Abstract
The assessment of biomarkers in biological samples from the lung has long been employed. Upon cooling water vapor present in exhaled breath, variable amounts of droplets of condensate (EBC) containing volatile and non-volatile compounds may be easily and non-invasively obtained from patients of any age.Objective of the present study was to compare the level of EBC conductivity determined for cohorts of individuals with different inflammatory lung disorders with that of healthy never-smoking individuals.The conductivity in EBC of PiZZ-Alpha-1-antitrypsin deficiency patients with a diagnosis of emphysema (PiZZ-AATD) was 3 fold lower than in spouse controls (54.5 ± 11.6 vs 165.3 ± 10.7 μS/cm). Non-PiZZ emphysema patients had conductivity in EBC of 59.6 ± 5.8 μS/cm and patients with sarcoidosis without airflow obstruction had EBC conductivity of 178,8 ± 6,2 μS/cm, not significantly different (p = 0.5) from healthy controls. Conductivity in serial EBC samples from patients with PiZZ-AATD emphysema and healthy controls was stable in 6 different samples collected over a period of 14 months. We conclude that conductivity values in EBC can be used as a correction factor for dilution of non-volatile components in EBC.
Abbreviations
AIR alpha1 international registry
COPD chronic obstructive pulmonary disease
EBC exhaled breath condensate
PiZZ-AATD PiZZ alpha-1-antitrypsin deficiency associated with pulmonary emphysema.
Introduction
The Netherlands started collecting baseline and prospective data right from the beginning of the web-based AIR patient database in 1998 (Citation1). Recorded data were obtained from local physicians or from patient visits at Leiden University Medical Center. To date, a total of 450 genotyped ZZ alpha-1-antitrypsin deficient patients are registered and 35 genotyped rare Null variants, including Heerlen, Bredevoort, Bellingham and Granite Falls. In the past 15 years, the registry has mainly been used to recruit patients for randomized placebo-controlled studies with new treatment regimens (Citation2). However, patients were also recruited for assessing the value of new biomarkers for severe alpha-1-antitrypsin deficiency associated with pulmonary emphysema (Citation3). The details of one such study are reported next.
The assessment of biomarkers in biological samples from the lung has long been employed. Sampling the broncho-alveolar compartment provides an opportunity to analyze the deep lung pathophysiological processes in PiZZ alpha-1-antitrypsin deficiency associated with pulmonary emphysema (PiZZ-AATD) (Citation4). A concern in using broncho-alveolar lavage or sputum induction by hypertonic saline in this patient population is the induction of additional inflammation in this condition, which is known for its relative high inflammatory burden in the lung (Citation4). In an effort to find more gentile approaches for patients, exhaled breath condensate (EBC) was evaluated as an attractive alternative to the fluids mentioned before (Citation5). In fact, upon cooling water vapour present in exhaled breath, variable amounts of droplets of condensate containing volatile and non-volatile compounds may be easily and non-invasively obtained from patients of any age.
Much of the variation in the concentration of mediators is, in fact, related to the dilution of respiratory droplets by the water of vaporization which requires a correction factor (Citation6). There is reason to believe that assessment of conductivity in EBC is an attractive correction factor (Citation6). In fact, measurement of EBC conductivity following collection and removal by lyophilisation of volatile NH4+ (the most abundant cation in the condensate), provides a good estimate of droplets dilution caused by water vapour. Objective of the present pilot study was to compare the level of EBC conductivity determined for cohorts of individuals with different inflammatory lung disorders with that of healthy never-smoking individuals.
Materials and methods
Reagents
Doubly distilled water used for reconstituting all EBC samples was obtained from a Millipore (Bedford, MA, USA) Milli-Q purification system. Unless otherwise stated, all other chemicals were of analytical grade and were used without further purification.
Subjects
A total of 31 patients who suffered from three different pulmonary diseases were enrolled for this study. All of them were enrolled by the Department of Pulmonology from Leiden University Medical Center, The Netherlands. The study protocol was approved by the Research Ethics Committee of this institute and each subject gave his informed consent before entering the study. Seven were PiZZ-AATD patients with pulmonary emphysema (4 men and 3 women; 51 ± 3 y; BMI 23 ± 3; FEV1 45 ± 3% pred; SVC 95 ± 4% pred and DLCO 53 ± 5% pred) were recruited from the Dutch AIR database; 18 were non-AATD COPD patients (10 male and 8 female; mean age 66 ± 7 y; BMI 25 ± 3; FEV1 53 ± 4% pred; SVC 96 ± 5% pred; DLCO 48 ± 6% pred);and 6 were patients with sarcoidosis, stage II (never smokers, 4 male and 2 female; mean age 59 ± 12 y; BMI 24 ± 2; FEV1 85 ± 7% pred; SVC 92 ± 3% pred; DLCO 87 ± 3% pred), both from LUMC Outpatient Clinic.
Diagnosis of sarcoidosis was confirmed by cytological assessment obtained by transbronchial lymph node sampling using EBUS. In addition, non-smoking healthy volunteers (7 subjects, 3 male and 4 female; mean age; 54 ± 8 years; BMI 29 ± 3; FEV1 92 ± 4% pred; SVC 94 ± 4% pred; DLCO 98 ± 3% pred, who were the spouses of the AATD patients) had no significant history of respiratory diseases, were analyzed as controls.
PiZZ-AATD patients and their spouses produced EBC at baseline and at 5 consecutive visits: week 2, 12, 24, 36 and 60 after baseline. The study protocol was approved by the Ethical Board of LUMC and all study participants gave written informed consent.
Sample collection
Exhaled breath condensate was collected using an RTube kit (Respiratory Research Inc., USA). Briefly, each subject was asked to breath for 10 minutes at tidal volume through a mouth piece into a polypropylene tube that was placed in an aluminum sleeve (Citation7). The latter was stored frozen at −40°C overnight before collection was started. This procedure allowed vapors, aerosols and moisture in exhaled breath to condense along the walls of the tube. Subjects were asked to breath in this device while wearing a nose clip. The volume of condensate collected was typically 1.0 ± 0.2 mL for controls and around 600–800 μL for (AATD) patients. This material was aliquotted and stored immediately at −80°C until analysis. Although the design of the system prevented salivary contamination, all EBC samples collected were examined for amylase activity by using the alpha-amylase ESP 1491300 kit (detection limit 0.003 U/mL; Boehringer Mannheim, Germany). Protein concentration in EBC was determined by using the micro BCA protein assay (Pierce, Rockford, USA).
Sample analysis
To remove all of the NH4+, each condensate was lyophilized to dryness in a SpeedVac concentrator system. The micro BCA assay performed before and after lyophilization (to check whether proteins were lost) confirmed that the protein concentration did not change upon lyophilization. EBCs were then reconstituted in 1 ml of doubly distilled water and conductivity was calculated by using the Electrochemical Impedance Spectroscopy (EIS) technique. Each measurement was carried out at room temperature with a Frequency Response Analyzer (FRA), model Solartron 1255 (Solartron Analytical, Farnborough, HAM, UK), in a frequency range between 0.1 Hz and 1 MHz at a potential of 50 mV. With the resistance values (Ω), obtained from the plots of complex impedance, given the value of the cell constant (k = 0.75 cm−1), it was possible to calculate the conductivity (S) of the samples by applying this relation:. Measurement of conductivity in each sample was done in triplicate.
Measurement of conductivity in each sample was done in triplicate.
Statistical analysis
Values are expressed as mean ± SD, unless otherwise mentioned. Statistical significant differences between groups were analyzed by ANOVA and significance set at p < 0.05.
Results
All subjects were able to deliver an adequate EBC sample with a mean volume of 500 μL for patients and 1 mL for controls. The conductivity in EBC of PiZZ-AATD patients was 3 fold lower than in spouse controls (54.5 ± 11.6 vs 165.3 ± 10.7 μS/cm). Next, we investigated COPD patients without AATD. The conductivity in their samples was 59.6 ± 5.8 μS/cm. Patients with an inflammatory lung disease but without airflow obstruction, like in sarcoidosis, had EBC conductivity of 178.8 ± 6.2 μS/cm, not significantly different (p = 0.5) from healthy controls (Figure ).
Figure 1. A. Conductivity in single EBC samples from healthy controls (spouses of AATD patients, n = 7), patients with sarcoidosis (stage II, n = 6), non-AATD COPD patients (n = 18) and PiZZ-AATD patients (n = 7). B. Conductivity in serial EBC samples from spouse controls (upper line) and PiZZ-AATD patients obtained at week 2, 12, 24, 36 and 60 after baseline visit.
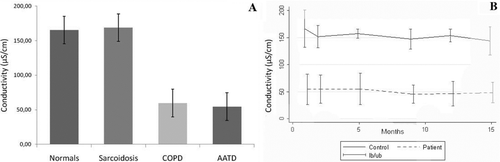
As a further step, we assessed the level of conductivity in EBC samples from PiZZ-AATD patients and from healthy spouses on 6 different occasions over a period of 14 months. The conductivity in EBC was fairly stable within the period investigated both in patients and in the spouse controls (Figure ).
In EBC from PiZZ-AATD subjects and in samples of their healthy spouse controls the conductivity correlated significantly (p = 0.001) with the level of alveolar ventilation (R2 = 0.8327), but not with FVC and FEV1 (Figure , panels A, B and C, respectively).
Figure 2. Correlation at baseline visit (V1) between alveolar volume (VA, 2A) obtained during single breath gas transfer measurement (in Liters on Y-axis), FVC (2B) and FEV1 (Figure C) and conductivity (μS/cm) in serial EBC samples from spouse controls (left panel) and PiZZ-AATD patients (right panel) obtained at week 2, 12, 24, 36 and 60 after baseline visit.

Discussion
Here we show for the first time the stability of conductivity in EBC in serial samples in both PiZZ-AATD emphysema patients and healthy spouse controls. This allows the future use of conductivity in EBC samples for correcting protein content or other chemical components by applying a dilution factor to generate the actual concentration of non-volatile content in EBC.
The use of the Dutch part of the AIR patient database is another example of its usefulness to rapidly conduct clinical studies in this rare patient population.
It is assumed that EBC is mainly derived from the airways (Citation8), and suggests it may be an ideal “container” of mediators which are likely to reflect the composition of the airway-lining fluid. If this assumption proves correct, the potential of EBC of acting as a diagnostic tool in clinical analysis can be expected to improve dramatically. We addressed the issue on how to approach variations in biomarker's concentration. First, great attention was turned to the optimization of methods of collection (Citation7).
Much of the variation in the concentration of non-volatile mediators is, in fact, related to the dilution of respiratory droplets by the water of vaporization (Citation6). Our data show a distinction of conductivity in samples obtained from patients with airflow limitation and those without such limitation. Clearly, the volume of alveolar ventilation correlated with the conductivity value in EBC and suggests that a correction factor is influenced by air from the deep lung. This is also reflected by the conductivity data in EBC from patients with stage II sarcoidosis. The lungs of such patients contain an ongoing inflammatory process. Yet the conductivity in their EBC was similar to that of healthy controls, suggesting again that ventilation is an important contributor to the conductivity in EBC fluid, despite the presence of inflammation in sarcoid lungs.
Being electrical conductivity of EBC given by the ions present in each sample, it quantifies ion content. Thus, measurement of conductivity is used to estimate the total concentration of the respiratory electrolytes in the condensates. However ammonia, heavily influenced by oral contamination, has been shown to be responsible for a great part of the electrical conductivity of EBC. For this reason, ammonia must be first removed by lyophilization and conductivity is determined in samples resuspended in bi-distilled water, thus being a measure of non-volatile ions and allowing to estimate EBC dilution by water vapour.
Conductivity is measured in milli (micro) Siemens/cm and the values we have reported are “normalized,” i.e., they consider the different volumes of original EBCs. As “reference” value we have used the conductivity value of the 150 mM NaCl solution, that is the plasma physiological solution. There may be other confounders that affect the level of conductivity, but our data on conductivity in serial EBC samples suggest that, if they are present, they are fairly stable over a 14-month period of time.
Previously, it was reported that care must be exercised when interpreting mediator measurements in exhaled breath condensate and that assays must be validated at concentrations relevant to those found within the biological fluid (Citation5). The lower limit of quantification of any biomarker is central to the measurement of mediators at these concentrations using current ELISA methodology. Recently, we used an exploratory proteomic approach aimed at generating fingerprints of biomarkers in EBC of subjects with alpha-1-antitrypsin deficiency related emphysema (Citation9). This exploratory study resulted in the generation of a panel of EBC proteins and provided a qualitative (not quantitative) impression of identified proteins, which are supposed to originate directly from the airways and the lung. Application of conductivity values as a correction factor for dilution may assist in the quantification of such results.
Funding
Funding for this study was obtained from Leiden University Medical Center and University of Pavia.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stockley RA, Luisetti M, Miravitlles M, Piitulainen E, Fernandez P, Alpha One International Registry (AIR) group. Ongoing research in Europe: Alpha One International Registry (AIR) objectives and development. Eur Respir J 2007; 29(3):582–586.
- Stolk J, Stockley RA, Stoel BC, et al. Randomised controlled trial for emphysema with a selective agonist of the γ-type retinoic acid receptor. Eur Respir J 2012; 40(2):306–312.
- Fregonese L, Ferrari F, Fumagalli M, Luisetti M, Stolk J, Iadarola P. Long-term variability of desmosine/isodesmosine as biomarker in alpha-1-antritrypsin deficiency-related COPD. COPD 2011; 8(5):329–333.
- Luisetti M, Piccioni PD, Donnetta AM, Bulgheroni A, Peona V. Protease-antiprotease imbalance: local evaluation with bronchoalveolar lavage. Respiration 1992; 59 Suppl 1:24–27.
- Bayley DL, Abusriwil H, Ahmad A, Stockley RA. Validation of assays for inflammatory mediators in exhaled breath condensate. Eur Respir J 2008; 31(5):943–948.
- Effros RM, Biller J, Foss B, et al. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med 2003; 168 (12):1500–1505.
- Horváth I, Hunt J, Barnes PJ, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 2005; 26(3):523–548.
- Bondesson E, Jansson LT, Bengtsson T, Wollmer P. Exhaled breath condensate-site and mechanisms of formation. J Breath Res 2009; 3:752–755.
- Fumagalli M, Ferrari F, Luisetti M, et al. Profiling the proteome of exhaled breath condensate in healthy smokers and COPD patients by LC-MS/MS. Int J Mol Sci 2012; 13(11):13894–13910.
