Abstract
In COPD patients a reduced daily activity has been well documented, resulting from both respiratory and non-respiratory manifestations of the disease. An evaluation by multisensory armband has confirmed that daily physical activity is mainly associated with dynamic hyperinflation, regardless of COPD severity. This aspect is crucial, since exercise capacity is closely correlated to life expectancy. Notwithstanding the causal key role of lung impairment in the patient's symptoms, some authors have suggested that other factors, such as systemic inflammation and co-morbidities, have an important role, particularly as mortality risk factors. Many studies suggest the efficacy of bronchodilators and rehabilitation in improving exercise capacity, and, speaking in terms of daily life, in increasing the number of days in which patients are able to perform their usual activities. On this evidence, the first aim in the management of COPD should be to improve exercise capacity and daily activity since these outcomes have direct effects on patients’ quality of life, co-morbidities (heart and metabolic diseases), and prognosis. Thus, improving physical activity represents a modern approach aimed at dealing with both pulmonary and systemic manifestations of the disease. It is however worth of notice to remember that in patients affected by COPD the relationship between the improvement of “potential” exercise capacity and daily physical activity has been found to be only moderate to weak. Obtaining a significant behavior modification with regard to daily physical activity, together with the optimization of therapy thus represents currently the true challenge.
Introduction
Chronic obstructive pulmonary disease (COPD) is now the fourth-leading cause of death worldwide, and it will become the third-leading cause of death by 2020 (Citation1). The hallmark feature of COPD is a reduction of expiratory flow, resulting from chronic bronchiolitis and emphysema; on this basis, the gold standard for diagnosis is spirometry (Citation2,Citation3), and bronchodilators are the first-line treatment for all patients, with inhaled steroids limited to more severe patients prone to frequent exacerbations (Citation3,Citation4). In COPD patients exercise limitation is mainly due to dynamic hyperinflation (Citation5), even if the contribution of other factors, such as an imbalance between respiratory and locomotor muscles for limited energy supply (Citation6), limb muscle dysfunction (Citation7), and co-morbidities (e.g., left ventricle diastolic dysfunction) (Citation8), can be clinically significant.
Notwithstanding the causal key role of lung impairment in the patient's symptoms, some authors have suggested that other factors, such as systemic inflammation (Citation9) and co-morbidities (Citation10), play roles of not negligible importance, particularly as mortality risk factors. The same authors pointed out that pulmonary functional impairment is not reversible and, therefore, relatively less relevant if compared with co-morbidities, which are directly linked to mortality, and can be treated (Citation9). This review is focused on the role of reduced exercise capacity and daily activity in COPD, both in the lung-related and systemic aspects of the disease, and on the clinical importance of their improvement.
Two different ways to consider COPD
The “lung-centered” view
As previously stated, the hallmark feature of COPD is airflow limitation, resulting from chronic bronchiolitis and emphysema (Citation2). The role of dynamic hyperinflation in the development of exercise limitation has been well documented, also in patients with mild obstruction (Citation11). Even if shortness of breath is a complex symptom (Citation12), several studies have confirmed that dyspnea severity is correlated with indexes of mechanical restriction such as dynamic hyperinflation and an increased ratio between tidal volume and inspiratory capacity (Citation13–Citation15), with an expected reduction of dyspnea after bronchodilation and lung volumes reduction (Citation16). This is why the GOLD document, such as many national guidelines, suggests bronchodilators as the first line treatment for all patients (Citation3,Citation4). These drugs have proven to provide long-term improvements in lung function, quality of life, exacerbations, reduction of lung hyperinflation and dyspnea (Citation17–23). Moreover, tiotropium, the long-acting bronchodilator with the highest number of studied patients in randomized clinical trials, demonstrated to significantly reduce mortality in the UPLIFT study (Citation24), even if not in the intention to treat analysis carried out 30 days after the end of the trial.
A reduced lung function has been demonstrated to significantly affect survival not only in COPD patients, but also in case of heart failure, both in out- (Citation25) and in-patient settings (Citation26). Moreover, the BODE index, a more comprehensive approach to better establish the prognosis of COPD patients, includes at least two parameters other than FEV1, linked to lung impairment (shortness of breath, and reduced exercise capacity) (Citation27).
In the “lung-centered view”, the extra-pulmonary conditions frequently detected in COPD patients are considered as: (Citation1) the direct complications of the lung impairment (e.g., pulmonary hypertension); (Citation2) the indirect effect of lung impairment (e.g., osteoporosis due to reduced daily activity), (Citation3) the clinical outcome of the exposure to the same risk factors (e.g., coronary heart disease and smoking), (Citation4) the side effects of the pharmacotherapy used in the treatment of COPD (e.g., steroids and osteoporosis, or muscle wasting) or, finally, (Citation5) the coincidental presence of another disease associated with the age of the patients.
The systemic inflammation and “co-morbidome” view
Even if the diagnosis and assessment of severity of COPD are based on the degree of airflow limitation at spirometry, increasing scientific evidence suggests that symptoms, prognosis, and airflow limitation are poorly correlated, and a comprehensive approach to the disease requires the evaluation of exercise tolerance and body-mass index other than spirometry (Citation27). Moreover, COPD is undoubtedly a disease that encompasses an abnormal inflammatory reaction in the lung, but accumulating evidence shows that it is also associated with systemic inflammation (Citation28,Citation29). It has been proved that systemic inflammation is increased during exacerbations of the disease (Citation30), even if not in all patients, also in case of severe obstruction (Citation31). This is the background that induced Leonardo Fabbri and Klaus Rabe to state in 2007 that “COPD can no longer be judged a disease only of the lungs”, with the proposal to add the term “chronic systemic inflammation syndrome” to the diagnosis of COPD (Citation32). This viewpoint stimulated the publication of hundreds of papers on COPD and systemic inflammation.
The consequences potentially related to systemic inflammation include cardiovascular disease, obesity, hypertension, type 2 diabetes, depression, deconditioning, malnutrition, atrophy and dysfunction of skeletal muscles, osteoporosis, anemia and bone marrow dysfunction (Citation33–Citation40), with some of these clinical conditions affecting the course of the disease (Citation10,Citation41). This new perspective points out the issue of the treatment of some frequent and clinically relevant co-morbidities (e.g., ischemic heart disease, chronic heart failure, hypertension, diabetes, osteoporosis, and depression). In 2012, Divo et al, who evaluated the role of co-morbidities on mortality, proposed the “co-morbidome,” a graphic expression of co-morbidities with a prevalence >10% in COPD. The 12 co-morbidities with the strongest association with an increased mortality resulted to be: cancer (lung, pancreatic, esophageal, and breast cancer), pulmonary fibrosis, atrial fibrillation/flutter, congestive heart failure, coronary artery disease, gastric or duodenal ulcers, liver cirrhosis, diabetes with neuropathy, and anxiety. Fabbri et al proposed a link between systemic inflammation, current smoking, obesity and co-morbidities, with the possibility that systemic inflammation itself may have an extra-pulmonary origin (e.g., abdominal fat in obese patients) (Citation9).
How to improve exercise capacity and daily activity in COPD
Approximately 60 years ago, the foundational works of Jeremy Morris and colleagues showed that the incidence of coronary heart disease in bus conductors who climbed up and down stairs of double-deck buses collecting tickets and in postal carriers who delivered the mail on foot was lower than that of the relatively inactive bus drivers or postal office workers who spent most of their occupational time sitting (Citation42,Citation43). Since then, numerous other investigators have confirmed the strong link between physical activity and health in a variety of populations. The American Heart Association includes physical inactivity as a major risk factor for coronary artery disease since 1992 (Citation44,Citation45). A recent report characterized the impact of physical inactivity as similar to that of smoking in relation to the burden of uncommunicable diseases worldwide (Citation46).
Exercise capacity is closely correlated to life expectancy, not only in patients with respiratory, cardiac of metabolic diseases, but even in healthy subjects (Citation47,Citation48). In COPD patients a reduced daily activity has been well documented, resulting from both respiratory and non-respiratory manifestations of the disease (Citation49,Citation50).
In COPD patients the level of physical activity is decreased after hospitalization, particularly when hospitalization is due to a COPD-related respiratory reason (Citation51,Citation52). Moreover, the level of physical activity itself is associated with the risk of acute exacerbation of COPD, hospitalization, and re-hospitalization (Citation53–Citation57). Finally, it has been demonstrated that reduced physical activity, together with respiratory failure and increased systemic inflammation, are factors associated with a higher healthcare resource utilization (Citation58).
The relationship between a reduced daily activity and COPD is probably complex. However, as previously discussed, exercise limitation is mainly due to dynamic hyperinflation (Citation5). By the use of multisensory armband it has recently confirmed that daily physical activity of patients with COPD is mainly associated with dynamic hyperinflation, regardless of COPD severity (Citation59): even in case of mild functional impairment (i.e., FEV1>80% of predicted values), dynamic hyperinflation can be detected.
The evaluation of exercise capacity in COPD patients is quite complex; several tools are available, from the relatively simple 6-minute walking test, to cardio-pulmonary exercise testing; the heterogeneity of the tests used to asses exercise capacity may explain the conflicting results described in the scientific literature (Citation60). Many studies, however, suggest the efficacy of bronchodilators in improving exercise capacity evaluated by constant load cardio-pulmonary exercise testing (Citation17,Citation21–Citation23,Citation61), the test characterized by the highest sensitivity (Citation23). This benefit can justify the increased number of days in which patients are able to perform usual activities after bronchodilation with one or two drugs, when compared with placebo (Citation62,Citation63).
However, as previously stated, reduced exercise capacity is not merely due to a respiratory impairment, since also non-respiratory manifestations of the disease significantly affect this attitude. In this context, exercise training, one of the key factors of pulmonary rehabilitation, has been proven to be beneficial in COPD patients in terms of exercise tolerance and quality of life by improving peripheral muscle function (Citation64–Citation67). Furthermore, pulmonary rehabilitation has proved to be effective in decreasing hospital admission rate and mortality, and in improving health-related quality of life in patients who have recently suffered a COPD exacerbation requiring hospitalization (Citation68), even if a recent study failed to demonstrate that early rehabilitation during hospital admission for chronic respiratory disease reduced the risk of subsequent readmission or enhanced recovery of physical function following the event over 12 months (Citation69).
Improving exercise capacity: Can it represent the link between the “lung centered” and “systemic inflammation and co-morbidome” views?
Both bronchodilators and pulmonary rehabilitation are able to improve exercise capacity in COPD, outcome important from the patients’ point of view, and crucial in the “lung-centered” perspective.
The deleterious effects of physical inactivity are associated with many chronic diseases, frequently linked to COPD, such as type 2 diabetes mellitus, hypertension, obesity, osteoporosis, and breast and colorectal cancers (Citation46). As indicated by the American Diabetes Association it is now well established that regular physical activity improves blood glucose control, preventing or delaying type 2 diabetes, improving lipid metabolism, blood pressure, quality of life and decreasing cardiovascular events, mortality (Citation70).
Physical activity reduces the risk of cardiovascular events (Citation71,Citation72); the association between higher levels of activity and lower cardiovascular disease rates can be explained in large part by renown risk factors, with inflammatory and haemostatic biomarkers making the largest contribution to lowering the risk, followed by blood pressure, lipids, and body mass index (Citation73). Chronic inflammation is an important risk factor for several diseases, such as cardiovascular diseases and type 2 diabetes mellitus. Inflammatory and pro-inflammatory cytokines are elevated in obesity, mainly in case of abdominal obesity (Citation74–Citation77). Thus so far, physical inactivity may represent a link between “lung” and “systemic centered” views of the disease.
Even if some aspects are still debated, in obese patients exercise training has demonstrated potential positive effects (Citation77) on: (i) muscle tissue, with an increased release of IL-6 responsible for improving balance between pro-inflammatory, and anti-inflammatory cytokines (Citation78); (ii) adipose tissue, with a reduction of macrophage infiltration due to an increase of angiogenesis, and blood supply (Citation79,Citation80); (iii) endothelial cells with a reduced vascular wall inflammation due to a reduced production of adhesion molecules and an increase of cell regeneration (Citation81–Citation83); and (iv) immune cells, with a reduction of toll-like receptors and inflammatory monocytes, together with an increase of regulatory T cells (Citation84). Through these mechanisms, exercise training reduces chronic inflammation in obese patients, independently of its effects on weight loss (Citation77). Finally, although the dose and domain of physical activity varied across studies, evidence suggests that even low doses of physical activity may be protective against depression (Citation85).
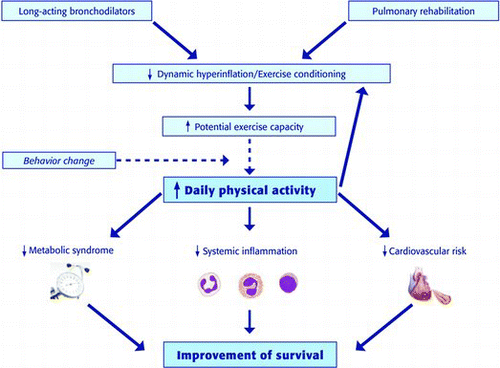
However, in COPD moderate-to-weak relationship between the improvement of “potential” exercise capacity and daily physical activity has been found (Citation86). In fact, even if a treatment with long-acting bronchodilators allows patients to climb stairs, they will continue to use the lift if they were used to have this behavior; obtaining a significant behavior modification with regard to daily physical activity is the true challenge.
Some potential limitations about our perspective should be underlined. First of all, the link between physical activity and “systemic inflammation” probably requests new literature evidence, since some papers highlight how physical activity has anti-inflammatory effect, but others suggest the opposite, mainly in muscle-wasted patients (Citation87). Moreover, in our review, we did not deal with the complexity of COPD, condition that includes patients with different features (e.g., prevalent emphysema vs. airway disease, or cachectic vs. non-cachectic). Finally, we generalized the effect of physical activity; actually, it is well known that the response to exercise training during a rehabilitation program can be different with responders and non-responders (Citation88).
Conclusions
As previously discussed, two distinct “views” of COPD are currently proposed: the first more focused on the lung, the second on systemic inflammation and co-morbidities, deemed not as a complication of the lung impairment but, rather, as an extra-pulmonary manifestation of COPD. If physical inactivity represents a link between “lung” and “systemic-centered” views of the disease, in the management of COPD patients the first aim should be to improve exercise capacity and daily activity since these outcomes have direct effects on patients’ quality of life, co-morbidities (heart and metabolic diseases), and prognosis. But how to reach this outcome in COPD patients if not by treating hyperinflation, the main cause of exercise limitation?
Moreover, due to the weak relationship between the improvement of “potential” exercise capacity, that can be achieved with bronchodilators and pulmonary rehabilitation, and daily physical activity, to reach the best possible results it is important to change COPD patients’ behavior (Figure ).
Declaration of Interest Section
FDM has received honoraria for lectures at national and international meetings from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompé, Guidotti/Malesci, GSK, Menarini, Novartis, Zambon. He has served as consultant for Astra Zeneca, Chiesi Farmaceutici, Novartis, and Zambon. He has received financial support for research from Novartis.
PS has received financial support for research from AirLiquide, Chiesi Farmaceutici and Almirall. He has received support for national and international meetings attendance from Chiesi Farmaceutici, Novartis, Menarini, AstraZeneca, Boehringer Ingelheim. He has received honoraria for lectures at national and international meetings from Chiesi Farmaceutici, Novartis, Zambon, AstraZeneca, Boehringer Ingelheim, Menarini, Almirall. He has served as consultant for Zambon, Astra Zeneca, Novartis, Chiesi Farmaceutici.
GS and FB have nothing to disclose.
SC has received honoraria for lectures at national and international meetings from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompé, Guidotti/Malesci, GSK, Menarini, Novartis, Zambon. He has served as consultant for Astra Zeneca, Chiesi Farmaceutici, Novartis, and GSK. He has received financial support for research from Novartis, and Pfizer.
The authors alone are responsible for the content and writing of the paper.
References
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349:1436–42.
- Di Marco F, Tantucci C, Pellegrino G, Centanni S. Chronic obstructive pulmonary disease diagnosis: the simpler the better? Not always. Eur J Intern Med 2013; 24:199–202.
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–65.
- Bettoncelli G, Blasi F, Brusasco V, et al. The clinical and integrated management of COPD. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31:3609.
- O'Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol (1985) 2008; 105:753–5; discussion 5-7.
- Aliverti A, Macklem PT. The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles. J Appl Physiol (1985) 2008; 105:749–51; discussion 55-7.
- Debigare R, Maltais F. The major limitation to exercise performance in COPD is lower limb muscle dysfunction. J Appl Physiol (1985) 2008; 105:751–3; discussion 5-7.
- Lopez-Sanchez M, Munoz-Esquerre M, Huertas D, et al. High prevalence of left ventricle diastolic dysfunction in severe COPD associated with a low exercise capacity: A cross-sectional study. PLoS One 2013; 8:e68034.
- Fabbri LM, Beghe B, Agusti A. COPD and the solar system: introducing the chronic obstructive pulmonary disease comorbidome. Am J Respir Crit Care Med 2012; 186:117–9.
- Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:155–61.
- Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177:622–9.
- Laviolette L, Laveneziana P, Faculty ERSRS. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J 2014; 43:1750–62.
- Laveneziana P, Webb KA, Ora J, Wadell K, O'Donnell DE. Evolution of dyspnea during exercise in chronic obstructive pulmonary disease: impact of critical volume constraints. Am J Respir Crit Care Med 2011; 184:1367–73.
- Laveneziana P, Webb KA, Wadell K, Neder JA, O'Donnell DE. Does expiratory muscle activity influence dynamic hyperinflation and exertional dyspnea in COPD? Respiratory physiology & neurobiology 2014; 199:24–33.
- O'Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 1997; 155:109–15.
- Di Marco F, Milic-Emili J, Boveri B, et al. Effect of inhaled bronchodilators on inspiratory capacity and dyspnoea at rest in COPD. Eur Respir J 2003; 21:86–94.
- Beeh KM, Singh D, Di Scala L, Drollmann A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis 2012; 7:503–13.
- Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J 2008; 31:869–73.
- Bedard ME, Brouillard C, Pepin V, et al. Tiotropium improves walking endurance in COPD. Eur Respir J 2012; 39:265–71.
- Brouillard C, Pepin V, Milot J, Lacasse Y, Maltais F. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur Respir J 2008; 31:579–84.
- O'Donnell DE, Casaburi R, Vincken W, et al. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med 2011; 105:1030–6.
- O'Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23:832–40.
- Oga T, Nishimura K, Tsukino M, Hajiro T, Ikeda A, Izumi T. The effects of oxitropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. A comparison of three different exercise tests. Am J Respir Crit Care Med 2000; 161:1897–901.
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–54.
- Mascarenhas J, Lourenco P, Lopes R, Azevedo A, Bettencourt P. Chronic obstructive pulmonary disease in heart failure. Prevalence, therapeutic and prognostic implications. Am Heart J 2008; 155:521–5.
- Iversen KK, Kjaergaard J, Akkan D, et al. The prognostic importance of lung function in patients admitted with heart failure. Eur J Heart Fail 2010; 12:685–91.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–12.
- Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 2006; 61:17–22.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59:574–80.
- Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003; 58:752–6.
- Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012; 7:e37483.
- Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 2007; 370:797–9.
- Bolton CE, Evans M, Ionescu AA, et al. Insulin resistance and inflammation - A further systemic complication of COPD. COPD 2007; 4:121–6.
- Laveneziana P, Palange P. Physical activity, nutritional status and systemic inflammation in COPD. Eur Respir J 2012; 40:522–9.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32:962–9.
- Palange P, Testa U, Huertas A, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J 2006; 27:529–41.
- Puente-Maestu L, Perez-Parra J, Godoy R, et al. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J 2009; 33:1045–52.
- Rana JS, Mittleman MA, Sheikh J, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 2004; 27:2478–84.
- Sabit R, Bolton CE, Edwards PH, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175:1259–65.
- Sevenoaks MJ, Stockley RA. Chronic Obstructive Pulmonary Disease, inflammation and co-morbidity—a common inflam-matory phenotype? Respir Res 2006; 7:70.
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33:1165–85.
- Heady JA, Morris JN, Kagan A, Raffle PA. Coronary heart disease in London busmen. A progress report with particular reference to physique. Br J Prev Soc Med 1961; 15:143–53.
- Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet 1953; 265:1111–20; concl.
- Fletcher GF, Balady G, Blair SN, et al. Statement on exercise: benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation 1996; 94:857–62.
- Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: Clinical and research applications: a scientific statement from the American Heart Association. Circulation 2013; 128:2259–79.
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012; 380:219–29.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from >6,000 consecutive referred male patients. Chest 2001; 120:1003–13.
- Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006; 119:21–31.
- Laveneziana P, Guenette JA, Webb KA, O'Donnell DE. New physiological insights into dyspnea and exercise intolerance in chronic obstructive pulmonary disease patients. Expert Rev Respir Med 2012; 6:651–62.
- Ramon MA, Gimeno-Santos E, Ferrer J, et al. Hospital admissions and exercise capacity decline in patients with COPD. Eur Respir J 2014; 43:1018–27.
- Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129:536–44.
- Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation–systematic review. Int J Chron Obstruct Pulmon Dis 2007; 2:241–51.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61:772–8.
- Garcia-Rio F, Rojo B, Casitas R, et al. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012; 142:338–46.
- Moy ML, Teylan M, Weston NA, Gagnon DR, Garshick E. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS One 2013; 8:e60400.
- Seidel D, Cheung A, Suh ES, Raste Y, Atakhorrami M, Spruit MA. Physical inactivity and risk of hospitalisation for chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2012; 16:1015–9.
- Garcia-Polo C, Alcazar-Navarrete B, Ruiz-Iturriaga LA, et al. Factors associated with high healthcare resource utilisation among COPD patients. Respir Med 2012; 106:1734–42.
- Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med 2009; 180:506–12.
- Aguilaniu B. Impact of bronchodilator therapy on exercise tolerance in COPD. Int J Chron Obstruct Pulmon Dis 2010; 5:57–71.
- O'Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006; 130:647–56.
- Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J 2013; 42:1484–94.
- Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011; 37:273–9.
- Rabinovich RA, Ardite E, Troosters T, et al. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164:1114–8.
- Scott AS, Baltzan MA, Fox J, Wolkove N. Success in pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Can Respir J 2010; 17:219–23.
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188:e13–64.
- Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172:19–38.
- Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011; CD005305.
- Greening NJ, Williams JE, Hussain SF, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. Br Med J 2014; 349:g4315.
- Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010; 33:e147–67.
- Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996; 276:205–10.
- Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med 2002; 347:716–25.
- Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 2007; 116:2110–8.
- Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol 1999; 277:E971–5.
- Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Janowska J, Zurakowski A. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-alpha and TNF soluble receptors in women with overweight and obesity. Metabolism 2004; 53:1268–73.
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999; 282:2131–5.
- You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity : current evidence and potential mechanisms. Sports Med 2013; 43:243–56.
- Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 2006; 12:6–33.
- Klimcakova E, Polak J, Moro C, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab 2006; 91:5107–12.
- Polak J, Klimcakova E, Moro C, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism 2006; 55:1375–81.
- Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004; 109:220–6.
- Sarto P, Balducci E, Balconi G, et al. Effects of exercise training on endothelial progenitor cells in patients with chronic heart failure. J Card Fail 2007; 13:701–8.
- Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism 2004; 53:377–81.
- Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 1999; 281:1722–7.
- Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med 2008; 46:397–411.
- Zwerink M, van der Palen J, van der Valk P, Brusse-Keizer M, Effing T. Relationship between daily physical activity and exercise capacity in patients with COPD. Respir Med 2013; 107:242–8.
- Van Helvoort HA, Heijdra YF, Thijs HM, Vina J, Wanten GJ, Dekhuijzen PN. Exercise-induced systemic effects in muscle-wasted patients with COPD. Med Sci Sports Exerc 2006; 38:1543–52.
- Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J 2006; 27:788–94.