Abstract
The Alpha-1 Foundation Research Registry has a long history of facilitating research studies in the United States. The current contact registry is used to invite participants to research studies. However, the next generation of individuals diagnosed with alpha-1 antitrypsin deficiency may look quite different from historical cohorts. This paper uses data from the Alpha Coded Testing (ACT) study, a home genetic testing program in which deficient individuals are invited to participate in the Registry, to demonstrate the impact that selection bias can introduce into registry data. Environmental tobacco smoke (ETS) exposure is rapidly declining in the United States. We queried whether consecutive non-smokers with or without childhood ETS in ACT (N = 801) had been diagnosed with COPD more often if deficiency genes were defined in subsequent testing. The prevalence of COPD was not different between cohorts with or without ETS exposure between normal (PiMM and PiMS), moderately deficient (PiMZ, PiMNull, and PiSS), and severely deficient (PiSZ, PiZZ, PiSNull, and PiZNull) genotypes. Surprisingly, age adjusted COPD Severity Scores in this cohort were higher for individuals with normal genotypes compared to moderately (P<0.001) and severely (P = 0.04) deficient genotypes. Ascertainment bias of testing within families (which yields the highest incidence of deficiency genotypes) also finds many family members without symptoms, even over the age of 40. We conclude that the future utility of registries will depend on accurate determination of testing mechanics. Larger database initiatives using the COPD Patient Powered Research Network are described.
Introduction
Registries have been impactful for alpha-1 antitrypsin deficiency (AATD) diagnosis and therapy in the United States. Disease recognition increased dramatically following purification, licensing, and introduction of augmentation therapy in the United States in the late 1980s. A unique partnership between industry, the Food and Drug Administration (FDA), and the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) formed to assimilate a prospective registry of individuals on or off augmentation therapy. The NHLBI Registry enrolled individuals with severe deficiency of alpha-1 antitrypsin (AAT) from 1989-1992 and followed them until 1996 on or off augmentation therapy (Citation1). The resulting dataset of 1129 individuals has anchored the science to power trials of augmentation therapy, understand the natural history of AATD, and advocate for expansion of targeted diagnostic efforts.
When the NHLBI Registry ended in 2006, efforts to advance a community of patients and health care professionals committed to better understand AATD led to the formation of the Alpha-1 Foundation, AlphaNet, and the Alpha-1 Foundation Research Registry. In the years that have followed, registry science has advanced, new therapies have been studied and introduced, and the number of individuals diagnosed continues to increase. The Alpha-1 Foundation Research Registry has a unique design optimized to efficiently enroll the research studies of tomorrow. This article will focus on current design, past successes, current process, integration with home diagnostic testing studies, and provide an example of efforts to generate new knowledge in AATD.
Current design
Because the natural history and demographics of the US AATD population was well defined by the NHLBI Registry, the Medical and Scientific Advisory Board of the Alpha-1 Foundation chose to enroll a contact registry that could be sustained for less money than a Registry dependent on serial lung function and CT densitometry testing. The Registry design allowed remote enrollment online to include everyone in the US with a deficient Alpha-1 allele, regardless of geography and distance from a clinical center with expertise in the disease.
The enrolled population is invited to participate in research studies performed in the US and Canada. Researchers desiring to perform a research study can invite registry participants to contact them for IRB approved studies based upon enrollment criteria. Stratification by genotype, geography, or self-reported lung or liver disease allows targeted invitations by letters approved by an IRB. Incentives for participation, travel monies, or study specific testing schedules are usually hosted by the study Principal Investigator.
Enrollment and current demographics
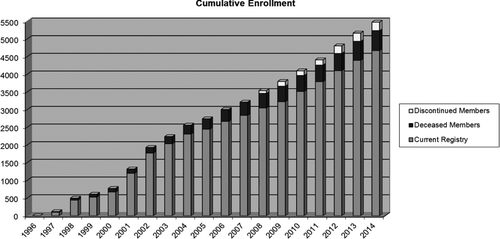
The current Alpha-1 Foundation Research Registry began enrollment in 1997. Enrollment of mildly deficient genotypes began in 2002 anticipating the increased interest in carriers of one severely deficient AAT gene. The current registry continues to enroll at approximately 300 individuals yearly (Figure ).
This allowed enrollment from every state in the United States and through an enrollment agreement with every province in Canada. The geographic density of the Registry population analyzed by mailing code is not different the density of the overall US population. Although AATD has the highest prevalence in populations of northern European descent, those individuals have assimilated throughout the United States.
Research studies
The efforts to build and populate any registry in AATD are insufficient unless thoughtful prospective research is facilitated. Because AATD is a rare disease, the number of patients that any single center can accumulate for research is limited. Although the impact of the Alpha-1 Foundation Research Registry on downstream research cannot be proven, the portfolio of research studies and downstream publications from those research studies would not have been possible without contact to the large number of participants willing to perform research in their disease. As of January 1, 2014, 82 recruitments have been processed for 56 different studies. Study preparatory work involved another 56 projects. Biennial newsletters are published, mailed, emailed, and hosted at www.alphaoneregistry.org.
Home diagnostic testing
In 2001, the burdens of family testing through physicians who knew little about AATD was very evident. Siblings of severely deficient individuals who visited their physicians in search of AAT genotyping were routinely dismissed as being healthy and not in need of testing. Against this backdrop, the Alpha-1 Foundation began the Alpha Coded Testing Study (Citation2). In short, individuals who desired confidential family testing with accurate diagnostic tests signed electronic consent with the Registry, answered questionnaires concerning the reasons and projected impact of testing, and were mailed a blood spot card, lancet, and instructions for blood spot acquisition. Blood spot cards were mailed to the Registry, anonymized, tested at the University of Florida Alpha-1 laboratory, with results returned to MUSC. Results were returned to the individuals with disease specific information, access to a genetic counselor for themselves and their family members, and specific invitations to join the Registry if AAT deficient. This program has the highest yield of diagnostic testing yet reported (Citation1).
Also important is that these individuals consent for recontact for future AAT research studies. Therefore, this population with AATD family members or those who are referred by their physicians for COPD represent two very different populations using the ACT Study. The family members are typically less symptomatic than the family index case and the participants with COPD have a low incidence of AATD, similar to other testing programs. In this backdrop, the ACT Study has a large number of PiMZ carriers available for future research and similar numbers of demographically matched PiMM controls. We present below an attempt to use this cohort to answer an important biologic question in AATD.
Does second-hand smoking cause COPD in AATD?
For years there has been wide support in the AATD community to counsel avoidance of environmental tobacco smoke (ETS) in all AAT deficient individuals (Citation3–5). Therefore, a questionnaire was placed proximal to AAT genotyping in the ACT Study to define whether ETS is associated with COPD in a nonsmoking population testing for AATD. ETS exposure was defined by at least one year exposure in the home for individuals before age 12 and at home or work for individuals ≥ age 12.
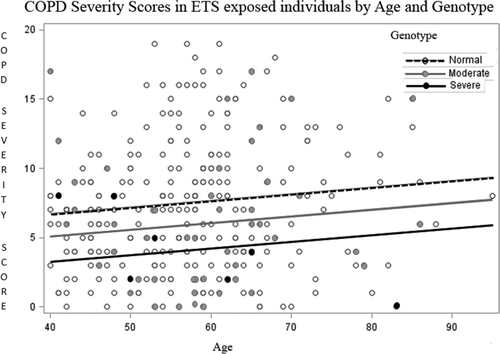
For this analysis the Alpha Coded Testing (ACT) Study enrolled and genotyped 2502 adult individuals for AAT alleles between 01SEP2010 and 05MAR2012 using the COPD Severity Score (COPDSS) questionnaire (Citation6). The COPDSS is a validated instrument querying 5 domains of respiratory symptoms, systemic corticosteroid use, other COPD medication use, previous hospitalization for respiratory disease, and home oxygen use with ranges of COPDSS scores between 0 and 35. The lower threshold to define spirometric COPD is not known. Statistical analysis was performed in SAS using a generalized linear model to adjust for age with group comparisons by Tukey's methods. ETS was reported in 80.3% of individuals and occurred in 71.4% before age 12, 72.6% ≥ age 12, and 64.0% both before and after age 12. ETS varied by year of birth with less ETS in younger individuals. After excluding ex-smokers of >100 cigarettes (N = 169), and individuals aged <40 years (N = 1532), COPD prevalence defined by a score > 0, or at any higher COPDSS threshold was not different between those exposed to ETS, or by clustered genotype (P = NS, Figure ).
Since COPD prevalence is highly age dependent, the severity scores were age adjusted and ETS exposed cohorts compared by normal (PiMM and PiMS), moderately deficient (PiMNull, PiMZ and PiSS) and severely deficient (PiZZ, PiSZ, PiSNull, and PiZNull) genotypes. COPD Severity Scores were weighted toward non-severe COPD in the majority of individuals. Individuals with severe deficiency had lower COPD severity scores than moderately deficient (p = 0.04) and normal genotype individuals (p < 0.0001) (Figure ).
The trends of more severe COPD in normal genotypes are presented to show some of the limitations of AATD registries. The ACT study is designed to allow testing from empowered individuals who desire to test family members for AATD and from individuals with COPD who choose to test confidentially. Those with established COPD are predominantly cigarette smokers and those testing within families are non-index cases, who typically have less severe disease. The paradox of selection bias in family testing studies requires rigid control for those entering with and without symptoms, following progression of disease outcomes for long times, and appropriate statistical analysis. For this reason, we are convinced that studies that convincingly show ETS as a risk for COPD in AATD will be difficult to perform. This data does not deter from the strong data that secondhand smoke is associated with childhood respiratory infections and adult lung cancer as reported in serial reports from the US surgeon general's taskforce.
Future design
Current funding has been secured for planning and design of a new Registry model in the US. Given the successes of the Cystic Fibrosis Foundation clinical trials built upon the backbone of the CF Registry, plans are ongoing for a similar structure to be used for the Alpha-1 Foundation to empower the Alpha-1 Foundation Clinical Resource Centers (CRC) distributed across the United States to obtain accurate physician entered data remotely submitted for longitudinal care. The database will have clinical trials capabilities, public facing data query tools, and co-enrollment capabilities with PCORNet, the large Registry effort initiated by the Patient Centered Outcome Research Institute (PCORI) for comparative effectiveness research. The COPD Foundation, sister organization to the Alpha-1 Foundation was able to secure a grant for one of the Patient Powered Research Networks (PPRN) of PCORNet in which 50,000–75,000 individuals with COPD will be enrolled in the COPD PPRN. Linkage between the Alpha-1 Registry and the COPD PPRN is IRB approved to allow bidirectional data flow once individuals with AATD have enrolled with permission for linkage from both Registries.
Conclusions
Sustainability of any registry is dependent upon the value obtained to the users, in this case the Alpha-1 community. We believe that the current Alpha-1 Foundation Research Registry, the Alpha Coded Testing (ACT) Study, and future enhancements to the Alpha-1 Foundation CRC Registry linked to the COPD PPRN of PCORNet will provide a robust platform to facilitate the large number of clinical trials in the pipeline for AATD.
Declaration of Interest Statement
CS has grants paid to the Medical University of South Carolina from the Alpha-1 Foundation, CSL Behring, Grifols and the NIH on the subject of Alpha-1 Antitrypsin Deficiency. He has consulted for CSL Behring, and Grifols on the subject of Alpha-1. DW is funded in part by the Alpha-1 Foundation and Grifols. LS and SK are funded by the Alpha-1 Foundation. RM and TB have nothing to report. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors thank the Alpha-1 Foundation for their long-term support for the Alpha-1 Foundation Research Registry.
References
- The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Amer J Respir Crit Care Med 1998; 158(1):49–59.
- Strange C, Dickson R, Carter C, et al. Genetic testing for alpha1-antitrypsin deficiency. Genet Med: Off J Amer Coll Med Genet 2004; 6(4):204–210.
- von Ehrenstein OS, Maier EM, Weiland SK, et al. Alpha1 antitrypsin and the prevalence and severity of asthma. Arch Dis Childhood 2004; 89(3):230–231.
- von Ehrenstein OS, von Mutius E, Maier E, Hirsch T, Carr D, Schaal W, et al. Lung function of school children with low levels of alpha1-antitrypsin and tobacco smoke exposure. Euro Respir J 2002; 19(6):1099–1106.
- Mayer AS, Stoller JK, Vedal S, et al. Risk factors for symptom onset in PI*Z alpha-1 antitrypsin deficiency. Inter J Chron Obstruct Pulmon Dis 2006; 1(4):485–492.
- Eisner MD, Trupin L, Katz PP, et al. Development and validation of a survey-based COPD severity score. Chest 2005; 127(6):1890–1897.