Abstract
Individuals with Alpha-1 antitrypsin deficiency (AATD) have mutations in the SERPINA1 gene causing genetic susceptibility to early onset lung and liver disease that may result in premature death. Environmental interactions have a significant impact in determining the disease phenotype and outcome in AATD. The aim of this study was to assess the impact of smoke exposure on the clinical phenotype of AATD in Ireland.
Clinical demographics and available thoracic computerised tomography (CT) imaging were detected from 139 PiZZ individuals identified from the Irish National AATD Registry. Clinical information was collected by questionnaire. Data was analysed to assess AATD disease severity and evaluate predictors of clinical phenotype.
Questionnaires were collected from 107/139 (77%) and thoracic CT evaluation was available in 72/107 (67.2%). 74% of respondents had severe Chronic Obstructive Pulmonary Disease (COPD) (GOLD stage C or D). Cigarette smoking was the greatest predictor of impairment in FEV1 and DLCO (%predicted) and the extent of emphysema correlated most significantly with DLCO. Interestingly the rate of FEV1 decline was similar in ex-smokers when compared to never-smokers. Passive smoke exposure in childhood resulted in a greater total pack-year smoking history. Radiological evidence of bronchiectasis was a common finding and associated with increasing age.
The Irish National AATD Registry facilitates clinical and basic science research of this condition in Ireland. This study illustrates the detrimental effect of smoke exposure on the clinical phenotype of AATD in Ireland and the benefit of immediate smoking cessation at any stage of lung disease.
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an autosomal co-dominant inherited condition that results in reduced circulating levels of AAT protein and predisposes affected individuals to early onset lung and liver disease. It was first described by Laurell and Eriksson in 1963(Citation1) and has since been recognised as one of the most common genetic conditions affecting people of Western European descent.
Despite its prevalence AATD is a condition that is rarely diagnosed. Detection rates in some countries are less than 10% of the at risk population (Citation2), this may relate to low awareness of AATD among physicians which can often lead to significant delays before the diagnosis is reached (Citation3). The WHO, ATS and ERS advocate targeted testing for AATD in all individuals with COPD, non-responsive asthma, cryptogenic liver disease and first degree relatives of individuals with AATD (Citation4). This approach has led to higher rates of detection in the populations most at risk of lung disease, though widespread under recognition of the condition remains (Citation5,6).
The development of national registries for individuals with AATD can address many of the shortcomings in our knowledge of the disorder; facilitate understanding of the natural history of AATD; promote patient education and dissemination of information; aid recruitment for clinical research studies; and assist international collaboration with colleagues through research initiatives (Citation7,8). In cystic fibrosis, a disease with a similar prevalence, the establishment of registries has led to significant improvements in patient related outcomes (Citation9).
Irish registry experience
The establishment of the Irish National AATD Targeted Detection Programme in 2004 facilitated an increased detection rate of AATD, and in 2007 the National AAT Registry was created. The registry is maintained by the Irish Alpha One Foundation. The national referral centre for AATD in Ireland is based in Beaumont Hospital, Dublin. Referrals are received from across the island of Ireland covering a population of 6.38 million people. Given the relatively small geographic size of the country, all individuals with AATD can potentially be assessed in a single centre.
Our programme has detected over 12,000 individuals, leading to the diagnosis of 250 PiZZ and 185 PiSZ individuals. In addition to the common AATD alleles, we have identified a number of rare mutations including two newly described null mutations, a Null/Null homozygote and MMalton/MMalton homozygote (Citation10). In the past year our registry information technology systems have been upgraded to enable more efficient data entry and extraction. In addition our website has been updated to be more user friendly and expand the resources and information available for individuals and family members of those affected by AATD (www.alpha1.ie).
AATD epidemiology in Ireland
The gene frequency for the Z protein is most prevalent in northern and western European countries with severe deficiency (PiZZ) affecting up to 1:1500 (Citation2), though it is less common in countries of predominantly Western European descent such as regions of North America and the Antipodes (Citation11,12). In Ireland 1:2104 individuals are PiZZ homozygotes, though in our targeted detection program 1:71 tested were PiZZ homozygotes highlighting the effectiveness of the targeted detection approach (Citation5).
The detection of asymptomatic individuals (non-index) through the family screening of probands is an opportunity to better understand the natural history of AATD (Citation13,14). Targeted detection will invariably detect many MZ heterozygotes and clarification of the true risk of lung disease in this population is important, particularly in smokers. Utilising index cases identified from the Irish Registry, it has been established that ever-smoking PiMZ individuals have an increased risk for COPD and this risk is attenuated in never-smokers (Citation15).
Clinical trial research
Augmentation therapy for AATD was approved for clinical use in Ireland in 2007; however, it remains inaccessible for patient use due to concerns about the significance of its clinical efficacy, the high cost of treatment, and lack of reimbursement for treatment by the Health Service in Ireland. The Irish National AATD Registry has proven to be an excellent resource for the recruitment of Irish patients with severe AATD into clinical trials. Our centre continues to participate in a number of international randomised controlled clinical trials, including intravenous AAT augmentation therapy (NCT00261833, NCT00670007) and nebulised AAT therapy (NCT01217671). The facilitation of clinical trial research into AATD in Ireland raises awareness of AATD, advances scientific knowledge, and importantly facilitates earlier access to augmentation therapy for patients affected by the condition.
Original Research
Aim
To assess the impact of smoke exposure on the clinical phenotype of AATD in Ireland.
Methods
Study population
The National AATD registry was used to identify all individuals with a confirmed PiZZ phenotype currently living in Ireland in January 2014. Registry participants who had undergone full clinical evaluation within the past 5 years at the national referral centre in Beaumont Hospital were selected for this study and clinical demographics were recorded. All participants provided written informed consent, which was approved by the Beaumont Hospital Research Ethics Committee.
Pulmonary function testing was performed in all participants according to American Thoracic Society standards (Citation16); post bronchodilator FEV1, FVC percent predicted, and gas transfer (DLCO) measured by the single breath carbon monoxide method were recorded. Pulmonary function test results from the preceding five years were recorded; the annualised rate of FEV1 decline was determined by regression analysis in those with three or more sequential results over a time period of one year or more.
Questionnaire
Cross-sectional data was collected from the study participants via a self reported questionnaire that was distributed by post, questionnaires were also distributed in the outpatient clinic. Each returned questionnaire was coded to enable matching to the relevant individual's clinical and radiological information and subsequently anonymised for analysis. Clinical parameters were recorded; age of diagnosis, symptomatic detection (index) vs. family screening (non-index), smoking status, passive smoke exposure, occupational exposure, frequency of pulmonary exacerbations, cough, sputum production, and oxygen use.
Dyspnoea scores were calculated using the 5-point modified Medical Research Council score (mMRC) and health status in the previous week was determined using a visual analogue scale (VAS) scored between 0 and 100. Pack-year smoking history was determined by the function of number of cigarettes smoked per day and total years smoked.
Radiology
High resolution CT images were obtained on a Siemens 16-slice scanner. All patients were imaged while supine and inspiratory images were obtained from the lung apices to the costophrenic angles. Scanning parameters were 120 kV and 90 mA. A modified version of Bhalla's scoring system for thin section CT in patients with AATD was applied to each scan (Citation17,18). All lobes were individually assessed for purposes of evaluating severity of emphysema, bronchiectasis and peribronchial thickening. All criteria were scored on a scale of 0–3. Once scores were assigned to each of the parameters, they were added to the patient's individual score to a maximum of 21. Higher scores therefore indicate greater severity. Two radiologists with a specialist interest in thoracic radiology reviewed all scans independently; a consensus opinion was then determined. Both radiologists were blinded to the clinical severity of AATD when scoring.
Statistical analysis
Data was analysed using GraphPad Prism v7.0 and STATA v13.0 was employed for stepwise multiple regression modelling. Statistical significance was determined as a two-tailed p-value < 0.05. Characteristics of the respondents were summarised using number and percentage of participants in each category. Pearson's correlation coefficients were used to identify significant bivariate relationships. To determine factors that influence the severity of disease, significant relationships identified on univariate analysis were entered as independent variables into a stepwise multiple regression analysis with ascertainment (index vs. non-index) and smoking status assessed separately as the dependent variables.
Results
Response
A total of 204 PiZZ individuals were identified from the National AATD Registry. Seven individuals (3.4%) died within the preceding twelve months. Questionnaires were distributed to 139 individuals who had attended Beaumont Hospital within the past 5 years. The response rate was 107/139 (77%) and clinical demographics were available for all respondents. Thoracic CT imaging was available in 72/107 (67.2%) and this was analysed separately. Then 21/107 (19.6%) of respondents were currently receiving intravenous AAT augmentation therapy.
GOLD classification
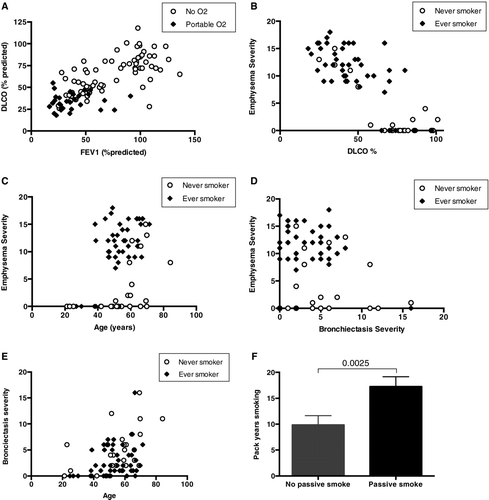
Respondents were classified according to the updated GOLD document (Citation19). Of 17 (16%) of respondents had no spirometric evidence of obstructive airway disease and were asymptomatic; 7(6.5%) were classified as GOLD group A, 4 (3.7%) group B, 33 (31%) group C and 46 (43%) as group D. Next, 83/107 (77.6%) of respondents reported usage of a combined corticosteroid/LABA inhaler, with 58/107 (54.2%) reporting inhaled LAMA usage. DLCO correlated well with FEV1 (r = 0.73, p < 0.0001), and emphysema (r = 0.83, p < 0.001), (Figures and 1B). Finally 31/107 (29%) of individuals used portable O2 and 17 of these were reported using long-term O2 therapy (LTOT). A DLCO value of <50% had a sensitivity of 96.7% and specificity of 76.3% to predict the requirement for portable O2 usage (Figure ).
Figure 1. (A) Correlation of DLCO with FEV1 (r = 0.73, p < 0.0001) and a DCLO value < 50% had a 96.7% sensitivity for the requirement for portable O2. (B) Significant correlation between emphysema severity and DLCO% predicted, r = 0.83, p < 0.0001. (C) Increased severity of emphysema detected at an earlier age is observed in smokers, milder emphysema severity is observed in never smokers occurring at a later age. (D) There was no significant relationship between severity of CT detected bronchiectasis and emphysema, r = -0.1664 (p = 0.1625). (E) Increased CT detected bronchiectasis with advancing age, no significant difference in ever-smokers vs. never smokers is observed. (F) Increased total pack-year smoking history in AATD individuals exposed to passive smoke in childhood (17.25 vs. 9.84 pack-years smoking, p = 0.003).
Exacerbations
Respondents reported a mean frequency of pulmonary exacerbations of 2.1/annum in the past year and 1.82/annum over a two-year period. Increased frequency of pulmonary exacerbation was associated with more severe impairment in FEV1, lower DLCO values and higher emphysema scores. Interestingly there was no association found with pulmonary exacerbations or radiologically assessed bronchiectasis, though some individuals with a more severe bronchiectatic phenotype did have a high number of exacerbations in the past 2 years.
The compliance rate with annual influenza vaccination programmes was high and increased on an annual basis over the preceding four years from 62/107 (58%) to 81/107 (75.7%). Uptake of the pneumococcal vaccine was also high at 76/107 (71%).
Radiological findings
A subgroup analysis of respondents was performed on those with available thoracic CT imaging in 72/107 respondents. The majority of respondents, 52/72 (72%), had radiological evidence of emphysema that was most severe in the lower lobes; smokers had more severe emphysema at an earlier age compared to never-smokers (Figure ). Radiological evidence of bronchiectasis was evident in 58/72 (80.6%) of respondents, this was mild in the majority of cases and there was no lobar preponderance. There was no correlation between the presence of emphysema and bronchiectasis, (r = -0.1664, p = 0.1625), (Figure ). Bronchiectasis increased with age (r = 0.41, p = 0.004), and while there was a similar age-adjusted prevalence of bronchiectasis in smokers and never-smokers, it was more significant in never-smokers (5.08 vs. 2.98, p = 0.02), (Figure ).
The emergence of different radiological phenotypes was evident; the individuals with the most severe bronchiectasis had little or no evidence of emphysema and those with the most severe emphysema had mild bronchiectasis. Multivariate regression analysis identified increasing age as the most significant risk factor for the development of bronchiectasis independent of smoking history (p < 0.001). Those with predominant radiological evidence of bronchiectasis reported less cough, sputum production and pulmonary exacerbations compared to those with evidence of emphysema indicating a milder clinical phenotype in this group.
Index versus non-index cases
Of the respondents 72/107 (67.3%) were index cases and the remaining 35 (32.7%) non-index cases were detected by family screening. Both groups were well matched in relation to symptoms of cough, sputum production, pulmonary exacerbations and health status measurement as both groups had a similar percentage of smokers and equal pack-year smoking history (see Supplementary Table 1). However on univariate analysis index cases appeared to have significantly lower FEV1 values (58% vs. 74%, p = 0.0273), DLCO values (51% vs. 64%, p = 0.0126) and a greater degree of airflow obstruction (47% vs. 58%, p = 0.01) despite similar rates of FEV1 decline (-36.4 mls/annum vs. -51.2 mls/annum, p = 0.49) (Supplementary Table ). In the univariate subgroup analysis of the thoracic CT data it was also found that index cases had higher mean emphysema scores (9.574 vs. 5.52, p = 0.011). However on adjustment for age, there was no significant difference observed in FEV1, airflow obstruction or emphysema between the groups (p = 0.51) (Supplementary Figure A). As both index and non-index cases had similar lifetime smoke exposure, the observed reduction in lung function in the index cases is explained by an older age in this group.
Table 1. Univariate analysis of ever-smokers vs. never-smokers
Supplementary Table 1. Univariate analysis of index vs. non-index cases
The effect of smoking
Of respondents, 71/107 (66.3%) reported a history of smoking for more than one pack-year. The majority of ever-smokers had quit prior to their diagnosis of AATD 37/71 (52%). The majority of people, 30/34 (88%), reported that a diagnosis of AAT helped them quit within a median time of 2 weeks. On univariate analysis ever-smokers had a marked reduction in FEV1, DLCO and degree of airflow obstruction, increased breathlessness, poorer health status, increased sputum production, and increased frequency of pulmonary exacerbations (Table ). Multivariate regression analysis identified the following independent variables in ever-smokers; increased emphysema, lower DLCO values, increased airflow obstruction and increased sputum production. This would be consistent with the classic phenotypes of emphysema and chronic bronchitis seen in COPD. Interestingly there was less bronchiectasis observed in the ever-smokers, a finding that persisted after multivariate analysis (-2.5, 95% C.I. -0.8 to -4.9, p = 0.047).
There were 36/107 (33.6%) never-smokers in this study with a mean FEV1 value of 83.8% and DLCO of 71%. There was no significant difference in the annualised rate of FEV1 decline between ever-smokers and never-smokers (Supplementary Figure B). This may be accounted for by a number of possible factors; all ever-smokers were now ex-smokers, there was a lower initial FEV1 in the smoker group, the modifying effect of medication use, and the survivor effects.
Subgroup analysis of never-smokers revealed that index cases have lower age adjusted FEV1 (75 vs. 95%, p = 0.042) and DLCO (63 vs. 83%, p = 0.006) values compared to asymptomatic never-smokers (Supplementary Figure C). Multivariate regression analysis confirmed the association for lower DLCO (-9.96%, 95% C.I -4.6 to -19.5%, p = 0.041), indicative of a more severe phenotype in symptomatic individuals with AATD (Supplementary Figure D).
Of the respondents 88/107 (82%) reported passive smoke exposure in childhood with a large proportion of these, 52/88 (59%), reporting parental passive smoke exposure. Parental smoke exposure did not emerge as an independent risk factor for poorer lung function in adulthood. This may be due to the high prevalence of parental smoking overall and insufficient power to detect a statistically significant difference in lung function in our study population.
Those who were exposed to passive smoke in childhood were more likely to smoke in adulthood, OR 2.650 (95% confidence interval 0.9645 to 7.279, p = 0.065), and had a significantly higher mean pack-year smoking history (17.25 vs. 9.84, p = 0.0025), see Figure . The impact on the workplace-smoking ban was assessed; 63/107 (59%) reported that passive smoke exposure had reduced as a result of the ban, 44/107 (41%) reported no change, while no subjects reported increased smoke exposure.
This data indicates that individuals with AATD who smoked have a similar clinical phenotype of COPD irrespective of their method of diagnosis (index vs. non-index). Symptomatic never-smokers have some mild impairment in pulmonary function that has brought them to medical attention, though they have a much milder clinical phenotype compared to those who ever-smoked. Importantly, our data shows that asymptomatic never-smokers have effectively normal lung function and no impairment in health status.
Occupational exposure
Most respondents, 55/107 (51.4%), were currently in full time employment. Then 28/107 (26.2%) of respondents reported that they had to change job or retire as a result of AATD, this group had significant impairment in lung function compared to those who continued in employment (mean FEV1 39% vs. 71%, p = 0.02). 47/107 (43.9%) of respondents reported occupational inhalational exposure during the course of their working life, the predominant exposure was to dust.
Discussion
Determination of clinical phenotype in AATD is essential for a greater understanding of the underlying pathophysiology of the disease, the correct stratification for research studies and therapy, and to prognosticate outcomes. The majority of respondents in this study had GOLD group D COPD reflecting the prevalence of a more severe phenotype within this AATD registry population. Objective quantification of pulmonary disease by thoracic CT imaging enables determination of the relationships between the primary pathophysiological process in AATD and health outcomes. In our study, pulmonary emphysema was the predominant radiological finding and it was primarily found in ever-smokers.
Regarding pulmonary function measurement, impairments in DLCO correlated most significantly with higher emphysema scores. A DLCO value below 50% predicted was found to be highly predictive of portable oxygen requirement and lower DLCO values correlated strongly with worsening dyspnoea and health status measurement outcomes. This implies that patients with DLCO values below 50% predicted may benefit from assessment regarding portable oxygen requirement. Recent recommendations regarding clinical trials in AATD suggest that serial CT densitometry be used as the primary endpoint to demonstrate stabilisation and prevention of progression (Citation20), however in clinical practice this is rarely feasible and our data would suggest that DLCO may be employed as a surrogate determinant of disease status.
There remains a significant proportion of smokers with AATD that have yet to be identified, and this may relate to widespread under recognition of the disorder in the medical profession (Citation3). In this study, no difference was observed between index and non-index cases in relation to pulmonary symptoms, measurements of lung function, and severity of emphysema. This is likely explained by the equivalent smoke exposure in both groups. Smoke exposure is the single biggest determinant for progression of emphysema in AATD. Our analysis of the influence of smoke exposure in AATD demonstrates the presence of emphysema, chronic bronchitis and resultant airflow obstruction with the resultant classic phenotypes of COPD presenting at a young age.
All respondents had stopped smoking at the time of this study. Individuals with AATD who quit smoking had similar rates of FEV1 decline compared to never-smokers despite initial lower FEV1 values. This is a matter of great encouragement to people with AATD who wish to give up smoking and reflects what is seen in non-hereditary emphysema.
To better understand the natural history of AATD in never-smokers, analysis of our registry revealed that lung function is normal and preserved beyond middle age in asymptomatic never-smokers. This is an important observation and it is consistent with published data suggesting they have a life expectancy approaching that of the general population (Citation13,Citation21). Though the confounding effects of genetic modifiers and environmental factors should be taken into consideration, never-smokers with symptomatic lung disease appear to have lower FEV1 and DLCO values indicating a more severe clinical phenotype than asymptomatic never-smokers. Our data implies that the natural history of AATD in never-smokers is altered at some point in symptomatic individuals, by an unknown precipitant, to worsen their condition and bring them to medical attention.
Bronchiectasis was recognised early to be a pulmonary complication of AATD, however the true prevalence and clinical significance of bronchiectasis in the AATD population remains poorly understood. Observational studies in populations of non-CF bronchiectasis have not demonstrated an increased prevalence of the condition implying that AATD as a cause of bronchiectasis is uncommon and that it occurs as a result of co-existent emphysema (Citation22). Our data would contradict these findings, demonstrating that radiologically detected bronchiectasis is both common in AATD and that there is no dependent relationship to emphysema or indeed a history of smoking. This is supported by similar studies on bronchiectasis and emphysema in AATD and also by registry data showing a high prevalence of bronchiectasis in the AATD population in Spain and Italy (Citation14,Citation23). Most people in our study had evidence of mild radiological bronchiectasis with minimal associated clinical symptoms such as increased frequency of cough or pulmonary exacerbation compared to those with emphysema, however those with more severe bronchiectasis did report significantly poorer health status outcomes. Determining why this occurs is an important area of research.
It has been 10 years since Ireland became the first country in the world to introduce a ban on smoking in the workplace (Citation24). Passive smoke exposure is a known risk factor for the development of emphysema and COPD, individuals with AATD are particularly at risk and have most to benefit from public health initiatives that reduce cumulative lifetime smoke exposure (Citation25). The fact that no individual reported an increase, and the majority reported a decrease, in passive smoke exposure speaks to the success of this programme pioneered in Ireland and emulated worldwide (Citation24). Apart from increasing the cumulative lifetime exposure to tobacco smoke, the results of this study indicate that passive smoke exposure in childhood, in particular parental smoking, influence smoking habits in adulthood by increasing the likelihood of ever-smoking and the total pack-year cigarette consumption. Further efforts to reduce passive smoke exposure in automobiles are welcome initiatives that are undergoing legislative implementation in some countries at present (Citation26).
The study of PiMZ individuals identified from probands in the Irish National AATD Registry has significantly improved our understanding of the risk of lung disease in this large population group (Citation15). A significant proportion of the severely deficient AATD population in the Irish registry are compound heterozygote PiSZ individuals, a finding reflected in reports from other national registries (Citation14). Despite a growing number of known PiSZ individuals there is a paucity of data in relation to outcomes of this AAT-deficient phenotype (Citation4). Airflow obstruction appears to be milder and less common compared to PiZZ individuals, however the PiSZ phenotype is associated with significant risk of COPD in smokers (Citation27).
Inclusion of rare SERPINA1 mutations in national registry databases permits epidemiological study regarding allele frequency and facilitates research collaboration to gain a deeper understanding of the effects of abnormal AAT protein production. Centres with expertise in genetic sequencing techniques have included increasing numbers of rare mutations identified through targeted detection programs (Citation28). In the Irish National Targeted Detection Programme, approximately 1.5% of AATD cases detected possess a rare SERPINA1 mutation.
Conclusion
National registries play an important role in evaluating the natural history and progression of pulmonary and liver disease in AATD and contribute to our understanding of the interaction between genetic susceptibility and environmental exposure in this population. This registry study illustrates the detrimental effect of smoke exposure on the clinical phenotype of AATD in Ireland and the benefit of immediate smoking cessation at any stage of lung disease. Our data would support the premise that asymptomatic individuals with AATD who are not exposed to smoke are likely to be unaffected by pulmonary complications in their lifetime. Public health initiatives to reduce smoking uptake, promote smoking cessation and reduce passive smoke exposure are likely to be of most benefit to individuals with AATD that have yet to be diagnosed.
Declaration of Interest Statement
MEOB received the eALTA award 2013 from Grifols. The other authors declare they have no competing interests to disclose. The authors are responsible for the content and writing of this paper.
Supplemental Material
A supplementary table and figure can be found at http://www.dx.doi.org/10.3109/15412555.2015.1021913.
ICOP_A_1021913_SUPP.pdf
Download PDF (47.4 KB)Funding
This work has been supported by funding from the Irish Government Department of Health and Children, Alpha One Foundation (Ireland), Alpha-1 Foundation (USA), and the Health Research Board (HRB) Ireland.
SUPPLEMENTARY MATERIALS FOR THIS PAPER ARE AVAILABLE ONLINE
References
- Laurell CB, Eriksson S. The electrophoretic alpha1 globulin pattern of serum in alpha1 antitrypsin deficiency. Scand J Clin Lab Invest 1963; 15:132–140.
- Luisetti M, Seersholm N. Alpha1-antitrypsin deficiency. 1: epidemiology of alpha1-antitrypsin deficiency. Thorax 2004; 59(2):164–169.
- Stoller JK, Sandhaus RA, Turino G, et al. Delay in diagnosis of alpha1-antitrypsin deficiency: a continuing problem. Chest 2005; 128(4):1989–1994.
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Resp Crit Care Med 2003; pp. 818–900.
- Carroll TP, O’Connor CA, Floyd O, et al. The prevalence of alpha-1 antitrypsin deficiency in Ireland. Respir Res 2011; 12:91.
- Luisetti M, Massi G, Massobrio M, et al. A national program for detection of alpha 1-antitrypsin deficiency in Italy. Gruppo I.D.A. Respir Med 1999; 93(3):169–172.
- Luisetti M, Miravitlles M, Stockley RA. Alpha1-antitrypsin deficiency: a report from the 2nd meeting of the Alpha One International Registry, Rapallo (Genoa, Italy), 2001. Eur Respir J 2002; 20(4):1050–1056.
- Stoller JK, Brantly M, Fleming LE, et al. Formation and current results of a patient-organized registry for alpha(1)-antitrypsin deficiency. Chest 2000; 118(3):843–848.
- FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr 1993; 122(1):1–9.
- Ferrarotti I, Carroll TP, Ottaviani S, et al. Identification and characterisation of eight novel SERPINA1 null mutations. Orphanet J Rare Dis 2014; 9(1):172.
- Silverman EK, Miletich JP, Pierce JA, et al. Alpha-1-antitrypsin deficiency. High prevalence in the St. Louis area determined by direct population screening. Am Rev Respir Dis 1989; 1 40(4):961–966.
- de Serres FJ, Blanco I, Fernández-Bustillo E. Genetic epidemiology of alpha-1 antitrypsin deficiency in North America and Australia/New Zealand: Australia, Canada, New Zealand and the United States of America. Clin Genet. 2003 Nov;64(5):382–97.
- Seersholm N, Kok-Jensen A, Dirksen A. Survival of patients with severe alpha 1-antitrypsin deficiency with special reference to non-index cases. Thorax 1994; 49(7):695–698.
- Piras B, Ferrarotti I, Lara B, et al. Clinical phenotypes of Italian and Spanish patients with α1-antitrypsin deficiency. Eur Respir J 2013; 42(1):54–64.
- Molloy K, Hersh CP, Morris VB, et al. Clarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotes. Am J Resp Crit Care Med 2014; 189(4):419–427.
- American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Resp Crit Care Med 1995; 1107–1136.
- Bhalla M, Turcios N, Aponte V, et al. Cystic fibrosis: Scoring system with thin-section CT. Radiology 1991; 179(3): 783–788.
- McMahon MA, O’Mahony MJ, O’Neill SJ, et al. Alpha-1 antitrypsin deficiency and computed tomography findings. J Comput Assist Tomogr 2005; 29(4):549.
- Vestbo J, Hurd SS, Agustí AG, et al. Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD Executive Summary. Am J Resp Crit Care Med 2013; 187(4):347–65.
- Dirksen A, Piitulainen E, Parr DG, et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J 2009; 33(6):1345–1353.
- Piitulainen E, Tornling G, Eriksson S. Effect of age and occupational exposure to airway irritants on lung function in non-smoking individuals with alpha 1-antitrypsin deficiency (PiZZ). Thorax 1997; 52(3):244–248.
- Cuvelier A, Muir JF, Hellot MF, et al. Distribution of alpha(1)-antitrypsin alleles in patients with bronchiectasis. Chest 2000; 117(2):415–419.
- Parr DG, Guest PG, Reynolds JH, et al. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Resp Crit Care Med 2007; 176(12):1215–21.
- McElvaney NG. Smoking ban—made in Ireland, for home use and for export. N Engl J Med 2004; 350(22):2231–2233.
- Mayer AS, Stoller JK, Vedal S, et al. Risk factors for symptom onset in PI*Z alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis 2006;v1(4):485–492.
- Kabir Z, Manning PJ, Holohan J, et al. Second-hand smoke exposure in cars and respiratory health effects in children. Eur Respir J 2009; 34(3):629–633.
- Turino GM, Barker AF, Brantly ML, et al. Clinical features of individuals with PI*SZ phenotype of alpha 1-antitrypsin deficiency. alpha 1-Antitrypsin Deficiency Registry Study Group. Am J Resp Crit Care Med 1996; 154(6 Pt 1):1718–1725.
- Ferrarotti I, Baccheschi J, Zorzetto M, et al. Prevalence and phenotype of subjects carrying rare variants in the Italian registry for alpha1-antitrypsin deficiency. J Med Genet 2005; 42(3):282–287.
