90°), a wide-stance squat and a lunge. Moreover, the program also contained four upper body exercises: front raise, bent over lateral, biceps curl and cross-over. The initial training volume and intensity were low and were progressively increased in accordance with the overload principle over the 12-week training period, by changing the duration of one vibration session (from 30 seconds to 1 minute), the number of repetitions of different exercises (from 1 to 3) and by shortening the rest periods. After 6 weeks, the static exercises were replaced by dynamic exercises.
Patients allocated to RT trained their quadriceps, hamstrings, deltoid, biceps brachii, triceps brachii and pectoral muscles on multigym equipment. Patients started at 70% of the initial one-repetition maximum (1RM) and performed 3 sets of 10 repetitions in the first week (Citation2). Along the program, the intensity and number of repetitions were progressively increased, in order to maintain a Borg-score between 4 to 6 for dyspnea and fatigue for each type of exercise. After 6 weeks of exercise, a new 1RM force tests were performed and resistance levels for each exercise were adjusted accordingly. All patients attended sessions on occupational therapy, nutrition, disease information and psychosocial items.
Measurements
Pulmonary function, body composition, exercise capacity, QF and QoL were assessed at intake and after 12 weeks of rehabilitation in both groups. FEV1, forced vital capacity (FVC) and DL,CO were measured using the Viasys Sensor medics VMAX, (Spectra, USA) in accordance with the ATS/ERS guidelines (Citation24). Results were expressed in absolute values or as % pred. (Citation25).
Maximal oxygen consumption (VO2max) and minute ventilation were measured breath by breath during an incremental symptom-limited exercise test (Jaeger cycle-ergometer). The ramp protocol used after 12 weeks of rehabilitation was the same as at study inclusion. Wmax and VO2max were compared with normal values (Citation26). The endurance time during cycling at 75% of Wmax (ET 75%) was also determined.
6MWD was measured under oxygen saturation monitoring according to the ATS-guidelines (Citation27). The best out of two attempts was retained and expressed as % pred. (Citation28).
QF was measured using an isometric handheld dynamometer (Microfet; Biometrics, Almere, the Netherlands) attached to a knee extension chair (Gymna, Bilzen, Belgium). Extension peak torque was evaluated at 60° of knee flexion. Patients were asked to perform a 5-second isometric contraction. The best out of three attempts was retained and expressed as % pred. (Citation29).
Health-related quality of life was determined using the validated Dutch translation of the CRDQ (Citation30).
Patient adherence to the program was assessed by computing the number of sessions, dropouts, and complications (Citation18).
The study was approved by the Ethics Committee of Ghent University Hospital (project approval number: 2010/157) and voluntary written informed consent was obtained.
Statistical analysis
The primary endpoint of this trial was the change in 6MWD in the experimental group (WBVT) after 12 weeks of rehabilitation. The power calculation of the present study was based on the results of the COPD patients in the REVALIS database (Citation20). According to that database, 25 patients were required to have a 80% chance to detect a significant difference in 6MWD
(p < 0.05). RT patients were acting as a control-arm with regard to patient characteristics in order to minimize selection bias and monitor the effect of the provided treatment.
Secondary outcomes were changes in QF, VO2max, Wmax, ET 75%, and CRDQ after the interventions, as well as the proportion of patients responding to the intervention. Responders were defined as patients reaching the MCID for 6MWD (≥54 m). We determined that a minimal of 50% of patients in WBVT had to reach the MCID for 6MWD to define that WBVT is a good alternative for RT. In addition, the number of responders as measured by MCID for Wmax (≥10 Watt) (Citation31) and QoL (≥10 points) (Citation32) were calculated. Statistical analyses were performed in the population that completed the intervention. To check whether the dropouts were at random, baseline characteristics of all patients who were initially included were compared with the baseline characteristics of the patients who completed the entire program.
Non-parametric statistics of the Statistical Package for the Social Sciences (SPSS 19.0) were used. The Wilcoxon test was used for intragroup comparison and the Mann–Whitney U test and Fisher's Exact test for intergroup comparison. A level of significance p < 0.05 was used throughout the data analysis. All values were expressed in median with interquartile range.
Results
Patient and treatment characteristics
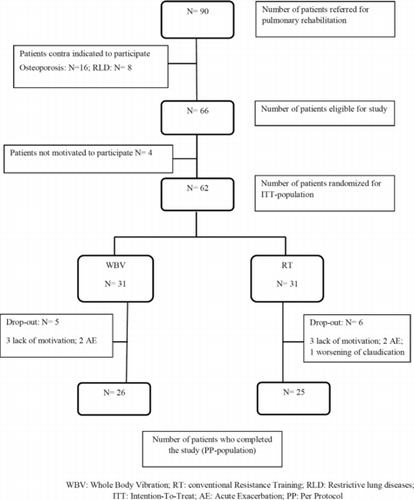
Ninety patients were included between April 2010 and November 2011 (Figure ) and final data collection was obtained in July 2012. Twenty-eight patients were excluded because of osteoporosis and/or a respiratory disorder other than COPD. Sixty-two COPD patients were randomly assigned to either WBVT or RT. Baseline characteristics were well balanced between both groups, except for a significantly higher BMI in the RT group (Table ). Twenty-six patients in the WBVT group and 25 patients in RT group completed the 12-week rehabilitation program (Table ). The baseline characteristics of the patients who completed the study (N = 51) were not significantly different from the dropouts (N = 11) (data not shown).
Table 1. Patient characteristics
Table 2. Effects of 12-week WBVT versus RT training in COPD patients
An acute COPD exacerbation, requiring treatment with antibiotics and/or systemic corticosteroids, occurred in three patients of the RT group and in six patients of the WBVT group of whom two needed hospitalization.
Patients who completed the entire program attended 24 (Citation10–Citation36) sessions in the WBVT group (N = 26) and 29 (Citation3–Citation38) sessions in the RT group (N = 25) (p = 0.287). Eleven patients were not evaluated after 12 weeks, because of lack of motivation (three patients in each group), an acute exacerbation (two patients in each group) and worsening of claudication in one patient of the RT group. Neither group reported injuries and soreness during or immediately after the interventions.
Patient-related outcomes
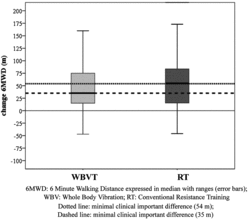
After 12 weeks 6MWD improved significantly with 35 (14–76) m (p = 0.003) in the WBVT group and with 60 (13–96) m (p < 0.001) in the RT group (Figure ). Wmax, ET 75% and CRDQ also improved significantly in both groups (p < 0.05) (Table ). QF improved by 9 (-16–29) Nm in the WBVT (p = 0.23) and by 12 (−3–44) Nm in the RT group (p = 0.009) (Table ). No significant differences in primary and secondary outcomes were observed between both groups. Changes in 6MWD tended to correlate with the changes in QF (R: 0.67,
p = 0.07).
Responders versus non-responders
The MCID for 6MWD was only reached in one third of the patients in the WBVT group versus two thirds in the RT group (p = 0.05) (Table ). Seven (27%) patients in WBVT and 5 (20%) patients in RT group met only the MCID criteria for Wmax and 11 (44%) patients in WBVT and 4 (15%) patients in RT met only the MCID for QoL. No significant differences were seen between both groups in number of patients reaching the MCID for Wmax and QoL.
Table 3. Responders to 12-weeks WBVT and RT training defined by MCID
Discussion
The present study shows that the effects on maximal and submaximal exercise capacity, muscle force and QoL obtained after 12 weeks in the conventional RT group were similar to the effects previously reported in the literature (Citation3, Citation33). The mean increase of 60 m even exceeded the 48 m reported in the Cochrane meta-analysis of Lacasse et al. (Citation33) and 60% of patients reached the MCID of 54 m proposed by Redelmeier et al. (Citation34).
The main finding of this controlled study is that the median increase in 6MWD after WBVT was 35 m and only 30% of patients reached the MCID for the 6MWD. Moreover, the quadriceps force did not significantly increase in the WBVT group. This further questions whether WBVT provided an adequate training stimulus to the peripheral muscles. Likewise, the study of Pleguezuelos et al. (Citation17) found no significant improvement of the quadriceps force after a 6-week outpatient WBVT program in COPD patients.
It also appears unlikely that the lower BMI in the WBVT-group may have interfered with the results of the primary outcome. Indeed, the outcome BMI did not significantly influence the change in 6MWD after 12 weeks of rehabilitation in the present study. Incidentally, baseline BMI does not allow to discriminate between patients who do and do not respond to a pulmonary rehabilitation program (Citation15, Citation35).
This finding not only suggests that differences between COPD patients and healthy and other patient populations may exist (Citation36–Citation38), but highlights the contribution of resistance training to the improvement of the 6MWD in COPD patients following a pulmonary rehabilitation program. Indeed, it remains unclear to which extent resistance training provided on top of endurance training really contributes to the clinical outcome of patients with COPD, admitted to a pulmonary rehabilitation program (Citation3, Citation5). Some studies even indicate that improvements in arm and/or leg muscle force, induced by resistance training, are not always translated into increases in walking distance, cycle endurance, or activity of daily life (Citation5). Our observations underline the importance of resistance training, since substantial gains in functional exercise capacity were only seen if improvements in muscle force were generated.
If our findings suggest that the relatively modest increases in 6MWD in the WBVT group might have been induced by an insufficient training stimulus provided to the peripheral muscles, this issue appears to be of lesser relevance for other outcome measurements such as cycling endurance or QoL. Possibly, these outcome measurements are more dependent on aerobic training alone or on respiratory variables, and less affected by peripheral muscle force. Interestingly, the present observations are in line with data indicating that QF and PImax are the most important critical determinants of the 6MWD (Citation39). Furthermore, WBVT is possibly not specific enough to increase walking distance. To reach a significant improvement in 6MWD, both muscle force and muscle power are required, something that is not obtained by performing static exercises on WBVT. Whether longer sets of dynamic training on WBVT in combination with conventional resistance training yield better results, remain to be investigated.
Our data strikingly differ with the two other published studies using WBVT in COPD patient. In one study 6MWD increased by 60 m (Citation7) after 3 weeks’ inpatient training and in the other study 6MWD improved with 80 m (Citation17) after 6 weeks’ outpatient rehabilitation. Important differences appear to exist between these studies and the present trial in terms of patient characteristics, study design and training modality. The 6MWD of the patients included in the Gloeckl et al. (Citation7) study averaged 340 m at intake compared to 420 m in our outpatient study, leaving less room for improvement in the present study. Moreover, they provided WBVT on top of an existing rehabilitation program, whereas in the present study WBVT was proposed as an alternative for RT during 12 weeks to an outpatient population.
The second study included COPD patients comparable with our patients in terms of patient characteristics, but they did not compare the WBVT-effect with an active control group. Moreover, the trials used other vibrating parameters, other vibrating platforms and different exercise protocols, regarding type, number and exercise duration. In both studies, only the quadriceps muscle was trained by WBVT for ten min per session, whereas in the present trial eight muscle groups were trained, with a maximum of three min per muscle-group per session.
Although our findings suggest that the lack of training effect on peripheral muscle force after WBVT might be in part responsible for the lower increase in 6MWD observed in that group, another factor might also have interfered with the study outcome. Indeed, some patients included in the WBVT group were hospitalized because of an acute exacerbation. Hospitalization of COPD patients acutely reduces their skeletal muscle force and results in losses of functional capacity (Citation40, Citation41), which may last for up to 3 months (Citation42).
Vibration training appeared to be well tolerated in COPD patients. The dropout rate was comparable with dropouts reported in studies using WBVT as training tool in other patient populations and in a geriatric population (Citation38, Citation43, Citation44). The number of attended sessions and adverse events were comparable to previous studies in COPD patients (Citation18, Citation19).
It could be argued that the criteria used to distinguish responders from non-responders were too stringent. Indeed, the median increase of 35 m in WBVT was similar to what has been reported in most studies containing an average of 28 rehabilitation sessions (Citation3), which has also been proposed as MCID by Puhan et al. (Citation45) and this MCID has been reached in two thirds of patients belonging to the RT and the WBVT-group.
Evaluation of the success of an intervention should also be based on other patient related outcomes as QoL: more than 40% of our patients reached the MCID for QoL without reaching the MCID for walking. Similar results were demonstrated in a study of Troosters et al.
who showed that patients who exhibited only a significant response in terms of QoL tended to have lower peripheral muscle force (Citation15). Anyway, if our study does not allow to estimate the exact contribution of WBVT or RT exercises on 6MWD, it appears that the effect of WBVT on the preceding 15 min of aerobic training was additive, whereas the effect of RT was synergetic. Possibly, WBVT did not induce a sufficient amount of fatigue of the quadriceps muscle (Citation40) or elicited too much fatigue of the quadriceps muscle, the latter resulting in a limitation of exercise tolerance (Citation46). Studies on the modulation of the training intensity by altering frequency, session duration and weight bearing could solve this issue.
Conclusion
The present study demonstrates that addition or replacement of one training modality or tool by another one should always be critically reassessed in terms of potential repercussions on clinically relevant outcome measurements, such as 6MWD or QoL. Integrating WBVT within a 12-week multidisciplinary pulmonary rehabilitation program, preceded by 15-minute aerobic training, improves 6MWD, maximal exercise capacity and QoL. However, a trend was seen that a lower number of patients reached the MCID of 54 m after WBVT compared to patients after RT. Further exploration of the use of WBVT in terms of training modalities and patient selection is required before the exact place of this device in a pulmonary rehabilitation program can be determined.
Acknowledgment
The authors thank the members of pulmonary rehabilitation: Wendy Van Loo, Sylvia Wittevrongel, Evelien De Burck, Stefanie Vermeersch, Sven Verschraegen and Philippe De Gryze for their involvement in the present study. This study was registered at www. ClinicalTrials.gov as NCT01135966.
Declaration of Interest Statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. The study was investigator-initiated and sponsored by institutional and departmental funding (Clinical Research Fund of Ghent University Hospital). We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Ms. B. Salhi contributed to the design, accrual and supervision of patients, achievement, collecting data, statistical analysis, interpretation of the data, drafting of the manuscript, revising it for important intellectual content and approving the final manuscript. Dr. Malfait contributed to the accrual and supervision of patients, revising the manuscript for important intellectual content and approving the final version. Prof. Dr. G. Van Maele contributed to the statistical analysis and approving the final version. Prof. Dr. G. Joos contributed to revising the manuscript for important intellectual content and approving the final version.
Prof. Dr. J. P. van Meerbeeck contributed to the interpretation of the data, drafting of the manuscript, revising it for important intellectual content and approving the final manuscript. Prof. Dr. E. Derom contributed to the concept and design of the study, accrual and supervision of patients, interpretation of the data, drafting of the manuscript and revising it for important intellectual content and approving the final manuscript.
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. All authors have read and approved of the manuscript, and none of the authors has any conflict of interest. Bihiyga Salhi is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
References
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, Garvey C, Goldstein R, Gosselink R, Lareau S, MacIntyre N, Maltais F, Morgan M, O'Donnell D, Prefault C, Reardon J, Rochester C, Schols A, Singh S, Troosters T. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173(12):1390–413.
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, Pitta F, Sewell L, Raskin J, Bourbeau J, Crouch R, Franssen FM, Casaburi R, Vercoulen JH, Vogiatzis I, Gosselink R, Clini EM, Effing TW, Maltais F, van der Palen J, Troosters T, Janssen DJ, Collins E, Garcia-Aymerich J, Brooks D, Fahy BF, Puhan MA, Hoogendoorn M, Garrod R, Schols AM, Carlin B, Benzo R, Meek P, Morgan M, Rutten-van Mölken MP, Ries AL, Make B, Goldstein RS, Dowson CA, Brozek JL, Donner CF, Wouters EF. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Amer J Respir Crit Care Med 2013; 188(8):E13–E64.
- Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172(1):19–38.
- Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J 1997; 10(2):417–23.
- O'Shea SD, Taylor NF, Paratz JD. Progressive Resistance Exercise Improves Muscle Strength and May Improve Elements of Performance of Daily Activities for People With COPD A Systematic Review. Chest 2009; 136(5):1269–83.
- Gosselink R, Troosters T, Decramer M. Exercise training in COPD patients: The basic questions. Eur Respir J 1997; 10(12):2884–91.
- Gloeckl R, Heinzelmann I, Baeuerle S, Damm E, Schwedhelm AL, Diril M, Buhrow D, Jerrentrup A, Kenn K. Effects of whole body vibration in patients with chronic obstructive pulmonary disease - A randomized controlled trial. Respir Med 2012; 106(1):75–83.
- Roelants M, Delecluse C, Goris M, Verschueren S. Effects of 24 weeks of whole body vibration training on body composition and muscle strength in untrained females. Int J Sports Med 2004; 25(1):1–5.
- Rauch F, Sievanen H, Boonen S, Cardinale M, Degens H, Felsenberg D, Roth J, Schoenau E, Verschueren S, Rittweger J. Reporting whole-body vibration intervention studies: Recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskel Neuron Inter 2010; 10(3):193–198.
- Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res 2004; 19(3):352–9.
- Olyaei G, Yoosefinejad AK, Shadmehr A, Mohajeri-Tehrani MR, Talebian S, Bagheri H. Effects of whole body vibration on balance and muscle strength in patients with diabetes type-2 associated with peripheral neuropathy. Euro J Neurol 2012; 19:586.
- Claerbout M, Gebara B, Ilsbroukx S, Verschueren S, Peers K, Van Asch P, Feys P. Effects of 3 weeks’ whole body vibration training on muscle strength and functional mobility in hospitalized persons with multiple sclerosis. Multip Scler J 2012; 18(4):498–505.
- Pang MYC, Lau RWK, Yip SP. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: A randomized controlled trial. Euro J Phys Rehabil Med 2013; 49(4):439–50.
- Burke D, Schiller HH. Discharge pattern of single motor units in tonic vibration reflex of human triceps surae. J Neurol Neurosurg Psych 1976; 39(8):729–41.
- Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from nonresponders. J Cardiopulm Rehabil 2001; 21(1):10–17.
- Furness T, Bate N, Welsh L, Naughton G, Lorenzen C. Efficacy of a whole-body vibration intervention to effect exercise tolerance and functional performance of the lower limbs of people with chronic obstructive pulmonary disease. Bmc Pulmon Med 2012; 12.
- Pleguezuelos E, Pérez ME, Guirao L, Samitier B, Costea M, Ortega P, González MV, Del Carmen VA, Ovejero L, Moreno E, Miravitlles M. Effects of whole body vibration training in patients with severe chronic obstructive pulmonary disease. Respirology 2013; 18(6):1028–34.
- Salhi B., Troosters T, Derom E, Behaegel M., Decramer M, Joos G. Effects of pulmonary rehabilitation in patients with restrictive lung diseases. Chest 2010; 137(2):273–279.
- Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med 2000; 109(3):207–12.
- Troosters T GRDE, Van Hove P BP, Pirnay F, Debruycker F, Smeets F, Neyrinck L DM. Efficacy of pulmonary rehabilitation in a clinical setting. Am J Respir Crit Care Med 2002; 165 A:735.
- Bolton CE, Bevan-Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, Greening NJ, Heslop K, Hull JH, Man WD, Morgan MD, Proud D, Roberts CM, Sewell L, Singh SJ, Walker PP, Walmsley S. British Thoracic Society Guideline on pulmonary rehabilitation in adults. Thorax 2013; 68: ii1–ii30.
- Merriman H, Jackson K. The effects of whole-body vibration training in aging adults: a systematic review. J Geriatr Phys Ther 2009; 32(3):134–45.
- Van Erck A, Vanden Bossche L, Witvrouw E, Van der Kelen V, Wojtowicz I, Adriaenssen J, De Camps T, Van Mieghem S, De Muynck M, Rimbaut S, Parlevliet T, Vanderstraeten G. Effect of whole body vibration on intracompartmental pressure in the lower leg. J of Orthop Science 2009; 14(5):618–22.
- Miller MR, Hankinson J, Brusasco V, Burgo F, Casaburi R, Coates A et al. [Standardisation of spirometry]. Rev Mal Respir 2007; 24(3 Pt 2):2S27–49.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16:5–40.
- Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 1985; 131(5):700–8.
- Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am J Respir Crit Care Med 2003; 167(9):1287.
- Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J 1999; 14(2):270–4.
- Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med 1994; 150(1):11–6.
- Gosselink HAAM, Wagenaar RC, Keimpema VA. The effects of a rehabilitation program in patients with COPD. Ned tijdschr Fysioth 1990; 100:193–9.
- Ries AL, Make BJ, Lee SM, Krasna MJ, Bartels M, Crouch R, Fishman AP. The effects of pulmonary rehabilitation in the national emphysema treatment trial. Chest 2005; 128(6):3799–809.
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987; 42(10):773–8.
- Lacasse Y, Martin S, Lasserson TJ, Goldstein RS. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A Cochrane systematic review. Eura Medicophys 2007; 43(4):475–85.
- Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997; 155(4):1278–82.
- Berton DC, Silveira L, Da Costa CC, De Souza RM, Winter CD, Teixeira PJZ. Effectiveness of pulmonary rehabilitation in exercise capacity and quality of life in chronic obstructive pulmonary disease patients with and without global fat-free mass depletion. Arch Phys Med Rehabil 2013; 94(8):1607–14.
- Raimundo AM, Gusi N, Tomas-Carus P. Fitness efficacy of vibratory exercise compared to walking in postmenopausal women. European Journal of Applied Physiology 2009; 106(5):741–8.
- Delecluse C, Roelants M, Diels R, Koninckx E, Verschueren S. Effects of whole body vibration training on muscle strength and sprint performance in sprint-trained athletes. Int J Sports Med 2005; 26(8):662–8.
- Madou KH, Cronin JB. The Effect of Whole body vibration on physical and physiological capability in special populations. Hong Kong Physiotherapy 2008; 26:24–38.
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996; 153(3):976–80.
- Burtin C, Saey D, Saglam M, Langer D, Gosselink R, Janssens W, Decramer M, Maltais F, Troosters T. Effectiveness of exercise training in patients with COPD: the role of muscle fatigue. Eur Respir J 2011; 146(2):318–27.
- Troosters T, Probst VS, Crul T, Pitta F, Gayan-Ramirez G, Decramer M, Gosselink R. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181(10):1072–7.
- Spruit MA, Gosselink R, Troosters T, Kasran A, Gayan-Ramirez G, Bogaerts P, Bouillon R, Decramer M. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003; 58(9):752–6.
- Roth J, Wust M, Rawer R, Schnabel D, Armbrecht G, Beller G et al. Whole body vibration in cystic fibrosis–a pilot study. J Musculoskelet Neuronal Interact 2008; 8(2):179–87.
- Rietschel E, van Koningsbruggen S, Fricke O, Semler O, Schoenau E. Whole body vibration: a new therapeutic approach to improve muscle function in cystic fibrosis? Inter J Rehabil Res 2008; 31(3):253–6.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J 2008; 32(3):637–43.
- Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J et al. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol 2009; 107(3):832–40.