Abstract
The Swedish national register of severe alpha1-antitrypsin (AAT) deficiency was established in 1991. The main aims are to prospectively study the natural history of severe AAT deficiency, and to improve the knowledge of AAT deficiency. The inclusion criteria in the register are age ≥18 years, and the PiZ phenotype diagnosed by isoelectric focusing. The register is kept updated by means of repeated questionnaires providing data to allow analysis of the mode of identification, lung and liver function, smoking-habits, respiratory symptoms and diagnoses as reported by physicians.
Until February 2014, a total of 1553 PiZZ individuals had been included in the register. The 1102 subjects still alive constituted about 20% of the adult PiZZ individuals in Sweden. Forty-three percent of the subjects had been identified during investigation of respiratory symptoms, 7% by an investigation of liver disease, 26% in an investigation of other pathological conditions, and 24% in a population or family screening. Forty five percent of the subjects had never smoked, 47% were ex-smokers, and 8% current smokers. Twenty-eight percent of the never-smokers, 72% of the ex-smokers, and 61% of the current smokers fulfilled the criteria for COPD with a FEV1/FVC ratio of <0.70.
Among the 451 deceased, the most common cause of death was respiratory diseases (55%), followed by liver diseases (13%). We conclude that the detection rate of severe AAT deficiency is relatively high in Sweden. Large numbers of subjects are identified for other reasons than respiratory symptoms, and the majority of these have never smoked.
Introduction
Severe alpha 1-antitrypsin (AAT) deficiency (PiZZ) is a hereditary condition characterized by reduced serum levels of AAT. This genetic defect results in polymerization of the AAT molecule in the hepatocytes, leading to an accumulation of the Z protein within the hepatocytes and a decreased release into the circulation. Panacinar emphysema is the most prevalent clinical disorder associated with severe AAT deficiency and the most frequent cause of disability and death. Liver disease is the second most frequent clinical manifestation of severe AAT deficiency and typically presents as cholestasis in infancy (Citation1).
In Sweden the prevalence of AAT deficiency is 1/1600, a figure firmly established by a nation-wide screening program of 200,000 new-borns over the period 1972-74, when 127 homozygotes were identified (Citation2).
The Swedish AAT registry was started in1991. It is administered by the Department of Respiratory Medicine and Allergology, Skåne University Hospital Malmö, Lund University (Citation3). The aims are to prospectively study the natural history of severe AAT deficiency, to improve the knowledge of AAT deficiency among physicians and the general public, to study lung and liver function, and other previously unrecognized organ manifestations in PiZZ individuals, to encourage attending physicians to perform family studies in conjunction with PiZZ individuals, and to create a pool of patients for clinical studies of AAT deficiency.
The register is collaboration with the Department of Clinical Chemistry (DCC), Malmö, which is the central laboratory for Pi phenotyping in Sweden. The laboratory reports continuously all individuals identified as PiZZ, PiZNull or PiNullNull to the register.
In March 1996, a WHO meeting of experts on alpha-1 antitrypsin (AAT) deficiency was held in Geneva, Switzerland. The meeting concluded that AAT deficiency is widely underdiagnosed, and only a small proportion of those predicted to have AAT deficiency have been diagnosed (Citation4). An international AAT coordinating registry, filing data from national registries, was recommended by the meeting. Since 1999, when The Alpha One International Registry (AIR) was started, all newly diagnosed Swedish PiZZ individuals are prospectively also included in the AIR (Citation5). Thus, the patients included in the Swedish registry before 1999 are included only in the national registry.
Our aims are to present the structure, and to summarize the results of the Swedish national AAT registry until February 2014.
Patients and methods
Patients
The criteria for inclusion in the register are age 18 years or older, and the carriage of the PiZZ, PiZNull or PNullNull phenotype. When the laboratory reports data to the register of a newly identified individual with the PiZ phenotype, the registry contacts the attending physician, encouraging him/her to offer the patient inclusion in the register. After returning a completed questionnaire and an informed consent form, the AAT-deficient individual is included.
Clinical examination, blood samples, and lung function tests are performed at the patients’ local hospitals, the results being reported to the registry via the questionnaire. After inclusion, a follow-up questionnaire is sent to the attending physician every two years.
The register is approved by the Ethical Review Board, Lund University, Sweden, and by the Swedish Data Inspection Board.
Questionnaire
The initial questionnaire includes the following items: characteristics of the patient (gender, date of birth), indication for the original AAT or plasma protein analysis, smoking habits (yes/no, age at start and stop of smoking), diagnoses of lung or other diseases, results of liver function tests, results of current and/or previous spirometric investigations, treatment for pulmonary disease (including replacement therapy), liver or lung transplantation, and occupation. After inclusion, a follow-up questionnaire was answered by the attending physician once a year till 1999, thereafter every 2 years.
In the present report, a smoker is defined as any subject who has smoked more than one cigarette per day for at least one year, or more than 20 packs of cigarettes during his/her life. Smoking history was available for all cases.
Diagnosis of AAT deficiency
Plasma AAT concentrations were initially determined by electroimmunoassay, and since 1989, by nephelometry. All Pi phenotypes were verified by isoelectric focusing at the Department of Clinical Chemistry, Malmö (Citation6). All individuals have been identified as phenotype PiZZ. Because DNA analysis is not performed, phenotypes PiZZ and PiZNull cannot be distinguished. A few possible cases of PiZNull, but no PiNullNull cases have been included in the registry. The Pi phenotype is available for all cases.
Lung function
Lung function tests were performed at the local hospitals. The results of the lung function tests are expressed as the percentage of predicted values according to European reference tables (Citation7). The FEV1/FVC ratios are expressed as absolute values. The results of the first spirometry at inclusion in the registry were analyzed. Only pre-bronchodilator values were analyzed, because a reversibility test with a bronchodilator was not consistently performed.
Causes of death
The date of death was obtained from the Swedish Registry of Deaths. Vital status was available for all patients until February 1, 2014. For each death, the medical records from the hospital at which the death occurred were reviewed to determine the causes of death.
Statistical analysis
Continuous variables were described as means, range and Standard Deviations. The comparison of continuous variables were analysed by ANOVA. The Tukey Honest Significant Difference (HSD) test was used for multiple comparisons. Statistical significance was defined as a two-sided p-value of < 0.05. Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS 21) software.
Results
Register increase
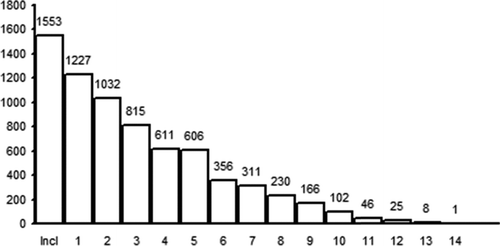
Until February 2014, a total of 2387 PiZ individuals have been diagnosed at the DCC, Malmö. Of these, 160 were below 18 years of age, 354 had died before inclusion in the register, and 320 could not been contacted for various reasons (e.g., had moved abroad, did not respond, were not interested). The remaining 1553 PiZZ individuals were included in the registry. They have been followed-up up to 14 times. Figure shows the number of patients per follow-up. The mean interval between diagnosis of AAT deficiency and inclusion in the register was 6 years (range 0–37 years).
Figure 1. Number of PiZZ individuals included in the Swedish national AAT register, and the number of subjects by follow-ups.
Demographic data, mode of identification, and lung function by smoking subgroups at inclusion are summarized in Table . Eighty five of the 128 current smokers (66%) quitted smoking during the follow-ups. Fifty-seven percent of the subjects were identified for reasons other than respiratory symptoms. The “other diseases” (26%) included renal diseases, joint symptoms, repeated infections other than respiratory tract infection, high sedimentation rate, or other signs and symptoms for which plasma protein analysis has been performed as part of the clinical investigation. In these individuals low plasma AAT concentration was found by plasma protein analysis (“electrophoresis”), which is a test frequently ordered in Sweden in investigations of a number of pathological conditions.
Table 1. Demographic data of the PiZZ individuals at inclusion in the Swedish AAT deficiency register
Twenty-four percent of the individuals were identified by screening, either population screening or family screening. Thus, a total of 205 PiZZ siblings were included in the registry. They represented 98 families. Two families had four siblings, and five families had three siblings.
Lung function at inclusion
One hundred and twenty-seven current smokers, 711 ex-smokers and 689 never-smokers had undergone spirometry at inclusion. Current and ex-smokers had significantly lower lung function than never-smokers (p < 0.001), Table .
Fifty-one percent (n = 787) of participants included in the register fulfilled the criteria for COPD, i.e., FEV1/FVC ratio < 0.70. Among the never-smokers, 28% (n = 194) had COPD. According to the GOLD criteria, 23% (n = 44) had GOLD stadium I, 12% (n = 82) GOLD stadium II, 8% (n = 53) GOLD stadium III, and 2% (n = 15) GOLD stadium IV. Among the ex-smokers, 72% (n = 515) had COPD. Eight percent (n = 42) had GOLD stadium I, 25% (n = 130) GOLD stadium II, 56% (n = 289) had GOLD stadium III, and 11% (n = 54) had GOLD IV.
Among the current smokers, 61% (n = 78) fulfilled the criteria for COPD. Ten percent (n = 8) had GOLD stadium I, 31% (n = 24) GOLD II, 28% (n = 22) GOLD stadium III, and 31% (n = 24) had GOLD stadium IV.
Physician-reported diseases at inclusion
Respiratory diseases
Respiratory diseases and symptoms were reported in 971 subjects (63%) at inclusion. Among them, 487 (50%) had a diagnosis of emphysema with or without chronic bronchitis, 215 (22%) had chronic bronchitis, 176 (18%) had asthma, 32 (3%) had bronchiectasis, 20 (2%) had lung fibrosis, and 32 subjects (3%) had other respiratory symptoms.
Liver diseases
The diagnosis of liver disease was reported in 65 subjects (4%). Liver cirrhosis was reported in 24 patients, hemochromatosis in 11, liver steatosis in 2, hepatocellular carcinoma in 2, hepatomegaly in 2, portal fibrosis in 1, Cholangitis in 1, and elevated liver enzymes without clinical symptoms in 22 patients. The prevalence of hemochromatosis is approximately 3.3% in Sweden. No data are available regarding prevalence of the other liver diseases in Sweden.
Other diseases
Inflammatory bowel disease (IBD) was reported in 23 PiZZ patients (1.5%). The prevalence of IBD in Sweden is 0.65%. The diagnoses of IBD were ulcerative colitis in 15 patients, and Crohn's disease in the remaining 8 patients. Panniculitis was reported in 2 patients (0.1%).
Fourteen patients (0.9%) had a diagnosis of c-ANCA positive Vasculitis, its prevalence in Sweden being 0.1/100 000 (0.0001%).
Rheumatoid arthritis was reported in15 patients (1%), and Morbus Bechterew in 4 patients (0.3%). In the general population in Sweden, the prevalence of rheumatoid arthritis is 0.6%, and that of Mb Bechterew is 5/100000 (0.00005%). Psoriasis was reported in 16 patients (1%), the prevalence in Sweden being 2%. Diabetes mellitus was reported in 30 patients (1.9%), and hypothyroidism in 16 patients (1.0%).
Augmentation therapy with AAT derived from human plasma
At inclusion 32 patients with COPD were treated with human AAT. Nine of them had undergone lung transplantation, and 15 had died during the follow-up. Six patients have discontinued the treatment for other reasons. In February 2014, two patients were on substitution therapy in Sweden. One of the two patients with Panniculitis has also been treated with human AAT during the follow-up.
Causes of death
During the follow-up period 451 (29%) of the patients died. Medical records were available for 419 decedents (93%). Autopsies were performed in 67 cases (16%). The main causes of death were respiratory diseases including respiratory failure and infections in 230 patients (55%). Liver diseases accounted for13% of deaths (n = 55) and other diseases for 32% (n = 134). Hepatic causes were liver failure in 23 decedents, hepatocellular carcinoma in 22, hepatic complications such as gastrointestinal bleeding in 7 and intra-abdominal infections in 3 decedents. The other diseases were cardio/cerebrovascular accident in 68, malignancy in 44, gastrointestinal bleeding and intra-abdominal infections in 13, suicide/accident in 6 and renal failure in 3 patients.
Discussion
The most important findings in the present analysis of PiZZ individuals included in the Swedish AAT registry are the relatively high detection rate and the substantial proportion of individuals identified during investigations for reasons other than the presence of respiratory symptoms.
The Swedish AAT registry differs from the other national AAT registries on several points (Citation8,9). First, the proportion of never-smokers is higher (45%) than in the other national registries. Second, a large proportion (57%) of the subjects is identified for reasons other than lung disease. The accuracy of the results drawn from the registry is increased by the fact that the majority of the patients are identified for reasons other than respiratory symptoms. Third, the Pi phenotype is available for all cases.
The prevalence of severe AAT deficiency being 1/1600 in Sweden, only approximately 20% of all 5000 adult PiZZ individuals are included in the AAT register. However, the identification rate is high compared with the other national registries, in which only 5–10% of PiZZ individuals are included.
There are several possible explanations for the relatively high identification rate in Sweden. First, the use of plasma protein analysis (“electrophoresis”) as a screening test in investigations of a variety of clinical symptoms other than lung disease results in fortuitous identification of a large number of AAT-deficient individuals. Second, a high level of local scientific interest in this deficiency since the 1960s, when AAT deficiency was discovered.
Since the 1980s, when Pi phenotyping by isoelectric focusing was developed at the laboratory in Malmö, the results of all individuals with the PiZ phenotype have been kept on record, which facilitated the initial recruitment of patients, and led to a rapid growth of the register. Furthermore, there is good cooperation with physicians who report data to the register.
A higher prevalence of the deficiency state in Sweden than in other countries might also be a contributory factor regarding the relatively high detection rate. It is well known that the PiZ gene frequency is highest in southern Scandinavia (Citation10), its prevalence being almost identical in Denmark and in Sweden. It is considerably lower in the United States (1/2,857 to 1/5,097) (Citation11), though the difference in prevalence is insufficient to explain the low detection rate in the United States.
The probability of identifying manifestations other than lung disease will increase when a large number of subjects without respiratory symptoms are included. Wegener's granulomatosis is a rare manifestation, which seems to be over-represented (14/1553). Our results support previous findings of a strong link between the PiZ gene and multisystemic vasculitic syndromes, c-ANCA-positive Vasculitis in particular (Citation12,13). Ulcerative colitis and rheumatoid arthritis are other inflammatory disorders that were over-represented, as compared with their prevalence in the general population. However, these high figures may be caused by selection bias because plasma protein analysis is often ordered for patients with symptoms leading to these diagnoses.
Fifty-one percent of the participants in the register fulfilled the criteria for COPD at inclusion. However, 28% of the never-smokers also had COPD at inclusion. This finding can be explained by the long time between the diagnosis of AAT deficiency and the inclusion in the register, the difference in mean age at diagnosis vs. inclusion in the registry being 8 years (37 years vs. 45 years).
The range in age at death is also high, and varies between 20 and 95 years. The high mean age at death of 73 years in never-smokers indicates that the prognosis of severe AAT deficiency is better than many previously published studies have described (Table ). Our previously published data have also shown that never-smoking PiZZ individuals identified by screening have the same life expectancy as the Swedish general population (Citation14). As expected, respiratory diseases are the most common cause of death (55%) in PiZZ individuals, followed by liver diseases (13%). Also these results are in accordance with our previously published data (Citation15).
In Sweden, only a few patients have been treated with intra venous substitution with human, purified AAT. Since 2007, the treatment is licensed in Sweden, but it is not reimbursed by the health care system. If a chest physician prescribes the treatment, the hospital has to cover the cost of the treatment. Until today, no decision is made to reimburse the cost of the treatment by the authorities despite some positive results of the placebo-controlled trials have been published (Citation16,17).
As all national AAT registries, also the Swedish register has weaknesses. Because only 20% of all adult PiZZ individuals in Sweden are identified, the conclusions drawn from the register do not present a real epidemiological data. However, the Swedish AAT registry is less biased regarding lung function and respiratory diseases than other national registries because the majority of subjects are identified for reasons other than respiratory symptoms. All smoking habits are self-reported by the patients. No objective tests, as the analysis of cotinine, have been used to identify smoking. The diagnoses are reported by the attending physician, and therefore under-reporting of many diseases is possible.
Conclusions
The detection rate of severe AAT deficiency is relatively high in Sweden, 20% of all AAT-deficient adults being included in the national register. The high detection rate contributes to the identification of a large number of never-smoking subjects without respiratory symptoms.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors gratefully acknowledge the expert secretarial assistance provided by Isabella Björk, and the reporting of patient data to the national AAT deficiency register undertaken by all Swedish physicians.
Funding
This work was supported by grants from The Swedish Heart-Lung Foundation and The Swedish Society of Medicine.
References
- American Thoracic Society/European Respiratory Society Statement. Standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168:818–900.
- Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med 1976; 294:1316–1321.
- Piitulainen E, Tornling G, Eriksson S. Effect of age and occupational exposure to airway irritants on lung function in non-smoking individuals with alpha 1-antitrypsin deficiency (PiZZ). Thorax 1997; 52:244–248.
- WHO. α1-Antitrypsin deficiency: Memorandum from a WHO meeting. Bull World Health Organ 1997; 75:397–415.
- Stockley RA, Luisetti M, Miravitlles M, Piitulainen E, Fernandez P. Alpha-1-antitrypsin deficiency International Registry group. Ongoing research in Europe: Alpha-1-antitrypsin deficiency International Registry (AIR) objectives and development. Eur Respir J 2007; 29:582–586.
- Jeppsson JO, Franzen B. Typing of genetic variants of alpha 1-antitrypsin by electrofocusing. Clin Chem 1982; 28:219–225.
- Quanjer P. Standardized lung function testing. Report working party. Bull Eur Physiopathol Respir 1983; 19(Suppl 5):1–95.
- Seersholm N, Kok-Jensen A, Dirksen A. Survival of patients with severe alpha 1-antitrypsin deficiency with special reference to non-index cases. Thorax 1994; 49:695–698.
- McElvaney NG, Stoller JK, Buist AS, Prakash UB, Brantly ML, Schluchter MD, Crystal RD. Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute Registry of alpha 1-antitrypsin deficiency. Alpha 1-Antitrypsin Deficiency Registry Study Group. Chest 1997; 111:394–403.
- Hutchison DCS. α1-antitrypsin deficiency in Europe: geographical distribution of Pi types S and Z. Respir Med 1998; 92:367–377.
- Stoller JK. Clinical features and natural history of severe α1-antitrypsin deficiency. Roger S Mitchell Lecture. Chest 1997; 111:123–128.
- Elzouki A-N, Segelmark M, Wieslander J, Eriksson S. Strong link between the alpha1-antitrypsin pizza allele and Wegener's granulomatosis. J Intern Med 1994; 236:543–548.
- Segelmark M, Elzouki A-N, Wieslander J, Eriksson S. The PiZZ gene of α1-antitrypsin as a determinant of outcome in PR3-ANCA-positive vasculitis. Kidney International 1995; 48:844–850.
- Tanash HA, Nilsson PM, Nilsson JA, Piitulainen E. Clinical course and prognosis of never-smokers with severe alpha-1-antitrypsin deficiency (PiZZ). Thorax 2008; 63: 1091–1095.
- Tanash HA, Nilsson PM, Nilsson JA, Piitulainen E. Survival in severe alpha-1-antitrypsin deficiency (PiZZ). Respir Res 2010; 11:44.
- Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DCS, Ulrik CS, Skovgaard LT, Kok-Jensen A, Rudolphus A, Seersholm N, Vrooman HA, Reiber JHC, Hansen NC, Heckscher T, Viskum K, Stolk J. A randomized clinical trial of alpha-1 antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999; 160:1468–1472.
- Dirksen A, Piitulainen E, Parr DG, Deng C, Wencker M, Shaker SB, Stockley RA. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha-1 antitrypsin deficiency. Eur Respir J 2009; 33:1345–1353.
