Abstract
The French registry of patients with alpha-1 antitrypsin deficiency (AATD)-associated emphysema was launched in 2006. Here, we aimed to report on the baseline characteristics of these patients, their health-related quality of life (HRQoL) and factors associated with HRQoL. Another goal was to survey the practices of French physicians regarding augmentation therapy. We included 273 patients with AATD, emphysema, obstructive-pattern [forced expiratory volume in 1 sec/forced volume capacity (FEV1/FVC) < 0.7], FEV1 ≤ 80% predicted. Mean (SD) age was 51.8 (11.1) years, 240 (87.9%) of patients were smokers or ex-smokers, mean (SD) FEV1 was 40.5% (15.7) predicted. Mean (SD) SGRQ score was 49.0 (20.0) and was higher for females than males (52.7 [20.7] vs 46.8 [18.2]; p = 0.01). Dyspnea showed the strongest association with SGRQ score (r = 0.65; p < 0.0001), followed by chronic bronchitis (r = 0.33; p < 0.0001) and wheezing (r = 0.32; p < 0.0001). Number of exacerbations in the year before inclusion was also significantly associated with SGRQ score (r = 0.36; p < 0.0001). The SGRQ score was associated with the 6-min walking distance (r = –0.53, p < 0.0001), FEV1 (% predicted, r = –0.53, p < 0.0001) and DLCO (% predicted, r = –0.52, p < 0.0001). It was also associated with the GOLD 2006 (r = 0.53; p < 0.0001) and GOLD 2011 (r = 0.63; p < 0.0001) classifications and with the BODE index (r = 0.37; p < 0.0001). Age, history of tobacco smoking or current smoking did not show any association with SGRQ total scores. On multivariate analysis, a model including age, chronic bronchitis, dyspnea (MRC scale), diffusing lung capacity and 6-min walking distance explained 57% of the variation in the score. The French registry provides important insights into the clinical characteristics of French patients with AATD-related emphysema.
Keywords:
Introduction
The Z mutation is believed to have occurred 2000 years ago in the Viking population and was disseminated over the world with the travels of the Vikings, first to Baltic regions, then throughout the world (Citation1). This mutation may have persisted over time, with high frequencies, because it conferred a selective advantage to its carriers, preventing infection in the pre-antibiotic era (Citation2). Thus, the prevalence of the Z mutation is highest in Sweden, where it affects about 2% of the population, but is intermediate in southern Europe. According to de Serres et al., the frequency of the S and Z alleles in France is close to 1.2% and 7%, for 7700 cases of the PiZZ mutation and almost 100,000 of the PiSZ mutation in this country (Citation3–5).
Augmentation therapy has been available in France for almost 20 years (Alfalastin – LFB). Alfalastin was granted marketing authorization in France in July 2005, after being sold under ATU (Temporary Authorization for Use) cohort status since January 1995. The therapeutic protein is obtained from human plasma by fractionation and purification.
Because knowledge on this disease was limited and because the awareness of the medical community was low, we decided in 2005 to set up a prospective cohort study named CONEDAT, “Cohorte nationale des emphysémateux déficitaires en alpha-1 antitrypsine” (Citation6). The goals of this cohort were to gather information from patients with AATD-related emphysema, to characterize the clinical and functional courses of these patients, and to determine associated prognostic factors. Other goals were to describe the practices regarding augmentation therapy and determine factors associated with the quality of life of patients. The aim of the present study is to describe the baseline characteristics of these patients together with their quality of life and associated factors.
Methods
The design of this study has been detailed elsewhere (Citation6–8). Briefly, this is an open, ongoing, prospective, multi-center cohort that includes all patients living in France who fulfill the following criteria: 1) AAT level <0.5 g/L; 2) emphysema detected on CT scan; 3) forced expiratory volume in 1 sec/forced vital capacity (FEV1/FVC) < 0.7; and 4) FEV1 < 80% predicted. Patients who underwent a lung or liver transplant were not eligible for the study. After the baseline visit, patients return to their study centers for follow-up assessments every 6 months for 10 years. Spirometry measurements and equations used to determine the predicted normal values for FEV1 agreed with the official statement of the European Respiratory Society for standardized lung function testing.
AAT protein concentration is assessed by immunoturbidimetrics or immunonephelometrics with commercially available kits (normal range 0.90–2.0 g/L). In most patients, AAT phenotype is assessed by isoelectric focusing electrophoresis on ready-to-use agarose gels with immunological detection, with a commercially available kit (Hydragel 18 AAT Isofocusing; Sebia, Evry, France). Alternatively, AAT genotype is determined by PCR with specific primers for the PIS and PIZ mutations in the SERPINA1 gene. Investigators involved in the study are listed at the end of this manuscript. All patients gave their informed consent to be included in the study, which was approved by the institutional review board for Paris Nord–Paris 7 hospitals. This study is registered at www.ClinicalTrials.gov (NCT00700934).
Data collected
The following data were collected: demographics (age, sex, education, employment, occupation history, smoking history), AAT level, phenotype and/or genotype, augmentation therapy history, symptom history (cough, phlegm, wheezing, breathlessness), self-reported hospitalizations and exacerbations (defined as a worsening in the respiratory condition leading to treatment modification with antibiotics and/or systemic steroids), physical examination (weight, height, body mass index), dyspnea (MRC scale), pulmonary function tests [FEV1 and FVC pre- and post-bronchodilator therapy, diffusing lung capacity (DLCO), total lung capacity (TLC), residual volume (RV)], and blood gas. Results for the 6-min walking distance were recorded.
Patients were classified according to the GOLD 2006 classification based on FEV1 and to the GOLD 2011 classification based on symptoms (dyspnea scale), and risk of exacerbations (airflow obstruction and exacerbation history) (Citation9,10).
Health-related quality of life (HRQoL) was assessed by the St. Georges Respiratory Questionnaire (SGRQ), with 3 dimensions: impact, activity and symptoms. A score is calculated for each dimension, to assess the impact of disease on each of the 3 dimensions. The result is the ratio of this score with the highest possible score for each dimension, on a scale from 0 to 100. A total score is obtained by dividing the sum of the 3 scores by the sum of the maximum scores for each dimension. A bio bank of genetic materials has been established in the 15 largest centers.
Statistical analysis
Continuous variables are described with means (SD) or median (interquartile ranges) and were compared using the t-test or Mann–Whitney U-test. The distribution of continuous variables was examined by plotting. Categorical variables are described by number (percentage). Associations between explanatory variables and SGRQ score were examined by bivariate and multivariate linear analysis. We used purposeful selection of covariates, as described by Hosmer and Lemeshow, to select the multivariate model.
The first step was the inclusion of all variables significant at the p = 0.20 on bivariate analysis as well as all variables known to be clinically relevant. The second step was to remove, one by one, variables that did not significantly contribute to the multivariate model on the basis of the Wald test p value and the change in coefficient of the remaining variables. We assessed the scale of continuous covariates by fitting general additive models including smoothing-splines transformations of the variables. Analyses involved use of R (http://www.R-project.org, the R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
In total, 312 patients from 56 centers were included in the cohort as of December 31, 2013; 30 had FEV1/FVC ratio >0.7 (n = 23) or FEV1 > 80% predicted (n = 8) and were excluded. In total, 281 patients from 52 centers remained for analyses; 273 patients (97.2%) had SGRQ data available at baseline. The number of patients by center ranged from 1 to 65. The main baseline characteristics of patients are in Table . All patients had AAT blood level <0.5 g.L−1; the AAT genotype was PIZZ for 231 patients (84.6%), SZ for 15 (5.5%), Null/Z for 3 (1.1%) and not available for 24 (8.4%). The median time between symptom onset and diagnosis of AATD was 6.7 years (range 0–39 years). All patients had respiratory symptoms: dyspnea in 261 (95.6%), chronic bronchitis in 100 (36.6%) and wheezing in 143 (52.4%). Overall, 153 (54.4%) patients showed 444 exacerbations requiring oral steroids and/or antibiotics in the year before inclusion in the study; 85 (19.1%) required hospitalization.
Table 1. Baseline characteristics of patients (n = 273)
Pulmonary function tests
Most patients had a severe ventilatory obstructive pattern with a mean FEV1 of 40.5% predicted (15.7), and 68.9% of patients having a FEV1 less than 50% predicted. Distension was common, with mean TLC 129.0% predicted (21.9). Mean (SD) 6-min walking distance was 434.3 (133.6) meters. The mean (SD) BODE index was 3.43 (1.4; range 1–8). As compared with patients who had smoked, patients who never smoked (n = 33, 11.7%) were older (mean [SD] 63.1 [9.0] vs 50.3 [10.4] years; p < 0.001) and had less severe FEV1 impairment (mean [SD] FEV1 = 49.7% [18.0] vs 39.4% [15.0] predicted; p < 0.001).
Treatment at inclusion
At inclusion, 249 patients (91.2%) were receiving long-acting bronchodilators, whereas 228 (83.5%) received inhaled corticosteroids (ICS) either alone (2.6%) or with bronchodilators; 88 patients (32.2%) received long-term oxygen therapy.
A total of 130 patients (47.6%) received augmentation therapy at the time of inclusion in the study. The administration scheme was weekly for 58 (44.3%), twice a month for 39 (29.0%), once a month for 30 (22.9%) and every 3 weeks for 2. Patients receiving augmentation therapy had a more severe airflow limitation [FEV1: 38.4 (14.7)% predicted vs 42.5 (16.4%); p = 0.03] and were more likely to receive inhaled steroids (89.3% vs 78.2%) than patients who did not receive augmentation therapy. After adjustment on age and FEV1, center was significantly associated with the likelihood of receiving augmentation therapy (p < 0.001).
Quality of life
The mean (SD) total SGRQ score was 49.0 (20.0) (range 4.7–97.4) and was higher for females than males (52.7 [20.7] vs 46.8 [18.2]; p = 0.01), which indicates worse QoL for females. Dyspnea showed the strongest association with SGRQ score (r = 0.65; p < 0.0001), followed by chronic bronchitis (r = 0.33; p < 0.0001) and wheezing (r = 0.32; p < 0.0001). Number of exacerbations in the year before inclusion was also significantly associated with SGRQ score (r = 0.36; p < 0.0001).
The SGRQ score was associated with the 6-min walking distance (r = -0.53, p < 0.0001), FEV1 (% predicted, r = -0.53, p < 0.0001) and DLCO (% predicted, r = -0.52, p < 0.0001). It was also associated with the BODE index (r = 0.37; p < 0.0001). Age, history of tobacco smoking or current smoking did not show any association with SGRQ total scores.
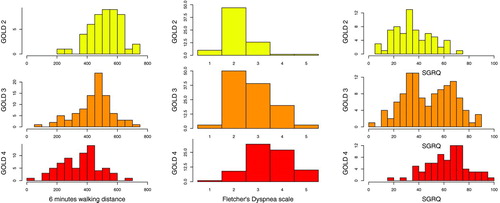
Figure shows the distribution of SGRQ according to GOLD 2006 stages. It must be noted that there was a considerable overlap in SGRQ scores along the 3 GOLD stages (GOLD II, III, IV). The mean scores were 63.3 (SD = 15.9), 47.6 (19.2) and 34.8 (15.0) for the GOLD 2006 II, III and IV categories respectively (r = 0.53; p < 0.0001). Stronger association was found with the GOLD 2011 classification (r = 0.63; p < 0.0001) with mean scores of 29.6 (13.5), 45.5 (14.8), 39.0 (15.8) and 60.9 (16.1) for classes A, B, C and D, respectively.
Multivariate determinants of SGRQ score
On multiple linear regression, a model including age (p = 0.0088), dyspnea scale (p < 0.0001), chronic bronchitis (p = 0.002), 6-min walking distance (p = 0.037) and DLCO (% predicted) (p < 0.001) explained 57% of the variability in total SGRQ. In this adjusted model, FEV1, sex and number of exacerbations in the previous year were not significantly associated with SGRQ score. Another model including only 3 variables—chronic bronchitis, dyspnea and DLCO (% predicted)—still explained 51.8% of the variability in SGRQ score.
Discussion
Numerous national registries of patients with AATD exist throughout the world. They differ widely in their goals, inclusion criteria and follow-up procedures. The French national registry of patients with AATD-related emphysema, launched in 2006, has currently accrued information for about 300 patients, with the inclusion of 500 patients expected in 3 years (Citation6–8). Because the aim of this registry was to better understand the natural history of the pulmonary disease related to the deficiency and to search for associated factors, we aimed to include a homogeneous group of patients with proven emphysema and an obstructive pattern, with FEV1 < 80% of predicted value. We report on the baseline characteristics of these patients, their HRQoL and factors associated with HRQoL, as well as the practices of French physicians regarding augmentation therapy.
Most registries use much broader criteria and do not require any pulmonary involvement. For instance, the largest registry published to date included all adult patients provided that they had serum AAT level < 11 μmol−1 or ZZ genotype (Citation11,12); 19.1% of these patients had a FEV1 > 80% predicted, and a substantial proportion likely have no pulmonary disease. The French registry includes many patients with severe respiratory impairment with almost 70% of patients having a FEV1 < 50% predicted, and 32.3% receiving long-term oxygen therapy at the time of inclusion in the study. The analysis of baseline characteristics of these patients provides some useful information on this disease.
Tobacco smoking was common and associated with more severe respiratory impairment. The number of never-smokers was low, and few of them had severe respiratory impairment, which underlines the major role played by tobacco smoking in these patients and the low risk of developing severe respiratory impairment in the absence of tobacco exposure. Most patients who used to smoke had quit by the time they were included in the study, which contrasts with what is commonly seen in patients with COPD. For instance, in the ECLIPSE study, following more than 2,000 COPD patients, more than 30% were current smokers, even those with the most severe respiratory impairment (Citation13).
This study suggests that knowledge of severe AAT deficiency could motivate smokers to quit smoking (Citation14). Diagnosis delay appears to be common, with a median time from symptom onset to AATD diagnosis close to 7 years. This feature, found in other studies (Citation12), may be explained at least in part by the high prevalence of wheezing in this population, which may favor a misdiagnosis of asthma.
Another motivation of the study was to survey the practices of physicians regarding augmentation therapy in France. At the time the study was launched, this treatment had been available for several years. Almost 50% of patients were receiving treatment at inclusion in the registry. Although the recommended dosage of antitrypsin augmentation therapy is 60 mg/kg body weight administrated once weekly, more than 50% of patients had another administration regimen (Citation7). At inclusion, patients receiving augmentation therapy had very similar characteristics than patients who did not, and practices appear to vary among centers.
Because assessment of airflow obstruction is insufficient to reflect the impact of lung disease in COPD patients, we included HRQoL measurement both at inclusion and during follow-up, using the SGRQ. Several studies have assessed HRQoL in patients with AATD related emphysema. Manca et al evaluated the HRQoL in patients with AATD compared to patients with non-AATD COPD using different instruments: COPD severity score (COPDSS), EuroQoL-5 Dimensions (EQ-5D), the living with COPD (LCOPD) and the COPD assessment test (CAT) (Citation15). They found a stronger correlation between FEV1 in the AATD group compared to the non-AATD COPD group. In patients with AATD, the correlations between FEV1 and HRQoL ranged between 0.50 and 0.57. We found very similar figures in our study with a Spearman correlation coefficient of 0.53. Pillai et al. reported on the HRQoL values of 309 patients with AATD according to the GOLD 2011 classification. They found that patients in the B and D categories had higher HRQoL impairment than patients in the A and C categories, with numbers close to that observed in the present study (Citation16). This result emphasizes the impact of exacerbations on the HRQoL of these patients.
We found dyspnea strongly associated with SGRQ. Among physiological variables, DLCO was strongly associated with SGRQ on both bivariate and multivariate analysis and remained the only physiological variable associated on multivariable analysis. However, the strong correlations we found between physiological variables (multicollinearity) preclude definitive conclusions. Although the severity of airflow obstruction appeared to be a major determinant in bivariate analysis, we found considerable overlap among GOLD 2006 categories (see Figure ). The GOLD 2011 classification showed a stronger association with outcome than the GOLD 2006 classification, emphasizing the impact of exacerbations on HRQoL.
In conclusion, the French registry for AATD-related emphysema included a homogeneous group of well-phenotyped patients with emphysema and airflow limitation. This registry allows for a better understanding of the natural course of this disease and associated factors. It highlights the large between-patient variability in symptoms and severity of airflow obstruction. Several studies are under way to search for factors such as occupational, pollution or genetic factors that could account for this large variability.
Declaration of Interest Statement
Pr. Cuvelier has received speaker fees from Novartis, Boehringer Ingelheim and consulting fees from LFB and Boehringer Ingelheim. Pr. Mornex has received consulting consulting fees from LFB and CSL Berhing, speaker fees from Boehringer Ingelheim, Pfizer, GSK, Actelion, LFB and research grants from LFB and Actelion.
Pr. Pison reports personal fees from LFB, non-financial support from GSK, Novartis, Pfizer, Actelion, Astra-Zeneca and Boerhinger-Ingelheim. Pr. Thabut has received speaking fees from Laboratoire Français du Fractionnement et des Biotechnologies (LFB), consulting fees from LFB and Novartis, and research grants from LFB. Dr. MC Pujazon has received consulting fees from LFB, Novartis, Stallergenes, and Chiesi.
The other authors have no interest to disclose.
Investigators
The investigators of the CONEDAT whose patients participated in this study are as follows (medical centers are in italics):
Thabut G, Fournier M, Mal H, Piperaud M, Jebrak G, Dauriat G, Biondi G, Ait Ilalne B, Hôpital Bichat, Paris; Mornex J-F, Hôpital Louis Pradel, Lyon; Pison C, Cherion C, Hôpital de Grenoble, Grenoble; Cuvelier A, Muir J-F, Hôpital de Bois-Guillaume, Rouen; Pujazon M-C, Carles P, Hôpital Larrey, Toulouse; Laffite J, Balduyck M, Grammont M, Wasielewsky E, Hôpital Calmette, Lille; Diot P, Marchand-Adam S, Pichon E, Henriet A-C, Hôpital Bretonneau, Tours; Chabot J-F, Guillaumot A, Beuraud N, Hôpital Brabois, Vandoeuvre les Nancy; Reynaud-Gaubert M, Ramadour M, Nieves A, Hôpital Sainte Marguerite, Marseille; Delaval P, Hôpital Pontchaillou, Rennes; Leroyer C, Hôpital Cavale Blanche, Brest; Quieffin J, Hôpital Jean Monod, Le Havre; Broussier P-M, Hôpital Charles Nicolle, Rouen; Mehdaoui A, Centre Hospitalier Général, Evreux; Morel H, Hôpital Broussais, Saint-Malo; Clément B, Nevers; Chiappa A-M, Hôpital de Quimper, Quimper; Angebault M, Coudray S, Centre Hospitalier Spécialisé en Pneumologie, Chevilly-Larue; Martin F, Lineau C, Hôpital Bretagne-Atlantique, Vannes; Charbonneau J, Hôpital Montbéliard, Montbéliard; Godard P, Hôpital Arnaud De Villeneuve, Montpellier; Kessler R, Hôpital Hautepierre, Strasbourg; Caillaud D, Hôpital Gabriel Montpied, Clermont-Ferrand; Lebas F-X, Goupil F, Latrouite L, Hôpital du Mans, Le Mans; Grignet J-P, Hôpital de Denain, Denain; Brun O, Perpignan; Perche A, Orléans; Gillet Juvin K, Hôpital Européen Georges Pompidou, Paris; Chanez P, Hôpital Nord, Marseille; Crequit J, Hôpital de Beauvais, Beauvais; Raymond S, Hôpital Belle Isle, Metz; Dot J-M, Hôpital d’Instruction des Armées Legouest, Metz-Armées; Steenhouwer F, Hôpital Victor Provo, Roubaix; Vaylet F, Hôpital d’instruction des armées, Clamart; Roa M, Hôpital Fréjus-Saint-Raphaël, Fréjus; Zalcman G, Hôpital Côte de Nacre, Caen; Guillou-Bideau B, Centre DELTA, Quimper; Soyez F, Bagneux; Iglesias E, Hôpital de la Source, Orléans; Bogdan G, Hôpital François Quesnay, Mantes la Jolie; Jounieaux V, Magois E, Hôpital d’Amiens, Amiens; Ouksel H, Hôpital d’Angers, Angers; Freymond N, Hôpital Lyon Sud, Pierre Benite; Eveilleau C, Brest; Terrioux P, Meaux; Bertholet C, Chambéry; Girard F, Hôpital les escartons, Briançon; Valcke J, Hôpital Privé Armand Brillard, Nogent sur Marne; Moutaux G, Tours; Tavernier J-Y, Hôpital de Douai, Douai; Briens E, Hôpital Yves Le Foll, Saint Brieuc; Caron F, Hôpital Poitiers, Poitiers; Claussner Paulignan M, Forbach; Lerolle U, Trélazé; Boudoux L, Hôpital Saint Vincent de Paul, Lille; Pourtout F, Courbevoie.
Funding
This study was supported by a grant from the Laboratoire français du Fractionnement et des Biotechnologies. The funding source had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
This study was supported by a grant from the LFB. The funder had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
References
- Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Amer J Respir Crit Care Med 2012; 185(3):246–259.
- Lomas DA. The selective advantage of alpha1-antitrypsin deficiency. Amer J Respir Crit Care Med 2006; 173(10):1072–1077.
- de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest 2002; 122(5):1818–1829.
- de Serres FJ, Blanco I, Fernandez-Bustillo E. Genetic epidemiology of alpha-1 antitrypsin deficiency in North America and Australia/New Zealand: Australia, Canada, New Zealand and the United States of America. Clin Genet 2003; 64(5):382–397.
- de Serres FJ, Blanco I, Fernandez-Bustillo E. PI S and PI Z alpha-1 antitrypsin deficiency worldwide. A review of existing genetic epidemiological data. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace/Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Universita di Napoli, Secondo ateneo. Dec 2007;67(4):184–208.
- Thabut G. [National cohort of patients with emphysema and alpha-1 antitrypsin deficiency]. Revue des Maladies Respir 2005; 22(6 Pt 1):1053–1057.
- Thabut G, Mornex JF, Cuvelier A, et al. [Characteristics of the patients included in the French cohort of patients with emphysema caused by alpha-1 antitrypsin deficiency]. Revue des Maladies Respir 2008; 25(9):1115–1122.
- Thabut G, Mornex JF, Pison C, et al. Performance of the BODE index in patients with alpha1-antitrypsin deficiency-related COPD. Euro Respir J 2014; 44(1):78–86.
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease; 2011. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed 01/15/2015.
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Amer J Respir Crit Care Med 2007; 176(6):532–555.
- A registry of patients with severe deficiency of alpha 1-antitrypsin. Design and methods. The Alpha 1-Antitrypsin Deficiency Registry Study Group. Chest 1994; 106(4):1223–1232.
- Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry Study Group. Amer J Respir Crit Care Med 1998; 158(1):49–59.
- Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. New Engl J Med 2011; 365(13):1184–1192.
- Carpenter MJ, Strange C, Jones Y, et al. Does genetic testing result in behavioral health change? Changes in smoking behavior following testing for alpha-1 antitrypsin deficiency. Ann Behav Med 2007; 33(1):22–28.
- Manca S, Rodriguez E, Huerta A, et al. Usefulness of the CAT, LCOPD, EQ-5D and COPDSS scales in understanding the impact of lung disease in patients with alpha-1 antitrypsin deficiency. COPD 2014; 11(5):480–488.
- Pillai AP, Turner AM, Stockley RA. Global Initiative for Chronic Obstructive Lung Disease 2011 symptom/risk assessment in alpha1-antitrypsin deficiency. Chest 2013; 144(4):1152–1162.