Abstract
Dynamic hyperinflation (DH) is a pathophysiologic hallmark of Chronic Obstructive Pulmonary Disease (COPD). The aim of this study was to investigate the impact of emphysema distribution on DH during a maximal cardiopulmonary exercise test (CPET) in patients with severe COPD.
This was a retrospective analysis of prospectively collected data among severe COPD patients who underwent thoracic high-resolution computed tomography, full lung function measurements and maximal CPET with inspiratory manouvers as assessment for a lung volume reduction procedure. ΔIC was calculated by subtracting the end-exercise inspiratory capacity (eIC) from resting IC (rIC) and expressed as a percentage of rIC (ΔIC %). Emphysema quantification was conducted at 3 predefined levels using the syngo PULMO-CT (Siemens AG); a difference >25% between best and worse slice was defined as heterogeneous emphysema.
Fifty patients with heterogeneous (62.7% male; 60.9 ± 7.5 years old; FEV1% = 32.4 ± 11.4) and 14 with homogeneous emphysema (61.5% male; 62.5 ± 5.9 years old; FEV1% = 28.1 ± 10.3) fulfilled the enrolment criteria. The groups were matched for all baseline variables. ΔIC% was significantly higher in homogeneous emphysema (39.8% ± 9.8% vs.31.2% ± 13%, p = 0.031), while no other CPET parameter differed between the groups. Upper lobe predominance of emphysema correlated positively with peak oxygen pulse, peak oxygen uptake and peak respiratory rate, and negatively with ΔIC%. Homogeneous emphysema is associated with more DH during maximum exercise in COPD patients.
Introduction
Expiratory flow limitation is the pathophysiologic hallmark of Chronic Obstructive Pulmonary Disease (COPD). One of the consequences of airflow limitation and permanent parenchymal destruction is lung hyperinflation (Citation1,Citation2). Static hyperinflation is due to the reduced elastic recoil of the lung. Dynamic hyperinflation (DH), which occurs independently or in addition to static hyperinflation, refers to the increase of lung volumes above their resting value that occurs with increases in respiratory rate due to expiratory flow limitation. It is associated both with excessive loading and mechanical disadvantage of inspiratory muscles and with restriction of normal tidal volume expansion during exercise (Citation2). Previous studies have indicated that lung hyperinflation contributes significantly to exercise limitation, exertional dyspnoea and exercise desaturation in COPD patients (Citation3,Citation4).
Thoracic computed tomography (CT) allows precise assessment of emphysema extent and distribution (Citation5,Citation6) and correlation of these parameters with functional and pathologic data (Citation7,Citation8). The distribution of parenchymal damage varies widely between individuals (Citation9), and its categorization in homogeneous and heterogeneous emphysema is of clinical significance, since it guides selection for surgical and bronchoscopic lung volume reduction procedures (LVR) (Citation10–Citation13). Although there are some data regarding the association between emphysema distribution, clinical features and resting lung function values in COPD patients of various severities (Citation9,Citation14), no study has yet investigated the potential impact of emphysema distribution on DH among COPD patients.
Thus, we conducted a retrospective study to identify: (a) differences in the degree of DH occurring during maximum cardiopulmonary exercise testing (CPET) between COPD patients with heterogeneous and matched COPD patients with homogeneous emphysema and (b) associations between emphysema heterogeneity and CPET parameters in the two patient groups. The primary hypothesis was that patients with homogeneous emphysema hyperinflate more due to the diffuse distribution of the emphysematous lung tissue destruction.
Material and Methods
Study population
Data were collected for COPD outpatients who had been assessed at the Respiratory Biomedical Research Unit of Royal Brompton Hospital between June 2009, and August, 2013 for potential eligibility to undergo a bronchoscopic lung volume reduction procedure. These included full lung function measurements, thoracic high resolution computed tomography (HRCT) and breath by breath data from maximal cycling CPET with inspiratory capacity manoeuvers every minute. The COPD patients were in stable clinical condition and optimally treated with combinations of β2-agonists, anticholinergic drugs and inhaled corticosteroids, according to guidelines. All patients had provided informed consent for their initial participation in the studies (Citation15–Citation18).
Study Measurements
Pulmonary Function Testing
Spirometry, gas transfer and lung volumes measurements by body plethysmography were conducted using a Compact Lab System (Jaeger, Hoechberg, Germany). Arterialized capillary blood samples were used to measure arterial blood gases (Citation19). The European Coal and Steel Community predicted values were used for lung function measurements (Citation20) and values of carbon monoxide diffusion capacity and transfer coefficient were adjusted for haemoglobin concentration (TLcoc and Kcoc, respectively) (Citation21). All pulmonary function testing (PFT) values used were measured prior to any bronchoscopic intervention and within a 6-month interval of both the HRCT and the maximal CPET.
HRCT acquisition and interpretation
Imaging was performed for clinical indications on 4-slice multidetector CT (Volume Zoom, Siemens, Erlangen, Germany), or 64-slice CT (Somatom Sensation 64, Siemens, Erlangen, Germany). Images were either acquired at 10-mm intervals (4-slice CT) or using a volumetric acquisition (64-slice CT) in a supine position from the lung apices to the bases at full inspiration without the use of intravenous contrast. Images were reconstructed at thin section width (1.0 mm to 1.5 mm) using a high spatial resolution algorithm and reviewed on a workstation at appropriate window settings for viewing the lung parenchyma (window centre = −500HU; window width = 1500HU).
Images were transferred to a post-processing workstation (Leonardo, Siemens) and quantitative lung density analysis was performed using the Pulmo CT program (Siemens AG), which automatically segments the lung and calculates pixel attenuation coefficients as previously described (Citation22) with a minimum segmentation threshold of -1024HU (Figure ). Three representative slices of the lungs were analysed: (a) at the level where the superior border of the aortic arch appears; (b) at the level of the main carina, where clear separation of the right and left main bronchi becomes visible; and (c) at the lowest level where neither diaphragm nor any abdominal viscera are visible.
Figure 1. (A) Upper slice (at the superior border of the aortic arch) transferred to the post-processing workstation, where quantitative lung density analysis was performed using the Pulmo CT program. (B) Histogram of distribution of lung attenuation values, measured in HU in the upper slice (at the superior border of the aortic arch) for several potential emphysema thresholds.
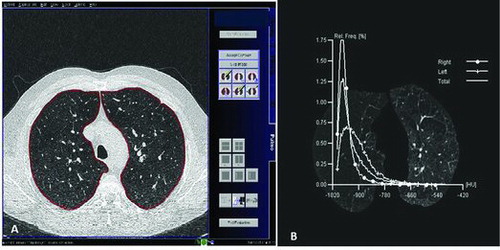
At each level, the program provides a total emphysema index, defined as the percentage of whole lung with attenuation values below a threshold of -900 HU, as well as a severe emphysema index, defined as percentage of whole lung with attenuation values below a threshold of -950 HU.(Citation23)(24)(25) An alternative threshold for emphysema definition of -960 HU has also previously been reported and we included this threshold as an additional quantitative parameter (Citation26).
To enable us to classify subjects as having either homogeneous or heterogeneous emphysema, we defined heterogeneous emphysema as a difference of >25% between the highest and lowest quantitative emphysema scores obtained, based on previous precedents of visual analysis (Citation27).
In addition, emphysema was treated as a continuous variable and specifically as a ratio of average ES of the upper and middle slice versus ES of the lower slice (UM/L) for both the 950 and 960 emphysema thresholds. A high ratio therefore represented upper lobe predominance.
Maximal CPET
Patients performed incremental symptom-limited CPET on a cycle ergometer (Jaeger Ergoline 800), with continuous monitoring of pulse oximetry (SaO2), heart rate (HR), and a 12-lead electrocardiogram. The test consisted of three minutes of rest, 1 minute of unloaded pedaling at 50–60 rpm, and then a ramp protocol with work rate (WR) increasing either 5 or 10 watts/minute, followed by 2 minutes of recovery (Citation28).
Gas exchange values and exercise parameters were collected breath-by-breath using a Jaeger Oxycon system (Citation29,Citation30), allowing measurement of: tidal volume (Vt), respiratory rate (RR), oxygen uptake (VO2), carbon dioxide production (VCO2), end-tidal oxygen (PETO2) and end-tidal carbon dioxide (PETCO2). The anaerobic threshold (AT), oxygen pulse (VO2/HR), respiratory exchange ratio (RER), minute ventilation (VE) and the ventilatory equivalent for carbon dioxide at peak VO2 (VE/VCO2@VO2 peak) were also calculated, as previously described (Citation29).
Each patient performed a total of four inspiratory capacity (IC) maneuvers at the beginning and after 1, 2, and 3 minutes of rest as previously described (Citation31); resting IC (rIC) was calculated by averaging these values. End-exercise IC (eIC) was calculated by another inspiratory maneuver which was conducted during the last 30 seconds of peak exercise. ΔIC was utilized as a measure of DH and was calculated as rIC-eIC. For every patient, ΔIC was expressed as a percentage of rIC, that is: ΔIC% = (ΔIC/rIC) × 100%. All exercise tests were performed before any bronchoscopic intervention took place and without oxygen supplementation.
Statistical analysis
Statistical analysis was conducted using the PASW (Predictive Analytics Software by SPSS Inc®) version 19 for Windows 2008. Distribution of values was assessed using the Shapiro-Wilk test of normality; continuous variables are described as mean±1 standard deviation or as median (minimum-maximum), accordingly. Group comparisons in PFTs, DH and exercise parameters were conducted utilizing either the independent samples t-test or the Mann–Whitney test, depending on the normality of their distribution. Pearson r or Spearman rho were used to describe parametric and non-parametric correlations between emphysema distribution indices, DH measures, and exercise parameters. Level of p < 0.05 was considered significant.
Results
Baseline characteristics
Sixty-four COPD patients (61.3 ± 7.3 years old; FEV1%predicted = 31.5 ± 11.2%, 61.8 male); fulfilled the enrolment criteria and constituted the final study population. Fifty patients (78.1%) presented with heterogeneous and 14 patients (21.9%) with homogeneous emphysema (group Het and group Hom correspondingly). An initial attempt for patients in Hom group to match the same number of patients in Het group (1:1 matching) for age, FEV1 and TLcoc was made. However, the two patient groups (N1 = 14 patients in Hom group and N2 = 50 patients in Het group) were found to be already matched not only regarding these three selected variables, but also regarding gender, body mass index (BMI), rest pulmonary function testing variables (PFTs) and gas transfer parameters. Results were identical with the use of either emphysema threshold (-950 HU or -960 HU). The baseline demographic characteristics of the two groups are presented in
Table 1. Baseline characteristics of the study population
Table 1. Baseline characteristics of the study population
Exercise parameters
Exercise parameters for both groups are presented in Table . Dyspnoea was the reason for CPET termination for all patients, and the degree of breathlessness did not differ between groups. Only 15 patients (11 from Het group and 4 from Hom group) reached their AT during maximal CPET (data not shown); the rest terminated the exercise before reaching AT, due to respiratory reserve depletion.
Table 2. Comparison of exercise parameters between patients with heterogeneous and homogeneous emphysema
Patients in the Hom group displayed more DH during exercise, as ΔIC% was significantly higher among Hom group compared to Het group; 39.8% vs 31.2% (p = 0.031). An additional analysis was undertaken, utilizing the change from rest to peak exercise values of End-Expiratory Lung Volume to Total lung capacity ratio (ΔEELV/TLC); Again, Hom group presented with significantly higher ratio, that is greater DH during exercise, compared to Het group (p = 0.035) (Table ). No other differences in CPET parameters were noted between the two groups (Table and Table ).
Table 3. Correlations between emphysema distribution and exercise parameters, for both emphysema thresholds
Effect of upper lobe predominance of emphysema distribution
The UM/L ratio of emphysema score for both the 950 and 960 emphysema thresholds was evaluated; a high ratio represents upper lobe predominance. As presented in Table , the UM/L ratio established a weak, inverse but significant correlation to ΔIC% for both emphysema thresholds (Spearman rho = -0.264, p = 0.049; Spearman rho = -0.246, p = 0.049, correspondingly). Moreover, peak VO2%predicted, peak VO2/HR %predicted and peak RR were all positively correlated to UM/L ratio (Table ).
Discussion
We aimed to investigate whether the distribution of emphysema has any impact on dynamic lung volumes during exercise in patients with COPD. The primary hypothesis was confirmed; we found that patients with homogenous emphysema hyperinflated more than those with heterogeneous disease, although no differences in other exercise parameters were noted. Furthermore, a measure of upper lobe predominance (UM/L ratio) correlated inversely with DH, and positively with peak oxygen consumption, peak oxygen pulse and peak RR.
Significance of findings
Emphysema distribution varies significantly among individuals and possibly represents different pathogenic patterns of disease development (Citation9). Three different subtypes of emphysema have been recognized: (a) centriacinar emphysema, which predominantly involves the upper lobes and is associated with long-standing cigarette smoking, (b) panacinar emphysema, which mainly involves the lower lobes and is frequently found in patients with alpha-1 antitrypsin deficiency and (c) distal acinar emphysema, which tends to occur adjacent to the pleura or the fibrous septa (Citation32).
These pathologic lesions are found in various combinations in each patient, comprising a heterogeneous or homogeneous emphysema pattern, as classified by CT imaging (Citation32,Citation33). Mair et al. indicated that emphysema distribution is associated with several clinical features, such as FEV1, BMI, BODE index and health status;(9) however, these associations were most evident among patients with core versus rind predominant, rather than upper versus lower predominant emphysema. Of note, the presence of heterogeneity has been repeatedly associated with improved outcomes and increased survival after a LVR procedure (Citation12,Citation13,Citation34). Although the theoretical background of these interventions is the restoration of lung elastic recoil and the improvement of lung mechanics, the impact of emphysema distribution itself on DH has not previously been assessed.
Both the pathological hallmarks of COPD, airway inflammation and parenchymal destruction contribute to the development of DH.(Citation35) During exercise, increased airway resistance and decreased elastic recoil result in increased time constants for alveolar units, so as RR and expiratory flow increase, the expiratory time available for exhalation becomes insufficient (Citation36,Citation37). In our study, patients with a heterogeneous pattern of emphysema, that is with unequally distributed parenchymal damage, experienced significantly less DH during maximal exercise.
In patients with heterogeneous emphysema, lung areas with distinct destruction coexist with areas where lung parenchyma is relatively well preserved (Citation32). We speculate that this coexistence poses a mechanical barrier to the further increase of end expiratory lung volume in emphysematous lung areas, resulting in less DH during maximal exercise. On the contrary, patients with homogeneous emphysema have, by definition, more widespread disease and diffuse floppy airways, meaning that mechanical restriction to whole lung hyperinflation is less.
Interestingly, no CPET parameter differed significantly between patients in the Het and Hom groups. It is well established that medical or other interventions targeting lung hyperinflation improve exercise tolerance among COPD patients (Citation38–Citation40). Tzani et al. reported that DH was associated with increased exertional dyspnea and reduced maximum exercise capacity in a cohort of COPD patients (Citation3); however, a relatively high value of end expiratory lung volume (≥75% TLC) was used as a threshold for group categorization, while resting TLC was significantly higher between patients who hyperinflated compared to those who did not.
In another study ΔIC was associated with exercise desaturation among male COPD patients with severe disease; nevertheless, this study utilized six-minute walking test, that is a submaximal exercise testing and not maximal CPET, so results are not easily comparable (Citation4). In our study, the highly selective patient population, the relatively small absolute difference of ΔIC (approximately 8.5%), and the presence of similarly increased resting lung volumes between the groups are the most probable causes for this lack of difference in measures of dyspnea and exercise capacity between patients with homogeneous and those with heterogeneous emphysema.
When emphysema distribution was treated as a continuous variable, weak but significant correlations were established with several CPET parameters. The higher the emphysema heterogeneity with upper lobe predominance, as manifested by increased UM/L ratio, the lower the ΔIC%, and the higher the peak O2 consumption and the peak oxygen pulse. Lung hyperinflation is an important cause of circulatory impairment during exercise, through several mechanisms (Citation3,Citation41,Citation42).
The development of an intrinsic positive end-expiratory pressure (PEEP) during active expiration and the high intrathoracic pressure swings that have to be generated to overcome the increased high elastic and resistive loads result in functional hypovolemia during exercise among COPD patients, which may have an impact on stroke volume. (Citation35)(41)(36)(43) Whether the unequal distribution of parenchymal destruction itself, as seen in heterogeneous emphysema, compromises cardiac functions less, irrespectively from the degree of DH, due to the potential compensatory effect of preserved lung areas, is a hypothesis that remains to be tested.
Methodological issues
Although data were analyzed retrospectively, they were collected prospectively minimizing recall bias. COPD patients who were included in the study were followed-up in a single tertiary hospital and were pre-screened for assessment as potential patients for a LVR procedure. Thus, they presented with severe disease and with a higher proportion of heterogeneous emphysema than expected. Testing was performed by an experienced and highly trained team of physiologists and researchers with strict quality control measures in place to minimize testing variability.
Moreover, patients were matched for other parameters which could potentially affect DH, such as resting lung function. Although the categorization of emphysema distribution was performed using a well-established technique, the use of UM/L ratio as a continuous variable to describe upper-lobe predominance in sub analysis has not been reported previously and needs to be further validated; Nevertheless its correlation with parameters of exercise capacity and DH is in accordance with published literature on emphysema heteroneneity (Citation3,Citation41), which strengthens the rationale of its use.
Conclusions
In conclusion, we found that patients with homogeneous emphysema hyperinflate significantly more during maximum exercise than to those with heterogeneous emphysema. Upper lobe predominance was also associated with less DH and with a higher peak VO2, peak VO2/HR and peak RR. Therefore, CT assessed emphysema pattern and degree of heterogeneity may have a role to play when phenotyping COPD; however, further prospective studies are needed to address this.
Abbreviations
BMIBody Mass IndexCOPDChronic obstructive pulmonary diseaseCPETCardio pulmonary exercise testDHDynamic hyperinflationESEmphysema scoreEELVEnd Expiratory Lung VolumeFEV1Forced Expiratory Volume in 1 secondFRCFunctional Residual CapacityFVCForced Vital CapacityHRHeart RateHUHounsfield unitHRCTHigh resolution computed tomographyeICEnd-exercise Inspiratory CapacityKcocCarbon Dioxide transfer coefficient corrected for haemoglobinpBorgpeak Borg scale scorePaO2arterial Oxygen Partial PressurePaCO2arterial Carbon Dioxide Partial PressurePETCO2End Expiratory Carbon Dioxide partial pressurePETO2End Expiratory Oxygen partial pressureppSPO2peak arterial Oxygen SaturationrBorgrest Borg scale scoreRERRespiratory Exchange RatiorICrest Inspiratory CapacityrSPO2rest arterial Oxygen SaturationRVResidual VolumeTLCTotal Lung CapacityTLcocCarbon Dioxide transfer factor corrected for haemoglobinUM/LRatio of upper and middle lobe emphysema score to lower lobeVCO2Carbon Dioxide ProductionVEMinute ventilationVE/VCO2Ventilatory Equivalent for Carbon DioxideVO2Oxygen ConsumptionVO2/HROxygen PulseVtTidal VolumeWRWork Rate
Authors’ contribution
AKB contributed to study design, data acquisition, analysis and interpretation and manuscript drafting. ZZ, AN, CD and DMH contributed to data acquisition and interpretation and they critically revised the manuscript. AJ contributed to study design and critically revised the manuscript. MIP and NSH contributed to study conception and design, data acquisition and interpretation and critically revised the manuscript. All authors approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors thank the clinical physiologists in the Respiratory BRU at Royal Brompton Hospital, London, UK, who performed the pulmonary function tests and conducted the cardiopulmonary exercise tests described in this article. During the conduction of this work AKB was a recipient of an ERS Short-Term Research Fellowship.
References
- FergusonGT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006; 3(2):176–179.
- O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3(2):180–184.
- Tzani P, Aiello M, Elia D, Boracchia L, MarangioE, Olivieri D, Clini E, Chetta A. Dynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patients. Respir Res 2011; 12(1):150.
- ZafarMA, Tsuang W, Lach L, Eschenbacher W, Panos RJ. Dynamic hyperinflation correlates with exertional oxygen desaturation in patients with chronic obstructive pulmonary disease. Lung 2013; 191(2):177–182.
- Coxson HO. Quantitative chest tomography in COPD research: chairman's summary. Proc Am Thorac Soc 2008; 5(9): 874–877.
- StolkJ, VersteeghMIM, Montenij LJ, Bakker ME, GrebskiE, TuticM, Wildermuth S, WederW, el BardijiM, Reiber JH, RabeKF, Russi EW, StoelBC. Densitometry for assessment of effect of lung volume reduction surgery for emphysema. Eur Respir J 2007; 29(6):1138–1143.
- Gould GA, Redpath AT, RyanM, Warren PM, BestJJ, FlenleyDC, MacNee W. Lung CT density correlates with measurements of airflow limitation and the diffusing capacity. Eur Respir J 1991; 4(2):141–146.
- Müller NL, StaplesCA, Miller RR, AbboudRT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988; 94(4):782–787.
- Mair G, Miller JJ, McAllisterD, MaclayJ, Connell M, MurchisonJ T, MacNee W. Computed tomographic emphysema distribution: relationship to clinical features in a cohort of smokers. Eur Respir J 2009; 33(3):536–542.
- McNulty W, Zoumot Z, Hopkinson NS. Bronchoscopic and percutaneous approaches to lung volume reduction. Clin Pulm Med 2013; 20(6):300–308.
- Murphy PB, Zoumot Z, Polkey MI. Noninvasive ventilation and lung volume reduction. Clin Chest Med 2014; 35(1):251–269.
- Sanchez PG, KucharczukJC, SuS, Kaiser LR, CooperJD. National Emphysema Treatment Trial redux: accentuating the positive. J Thorac Cardiovasc Surg 2010; 140(3):564–572.
- McKenna RJ Jr, BrennerM, FischelRJ, SinghN, YoongB, Gelb AF, Osann KE. Patient selection criteria for lung volume reduction surgery. J Thorac Cardiovasc Surg 1997; 114(6):957–964; discussion 964–967.
- De TorresJP, Bastarrika G, ZagacetaJ, Sáiz-MendigurenR, Alcaide AB, SeijoLM, CampoA, ZuluettaJJ. Emphysema presence, severity, and distribution has little impact on the clinical presentation of a cohort of patients with mild to moderate COPD. Chest 2011; 139(1):36–42.
- ClarkSJ, ZoumotZ, BamseyO, PolkeyMI, Dusmet M, LimE, JordnS, HopkinsonNS. Surgical approaches for lung volume reduction in emphysema. Clin Med Lond 2014; 14(2):122–127.
- ShahPL, ZoumotZ, Singh S, Bicknell SR, Ross ET, QuiringJ, HopkinsonNS, Kemp SV; RESET trial Study Group. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013; 1(3):233–240.
- DaveyC, ZoumotZ, JordanS, Carr DH, PolkeyMI, Shah PL, Hopkinson NS. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (The BeLieVeR-HIFi trial): study design and rationale. Thorax 2014 Mar 24; doi: 10.1136/thoraxjnl-2014-205127. [Epub ahead of print]
- ZoumotZ, KempSV, CanejaC, SinghS, ShahPL. Bronchoscopic intrabullous autologous blood instillation: a novel approach for the treatment of giant bullae. Ann Thorac Surg 2013; 96(4):1488–1491.
- Zavorsky GS, Cao J, Mayo NE, Gabbay R, Murias JM. Arterial versus capillary blood gases: a meta-analysis. Respir Physiol Neurobiol 2007; 155(3):268–279.
- Quanjer PH, Tammeling GJ, Cotes JE, PedersenOF, PeslinR, YernaultJC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16:5–40.
- Clark EH, WoodsRL, Hughes JM. Effect of blood transfusion on the carbon monoxide transfer factor of the lung in man. Clin Sci Mol Med 1978; 54(6):627–631.
- GieradaDS, YusenRD, Pilgram TK, Crouch L, SloneRM, BaeKT, Lefrak SS, Cooper JD. Repeatability of quantitative CT indexes of emphysema in patients evaluated for lung volume reduction surgery. Radiology 2001; 220(2):448–454.
- Gevenois PA, de MaertelaerV, De VuystP, ZanenJ, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995; 152(2):653–657.
- Gietema HA, Müller NL, Fauerbach PVN, Sharma S, Edwards LD, Camp PG, et al. Quantifying the extent of emphysema: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol 2011; 18(6):661–671.
- WangZ, Gu S, LeaderJK, KunduS, Tedrow JR, SciurbaFC, GurD, Siegfried JM, Pu J. Optimal threshold in CT quantification of emphysema. Eur Radiol 2013; 23(4):975–984.
- GieradaDS, Slone RM, Bae KT, Yusen RD, Lefrak SS, CooperJD. Pulmonary emphysema: comparison of preoperative quantitative CT and physiologic index values with clinical outcome after lung-volume reduction surgery. Radiology 1997; 205(1):235–242.
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung volume reduction surgery. N Engl J Med 2001; 345(15):1075–1083.
- American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167(2):211–277.
- WassermanK. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Lippincott Williams & Wilkins, Philadelphia, PA; 2005. 620 p.
- Beaver WL, Wasserman K, WhippBJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986; 60(6):2020–2027.
- GuenetteJA, Chin RC, CoryJM, Webb KA, O'Donnell DE. Inspiratory Capacity during exercise: measurement, analysis, and interpretation. Pulm Med 2013; 2013:956081
- Russi EW, Bloch KE, Weder W. Functional and morphological heterogeneity of emphysema and its implication for selection of patients for lung volume reduction surgery. Eur Respir J 1999; 14(1):230–236.
- Weder W, ThurnheerR, Stammberger U, BürgeM, RussiEW, Bloch KE. Radiologic emphysema morphology is associated with outcome after surgical lung volume reduction. Ann Thorac Surg 1997; 64(2):313–319
- Hopkinson NS, KempSV, Toma TP, Hansell DM, Geddes DM, ShahPL, Polkey MI. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J 2011; 37(6):1346–1351.
- Puente-Maestu L, Stringer WW. Hyperinflation and its management in COPD. Int J Chron Obstruct Pulmon Dis 2006; 1(4):381–400.
- GagnonP, Guenette JA, Langer D, Laviolette L, MainguyV, MaltaisF, Ribeiro F, SaeiD. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014; 9:187–201.
- O'Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD 2006; 3(4):219–232.
- MaltaisF, HamiltonA, MarciniukD, HernandezP, SciurbaFC, Richter K, KestenS, O'DonnellD. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005; 128(3):1168–1178.
- HopkinsonNS, Toma TP, Hansell DM, GoldstrawP, MoxhamJ, GeddesDM, PolkeyMI. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med 2005; 171(5):453–460.
- O'Donnell DE, Lam M, WebbKA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158(5 Pt 1):1557–1565.
- Vassaux C, Torre-Bouscoulet L, ZeineldineS, Cortopassi F, Paz-Díaz H, CelliBR, Pinto-Plata VM. Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur Respir J 2008; 32(5):1275–1282.
- MillerJD, PegelowDF, Jacques AJ, Dempsey JA. Effects of augmented respiratory muscle pressure production on locomotor limb venous return during calf contraction exercise. J Appl Physiol 2005; 99(5):1802–1815.
- Montes de Oca M, RassuloJ, Celli BR. Respiratory muscle and cardiopulmonary function during exercise in very severe COPD. Am J Respir Crit Care Med 1996; 154(5):1284–1289.
