Abstract
The Behavioral Risk Factor Surveillance System (BRFSS) survey is used to estimate chronic obstructive pulmonary disease (COPD) prevalence and could be expanded to describe respiratory symptoms in the general population and to characterize persons with or at high risk for the disease. Tobacco duration and respiratory symptom questions were added to the 2012 South Carolina BRFSS. Data concerning sociodemographics, chronic illnesses, health behaviors, and respiratory symptoms were collected in 9438 adults ≥ 35 years-old. Respondents were categorized as having COPD, high risk, or low risk for the disease. High risk was defined as no self-reported COPD, ≥ 10 years’ tobacco use, and ≥ 1 respiratory symptom (frequent productive cough or shortness of breath (SOB), or breathing problems affecting activities). Prevalence of self-reported and high-risk COPD were 9.1% and 8.0%, respectively. Overall, 17.3%, 10.6%, and 5.2% of all respondents reported activities limited by breathing problems, frequent productive cough, and frequent SOB, respectively. The high-risk group was more likely than the COPD group to report a productive cough and breathing problems limiting activities as well as being current smokers, male, and African-American. Health impairment was more severe in the COPD than the high-risk group, and both were worse than the low-risk group.
Conclusions: Persons at high risk for COPD share many, but not all, of the characteristics of persons diagnosed with the disease. Additional questions addressing smoking duration and respiratory symptoms in the BRFSS identifies groups at high risk for having or developing COPD who may benefit from smoking cessation and case-finding interventions.
Introduction
Chronic obstructive pulmonary disease (COPD) may be the most preventable major cause of death from chronic disease in the United States, as it has one of the highest rates of smoking-attributable mortality (Citation1). Although chronic lower respiratory disease (> 95% COPD) is the third-leading cause of mortality in the United States (Citation2), the burden of COPD is even more substantial as a co-morbidity in other common causes of deaths (Citation3,Citation4). Clearly, more extensive efforts are needed not only for primary prevention, but also for secondary prevention through greater recognition of undiagnosed disease. It has been reported that there are as many or more persons with undiagnosed COPD as are diagnosed with the disease (Citation5–Citation7). Strategies to help target undiagnosed patients include the use of risk-defining questionnaires (Citation7–Citation11) and spirometry for case-identification among persons at risk for COPD (Citation12,Citation13).
Surveillance studies used to estimate the prevalence and impact of COPD in the United States include the National Health and Nutrition Examination Survey (NHANES) (Citation5), National Health Interview Survey (NHIS) (Citation14), and more recently the Behavioral Risk Factor Surveillance System (BRFSS) (Citation14–Citation16). The BRFSS is an annual, state-based, telephone health survey that collects data on sociodemographics as well as many of the behaviors and conditions that place adults at risk for chronic disease, typically greater than 10,000 respondents in each state, and collectively in almost one-half million nationally. In 2007, a COPD prevalence question and a COPD impact module were added to one state's (North Carolina's) BRFSS (Citation15). The prevalence question used in North Carolina was subsequently introduced in all states’ BRFSS in 2011, with numerous states also incorporating the optional COPD impact module (Citation16).
In 2012, we added an additional COPD module to the South Carolina (SC) BRFSS, termed the COPD at-risk module, which included questions about respiratory symptoms and duration of tobacco use. Through the addition of the COPD at-risk module with other COPD-related questions previously incorporated in the BRFSS, we hypothesized that the survey could be a valuable tool to describe respiratory symptoms in the general adult population, health-related characteristics of persons with undiagnosed or at significant risk for developing COPD as well as those with self-reported provider-diagnosed COPD.
Methods
Study population and survey
The BRFSS, a random digit-dialed telephone survey of non-institutionalized adults (≥18 years), is conducted annually with assistance from the Centers for Disease Control and Prevention (CDC) by state health departments as well as the District of Columbia and U.S. territories. In 2012, the South Carolina Department of Health and Environmental Control and North Carolina COPD Taskforce collaborated to include the COPD at-risk questions as a supplementary module (COPD at-risk module). The module was primarily intended to identify respondents at high risk of having or developing COPD and consisted of 4 additional questions: 3 regarding respiratory symptoms and 1 to define lifetime smoking duration. A secondary objective was to describe respiratory symptoms in a state's general adult population. The at-risk questions were derived from previously published COPD screening questionnaires (Citation8–Citation11).
The survey was administered by the University of South Carolina Institute for Public Service and Policy Research to 12,795 SC adults (9208 landline and 2606 cellular telephone respondents completed the survey, and 620 landline and 361 cellular telephone respondents partially completed the survey). Response rates for BRFSS were calculated using the American Association of Public Opinion Research Response Rate Formula #4 standards. (http://www.aapor.org/Standard_Definitions2.htm). The response rate for SC in 2012 was higher than the national median rate (48.6% vs 45.2%) (Citation17). Due to the low prevalence and risk for COPD in younger adults, we limited our analyses to adults aged ≥ 35 years (n = 10,863). Respondents were excluded if they were missing data on COPD (n = 3), smoking status (n = 218), smoking duration (n = 584), or one or more respiratory symptoms (n = 1085) (total excluded = 1425). The final sample size for data analysis included 9438 adults (87% of respondents ≥ 35 years). Beginning in 2011, a new weighting methodology (raking, also known as iterative proportional fitting) replaced post-stratification for calculation of sample weights. BRFSS uses 8 demographic variables (age group by gender, race/ethnicity, education, marital status, tenure, gender by race/ethnicity, age group by race/ethnicity, and phone ownership) in the raking procedure. Raking adjusts for non-coverage and non-response (Citation18). The BRFSS has been approved as exempt research by the CDC's institutional review board.
Respiratory symptoms in the at-risk module
Respondents were asked, “How often do you cough up mucus or phlegm?” Frequent productive cough was defined as either most days a week or every day. Respondents were also asked, “During the past 30 days, how often did you feel short of breath?” Frequent shortness of breath (SOB) was defined as most of the time or all of the time. Finally, respondents were asked, “Thinking about your physical activity during the last 12 months, do you agree slightly or strongly or disagree slightly or strongly with the following statement—I do less now than I used to because of my breathing problems.” Respondents who agreed strongly or slightly were characterized as agreeing that breathing problems limited their physical activity. These questions were derived from prior COPD screening questionnaires (Citation8–Citation10).
Tobacco use and duration
All respondents were asked, “Have you smoked at least 100 cigarettes in your entire life?” Persons with a negative response were categorized as never smokers. Persons with an affirmative response were further asked, “Do you now smoke cigarettes every day, some days, or not at all?” to define current and former smokers. Current and former smokers were asked, “Over your lifetime, how many years have you smoked tobacco products?”
Prevalence and risk of COPD
All respondents were asked, “Has a doctor, nurse, or other health professional ever told you that you had chronic obstructive pulmonary disease or COPD, emphysema, or chronic bronchitis?” Persons with self-reported COPD were also asked whether they had ever undertaken a diagnostic breathing test and if SOB affected their quality of life. Three COPD risk categories were defined based upon prior diagnosis, respiratory symptoms, and tobacco use duration: 1) Diagnosed COPD: self-report of provider-diagnosed COPD; 2) High-risk: respondents with no COPD diagnosis, ≥ 10 years of tobacco product use, and ≥ 1 respiratory symptom (frequent productive cough, frequent SOB, or decreased physical activity due to breathing problems); and 3) Low-risk: neither diagnosed with COPD nor at high risk. In addition to sociodemographic variables, all respondents were also asked about their access to health care, chronic conditions, and overall health status.
Statistical analysis
All analyses were conducted using SAS-callable SUDAAN (version 11.0.0, RTI International, Research Triangle Park, NC) to account for the complex sampling design. First, we calculated the prevalence and 95% confidence intervals (CI) of respiratory symptoms and COPD by selected characteristics. For comparisons of prevalence between subgroups, statistical significance was determined using t-tests. We also tested for linear trends across age and smoking duration categories using the Wald F-statistic. Age-adjusted prevalence and 95% CI of selected characteristics by COPD risk status were then calculated. Prevalences were compared by risk status using pairwise t-tests. A p-value <0.05 was considered statistically significant for all tests.
Results
Among 9438 adults aged ≥35 years, 9.1% were diagnosed with COPD, 8.0% were high risk for COPD, 10.6% reported frequent productive cough, 5.2% reported frequent SOB, and 17.3% agreed that physical activity was limited over the past 12 months due to breathing problems. In persons with COPD, 82.0% (78.4–85.3) reported having a diagnostic breathing test, 70% (65.2–74.3) reported SOB affected their quality of life. Table shows the prevalence of each respiratory symptom, if frequent, and COPD for groups defined by selected characteristics. The prevalence of respiratory symptoms did not differ significantly by gender; however, diagnosed COPD was more common among women than men (9.9% vs. 8.1%, p = 0.03). The prevalence of all respiratory symptoms and COPD increased with age (linear trend p < 0.001 for frequent productive cough, breathing problems limited physical activity, and COPD; p = 0.009 for frequent SOB). Compared to Whites, African-Americans were more likely to report frequent SOB and that breathing problems limited physical activity, but less likely to report being diagnosed with COPD. Those who were unable to work reported the highest prevalence of each respiratory symptom and COPD. Among those who were unable to work, half agreed that breathing problems limited their physical activity and more than a quarter had COPD. There was a higher prevalence of respiratory symptoms and COPD among individuals in lower education groups. Frequent respiratory symptoms and COPD were more common among current smokers than former smokers and among former smokers than never smokers (p < 0.01). The prevalence of each respiratory symptom and COPD also increased with years of smoking duration (linear trend p < 0.0001 for all).
Table shows characteristics of SC adults aged ≥ 35 years among the three risk categories. Compared with adults diagnosed with COPD, those at high risk were more likely to be male, African-American, and current smokers and less likely to be female, or unable to work. Combined, nearly 60% of persons at high-risk were in the 45–54 and 55–64-year-old age groups. Prevalence of various health-related characteristics and respiratory symptoms were also assessed by COPD risk status (Table ). Compared with adults diagnosed with COPD, those at high risk were more likely to report fewer chronic conditions including asthma, arthritis, cancer, and depression; frequent productive cough; and that breathing problems limited physical activities.
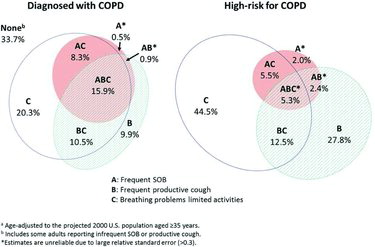
Health impairment measures such as frequent physical distress were less prevalent in the high-risk than the COPD group, and both groups were substantially worse than the low-risk group. The Venn diagrams in Figure show the relationships of the prevalence for each symptom (frequent only) and overlap for the COPD and high-risk groups. Persons with COPD were three-fold more likely to report all three symptoms than those at high-risk. One-third of persons with COPD did not report frequent productive cough, frequent SOB, nor significant effects of breathing problems on physical activities.
Table 1. Percentage of respiratory symptoms and chronic obstructive pulmonary disease (COPD) among South Carolina adults aged ≥35 years by selected characteristics–BRFSS, 2012a
Table 2. Age-adjusteda prevalence and 95% confidence intervals (95% CI) of selected characteristics among South Carolina adults aged ≥35 years by COPD risk statusb-BRFSS, 2012
Table 3. Age-adjusteda prevalence and 95% confidence intervals (95% CI) of health characteristics and respiratory symptoms among South Carolina adults aged ≥35 years by COPD risk statusb-BRFSS, 2012
Discussion
We undertook the first use of the BRFSS to assess respiratory symptoms in the adult population, primarily to describe individuals at high risk for COPD as well as those with COPD. Among all subjects, the most frequent respiratory symptom reported was physical activities limited by breathing problems. Respiratory symptoms were uncommon in persons who smoked less than 10 years and did not self-report COPD (low-risk group). Among all survey participants, we found 1 in 6 adults aged ≥35 years in SC reported COPD or were at high risk of having or developing the disease based upon respiratory symptoms, age, and tobacco history; furthermore, there were nearly as many adults in the high-risk as in the COPD group.
The high-risk group likely includes those with undiagnosed COPD or at significant risk of ultimately developing the disease. The high-risk and COPD groups shared many sociodemographic and health characteristics, however some measures differed. One key difference was gender; in contrast to those with diagnosed COPD where the percentage of females was greater, males were more likely to be at high risk than females. In addition, African-Americans were also more likely to be at high risk for COPD.
Low socioeconomic status was evident in both the high-risk and COPD groups. Healthcare access was similar between these two groups, suggesting the need for better case-finding efforts in clinical settings and ready availability of spirometry. The inclusion of COPD screening questions in the BRFSS could be used to describe persons for whom population-based and case-finding efforts for COPD might be undertaken at local and state levels. Further, this approach helps better define respiratory symptoms in a general adult population for which there is a paucity of data in the United States.
There are few published studies concerning respiratory symptoms, particularly SOB, in adults at risk for or with COPD in a general adult population (Citation5,Citation19). In our study, frequent productive cough was reported in ∼10% of all respondents, one-third of those with COPD, and one-half of the high-risk group. In a study using 2007–2010 NHANES data, frequent phlegm or cough were reported in 7.4% and 10.8% of all subjects (n = 5139), 9.1% and 9.3% of those with mild airflow obstruction, and 19.8% and 24.2% in moderate to severe airflow obstruction, respectively (Citation19). In our study, the more frequent productive cough in high-risk persons compared to diagnosed COPD and the low-risk group may be related to differences in current smoking rates. Respiratory symptoms, particularly productive cough, are common in smokers, especially in those with diagnosed (Citation20) or undiagnosed COPD (Citation21).
Similar to our results, a large international study reported 34.6% of COPD patients had chronic bronchitis (defined as “phlegm on most days for 3 or more consecutive months during the year and trouble with phlegm for 2 or more years”) (Citation22). Our findings also agree with a study in which COPD patients with chronic bronchitis were younger and more likely to be current smokers compared with those without (Citation23). Chronic bronchitis is associated with poor quality of life, more exacerbations (Citation19,Citation24) and greater mortality (Citation25). Dyspnea has been reported in 30-50% of persons with undiagnosed airflow obstruction (Citation6,Citation26). Frequent dyspnea, seen in a higher portion of COPD than high-risk persons in this study, is likely reflective of more advanced disease, increasing the probability of diagnosis (Citation26). Hanania et al. reported that a combination of both dyspnea and cough are predictive of airflow obstruction in COPD patients (Citation10). We found that the minority of persons in both the COPD and high-risk groups reported two or more frequent respiratory symptoms (Figure ). People with frequent productive cough and limitation of activities due to breathing problems, but not frequent SOB, may represent earlier stages of COPD.
A diagnosis of COPD is reported in 6–7% of U.S. adults (Citation5,Citation16). We found the age-adjusted prevalence of self-reported COPD in SC to be 9.1%, among whom more than 80% indicated they had had a diagnostic breathing test, presumably spirometry. Prior studies have reported the relationship between self-reported, provider-diagnosed COPD and spirometrically confirmed disease (Citation27,Citation28). One study of a cohort of nurses has shown a good correlation between self-reported and spirometry-confirmed COPD (78% sensitivity) (Citation27). In a NHANES study of a representative sample of the U.S. adult population, > 95% of persons with self-reported, provider-diagnosed chronic bronchitis or emphysema had evidence of mild or moderate airflow obstruction by spirometry (Citation28).
Among a sample of the Swedish general population, self-reported physician diagnosis of COPD had high specificity (99%) and good positive predictive value for airflow obstruction assessed by spirometry, but low sensitivity, which could result in underestimated prevalence in the general population (Citation29). Based on the at-risk module, without spirometry, we found the prevalence of high risk for COPD to be almost as high as that of diagnosed COPD. The NHANES health surveys have reported that there are more persons with airflow obstruction based upon spirometry than those known to have asthma or COPD (Citation5,Citation29). Whether based on population screening including spirometry as done in NHANES or using a symptom and exposure-based questionnaire like the current study, the number of probable undiagnosed COPD patients is substantial.
Similar to studies that compared diagnosed to undiagnosed COPD patients (Citation6,Citation7) the COPD and high-risk groups in our study shared many characteristics. Prolonged smoking, greater than 20 years, was common in both the high-risk and COPD groups. Like other studies, we found that low socioeconomic status is common in both diagnosed (Citation30) and high-risk persons, likely related to higher smoking rates (Citation7,Citation31), and that females were more prevalent in the diagnosed COPD group (Citation5). Our data in the high-risk group agrees with a study of undiagnosed COPD where males were more prevalent (Citation6); however, the same study also found a higher prevalence of diagnosed COPD among men. Our observation concerning African-Americans is a new finding. Although tobacco use rates are similar between whites and African Americans (Citation1), COPD-related mortality is most evident in the former group (Citation14,Citation32). Our data suggest this may be partially related to under-recognition. As most COPD patients are managed in the primary care setting (Citation33), general practitioners should be able to recognize characteristics of high-risk patients to assist in case-finding efforts.
COPD is a complex respiratory disease associated with systemic manifestations and co-morbidities including asthma, heart disease, musculoskeletal disease, depression, and cancer (Citation34–Citation37). Little has been published about the type or prevalence of co-morbidities in undiagnosed COPD (Citation38). We found that some co-morbidities were more common in the COPD group than either the high-risk or low-risk groups, particularly asthma, cancer, and depression. Coronary heart disease, diabetes mellitus, stroke, and kidney disease occurred at similar frequencies between the COPD and high-risk groups. Multiple (> 3) co-morbidities were more common in both the COPD and high-risk groups compared to the low-risk group. The increased risk of co-morbidities in the high-risk group compared to the low-risk group is important considering the significant impact on health status, healthcare utilization, hospitalization, and mortality in COPD (Citation34,Citation35).
Our approach to identify at-risk persons based on age, tobacco use, and respiratory symptoms is consistent with COPD guidelines that recommend case-finding in symptomatic persons with an exposure history, but discourage population screening in asymptomatic persons (Citation39,Citation40). It has been shown that the majority of asymptomatic persons with airflow obstruction have mild disease (Citation7,Citation33), and there is little evidence that diagnosing asymptomatic smokers leads to better outcomes (Citation40). However, health impairment is often present in people with undiagnosed COPD (Citation6,Citation7), and spirometry may increase smoking cessation effectiveness (Citation41). We also observed significant health impairment in the high-risk group, although the contribution of respiratory symptoms versus other etiologies is unclear.
When using respiratory symptoms and/or smoking history to identify undiagnosed COPD patients in primary care settings, 13–27% of those at-risk were spirometrically diagnosed (Citation10,Citation13,Citation22,Citation42,Citation43). Patient care settings such as indigent clinics with higher smoking frequencies may yield higher rates of COPD diagnosis. The validity of using a symptom-based method to screen for COPD is supported by studies showing dyspnea (Citation44), chronic bronchitis (Citation45), or a modified BODE score (Citation46) (multiple measures including dyspnea) were a stronger predictor than FEV1 alone for mortality in COPD.
Our study had several limitations. Responses were obtained by telephone survey; therefore we relied on self-report of symptoms, medical conditions, and smoking history. Reports of COPD diagnoses were not confirmed with spirometry or review of medical records, which could result in misclassification of respondents. However, the validity of COPD by self-report in the SC BRFSS respondents is supported by:
82% reported a prior diagnostic breathing test;
70% indicated SOB affected their quality of life;
Disease prevalence is similar to another U.S survey, National Health Information Survey, for the East South Central states (9.1% vs 7.5%) (Citation47);
Frequency of current tobacco use and co-morbidities including asthma and diabetes mellitus in the current study were similar to a study conducted in the southeastern Kentucky adult population (Citation48); and,
Two prior studies report that > 95% of persons with a self-report of provider-diagnosed COPD have airflow obstruction, when confirmatory spirometry was performed (Citation28,Citation29).
Although pack-years (product of intensity and duration) is the most frequent clinical measure for tobacco exposure, we used smoking duration, as two survey questions would have been required otherwise.
A study comparing the effects of smoking status, duration, and intensity on lung cancer risk reported current smoking and intensity were the best predictors of risk, however duration was only slightly less predictive (Citation49). In addition, a recent study assessing tobacco-related mortality over 50 years showed that smoking duration and intensity were both associated with increased COPD mortality (Citation50). We did not include persons with no or <10 years of smoking with frequent symptoms as part of the high-risk group, thus missing a potentially important group since a quarter of patients with COPD are never-smokers (Citation51). The addition of cellular telephones to target respondents began in 2011 and the BRFSS survey response rates have decreased to less than 50%. Inclusion of these households has improved coverage of respondents with lower income or education levels, as well as younger respondents (Citation52). Finally, this survey was performed in one state and additional studies will be undertaken in 2015 to determine the external validity and generalizability of these findings.
Conclusions
By using a questionnaire incorporated into the BRFSS to estimate the frequency of cough, SOB, physical impairment associated with breathing problems, and years of tobacco use, we were able to describe respiratory symptoms in a general adult population, better characterize persons with COPD, and to also identify a target population at high risk for having or developing COPD. We found there were many similarities between persons who reported a provider-diagnosis of COPD and those who were at high risk. However, males and African-Americans appear to be at greater risk for having undiagnosed COPD than their comparators. Our surveillance approach addresses several goals stated in the USA CDC's Public Health Strategic Framework for COPD Prevention (Citation53) and could provide a forum for advocacy, policy change, and for influencing clinical practice at local and state levels. An approach that combines public health (prevention, surveillance) and healthcare (case finding, guideline-based care) strategies can potentially have a significant impact on COPD, the most preventable cause of death from a chronic disease in the United States.
Acknowledgments
The inclusion of questions in the South Carolina Behavioral Risk Factor Surveillance System was supported by the Learn More Breathe Better COPD Campaign.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of Interest Statement
RAP has served as consultant for Astra Zeneca and research grants from Glaxo Smith Kline and Boehringer Ingelheim. JAO - advisory boards for Boehringer-Ingelheim, CSL Behring, Astra Zeneca, and Novartis. CS -consultant for AstraZeneca PLC, CSL Behring, and Grifols on topics related to COPD, outside of the current work. He has grants in COPD outside of the current work from the Alpha-1 Foundation, CSL Behring, Entera Health, Grifols and the National Institutes of Health. DMM - has received honoraria/consulting fees and served on speaker bureaus for GlaxoSmithKline plc, Novartis Pharmaceuticals, Pfizer Inc. Boehringer- Ingelheim, Astra Zeneca PLC, Forest Laboratories Inc., Merck, Amgen and Creative Educational Concepts. Furthermore, he has received royalties from Up-to-Date and is on the Board of Directors of the COPD Foundation. MK - Monica Kraft receives grant/research support from Chiesi, Genentech, GlaxoSmithKline and Merck and other financial or material support from NIH. KH, AGW, JBC, and WL have no disclosures.
More than 2 authors (RAP, AGW) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors alone are responsible for the content and writing of the paper.
References
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Printed with corrections, January 2014.
- Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2010. Natl Vital Stat Rep 2013; 611–699.
- Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J 2003; 22:809–814.
- Zielinski J, MacNee W, Wedzicha J, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis 1997; 52:43–47.
- Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: Data From the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2000; 160:1683–1689.
- Coultas DB, Mapel D, Gagnon R, Lydick E. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med 2001; 164:372–377.
- Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: Impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64;863–868.
- Price D, Tinkelman DG, Nordyke RJ, et al. Scoring system and clinical application of COPD diagnostic questionnaires. Chest 2006; 129:1531–1539.
- Martinez FJ, Raczek AE, Seifer FD, et al. Development and initial validation of a self-scored COPD population screener questionnaire (COPD-PS). COPD 2008; 5:85–95.
- Hanania NA, Mannino DM, Yawn BP, et al. Predicting risk of airflow obstruction in primary care: Validation of the lung function questionnaire (LFQ). Respir Med 2010; 104:1160–1170.
- Calverley PM, Nordyke RJ, Halbert RJ, et al. Development of a population-based screening questionnaire for COPD. COPD 2005; 2:225–232.
- Price D, Crockett A, Arne M, et al. Spirometry in primary case-identification, diagnosis and management of COPD. Prim Care Respir J 2009; 18:216–223.
- Saad N, Sedeno M, Metz K, Bourbeau J. Early COPD diagnosis in family medicine practice: how to implement spirometry? Int J Family Med 2014; Article ID 962901.
- Ford ES, Croft JB, Mannino DM, et al. COPD Surveillance—United States, 1999–2011. Chest 2013; 144:284–305.
- Herrick H, Pleasants R, Wheaton AG, et al. chronic obstructive pulmonary disease and associated health-care resource use – North Carolina, 2007 and 2009. MMWR Morb Mortal Wkly Rep 2012; 61:143–146.
- Kosacz NM, Punturieri A, Croxton TL, et al. Chronic obstructive pulmonary disease among adults — United States, 2011. MMWR Morb Mortal Wkly Rep 2012; 61:938–943.
- Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System 2012 Summary Data Quality Report. Available from: http://www.cdc.gov/brfss/annual_data/2012/pdf/SummaryDataQualityReport2012_20130712.pdf ( accessed 3 July, 2013).
- Mawokomatanda T, Flegel D, Pierannunzi C, et al. Centers for Disease Control and Prevention (CDC). Surveillance for certain health behaviors among states and selected local areas—Behavioral risk factor surveillance system, United States, 2011. MMWR Surveill Summ 2014; 63:1–149.
- Wheaton AG, Ford ES, Thompson WW, et al. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the United States–National Health and Nutrition Examination Survey 2007–2010. BMC Public Health 2013; 13:854.
- Pelkonen M, Notkola IL, Nissinen A, et al. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: A follow-up in middle-aged rural men. Chest 2006; 130:1129–1137.
- Ohar JA, Sadeghnejad A, Meyers DA, et al. Do symptoms predict COPD in smokers? Chest 2010; 137:1345–1353.
- Agusti A, Calverley PM, Celli B, et al. Characterization of COPD heterogeneity in the ECLIPSE Cohort. Respir Res 2010; 11:122.
- Kim V, Han MK, Vance GB, et al. The chronic bronchitis phenotype of COPD: An analysis of the COPD Gene Study. Chest 2011; 140:626–633.
- Martinez CH, Kim V, Chen Y, et al. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014; 108:491–499.
- Ekberg-Aronsson M, Pehrsson K, Nilsson JA, et al. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005; 6:98.
- Nascimento OA, Camelier A, Rosa FW, et al. Chronic obstructive pulmonary disease is underdiagnosed and undertreated in Sao Paulo (Brazil): Results of the PLATINO Study. Braz J Med Biol Res. 2007; 40:887–895.
- Barr RG, Herbstman J, Speizer PE, et al. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155:965–971.
- Halldin CN, Doney BC, Hnzido E. Changes in prevalence of chronic obstructive pulmonary disease and asthma in the US population and associated risk factors. Chron Respir Dis 2014;1–14.
- Murgia N, Brisman J, Claesson A et al. Validity of a questionnaire-based diagnosis of chronic obstructive pulmonary disease in a general population-based study. BMC Pulm Med 2014; 14:49–55.
- Tilbert T, Paulose-Ram R, Brody DJ. Lung obstruction among adults 40–79: United States, 2007–2012. NCHS data brief, no. 180. Hyattsville, MD: National Center for Health Statistics, 2015
- Schirnhofer L, Lamprecht B, Firlei N, et al. Using targeted spirometry to reduce non-diagnosed chronic obstructive pulmonary disease. Respiration 2011; 81:476–482.
- Heron M. Deaths: Leading causes for 2007. Natl Vital Stat Rep. 2011; 59:1–95.
- Heins-Nesvold J, Carlson A, King-Schultz L, Joselyn KE. Patient identified needs for chronic obstructive pulmonary disease versus billed services for care received. Int J Chron Obstruct Pulmon Dis 2008; 3:415–421.
- Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:155–161.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of comorbidities. Eur Respir J 2006;28:1245–1257.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension, and cardiovascular disease in COPD. Eur Respir J 2008; 32:962–969.
- Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med 2013; 1:73–83.
- Van Remoortel H, Hornikx M, Langer D, et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189:30–38.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of COPD [online] 2014. Available from http://www.goldcopd.com/ ( accessed 25 May, 2014).
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155:179–191.
- Bednarek M, Gorecka D, Wielgomas J, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax 2006; 61:869–873.
- Tinkelman DG, Price D. Nordyke RJ, Halbert RJ. COPD screening efforts in primary care: What is the yield? Prim Care Respir J 2007; 16:41–48.
- Yawn B, Duvall K, Peabody J, et al. The impact of screening tools on diagnosis of chronic obstructive pulmonary disease in primary care. Am J Prev Med 2014; Sept 15. (Epub ahead of print).
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121:1434–1440.
- Guerra DL, Sherrill C, Venker C, et al. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009; 64:894–900.
- Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173:1326–1334.
- Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS data brief, no 63. Hyattsville, MD: National Center for Health Statistics, 2011.
- Methvin J, Mannino D, Casey B. COPD prevalence in southeastern Kentucky: The burden of lung disease study. Chest 2008; 135:102–107
- Leffondré K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling Smoking History: a Comparison of Different Approaches. Am J Epidemiol 2002; 156:813–823.
- Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013; 368:351–364.
- Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the third National Health and Nutrition Examination Survey. Am J Med 2005; 118:1364–1372.
- Centers for Disease Control and Prevention. Methodologic Changes in the Behavioral Risk Factor Surveillance System in 2011 and Potential Effects on Prevalence Estimates. MMWR Morb Mortal Wkly Rep 2012; 61:410–413.
- Centers for Disease Control and Prevention. Public Health Strategic Framework for COPD Prevention. Atlanta, GA: Centers for Disease Control and Prevention, 2011.