Abstract
Polymorphisms in the nicotinic acetylcholine receptor gene (CHRNA5/CHRNA3 locus) have been associated with several smoking related traits such as nicotine dependence, cigarette consumption, smoking cessation, lung cancer, and COPD. The aim of this candidate gene study was to study the locus among the Finnish COPD patients and long-term smokers with regard to COPD risk, smoking behavior, cancer, and all-cause mortality. Genotyping of rs1051730, the locus tagging SNP was done in two longitudinal cohorts: Finnish COPD patients (N = 575, 74% men) and long-term smokers, all men (N = 1911). Finnish population sample (N = 1730) was used as controls. The analyses were done using logistic and Cox regression. The main findings were that the minor allele increased the risk of COPD when compared to the Finnish population at large (OR = 1.4, 95% CI 1.2-1.7, p = 3.2 × 10-5). Homozygosity for the risk allele was associated in both cohorts with all-cause mortality (crude HR 2.2, 95% CI 1.2–3.8 and 1.3, 95% CI 1.1–1.5, respectively), with any type of cancer (crude OR 2.3, 95% CI 1.0–5.1) among the COPD patients and with the number of pack-years (crude OR 1.4, 95% CI 1.1–1.9) among the male smokers. CHRNA5/CHRNA3 locus tagged by rs1051730, which has been previously associated with several smoking related diseases was now shown to be associated also with increased all-cause mortality among long-term smokers with or without clinical COPD further emphasizing the clinical importance of the finding.
Introduction
Cigarette smoking is a known risk factor for chronic obstructive pulmonary disease (COPD), several types of malignant diseases, ischemic heart disease, and stroke (Citation1–3). However, the susceptibility to these smoking associated diseases varies largely between individuals. Three neuronal nicotinic acetylcholine receptors encoding genes (CHRNA3-CHRNB4-CHRNA5) form a cluster on the chromosome 15q25. Several single nucleotide polymorphisms (SNPs) have been described in tight linkage disequilibrium (LD) and many of those have shown a consistent association to nicotine dependence and multiple smoking related traits (Citation4, Citation5). SNP rs1051730 is located in the exon of CHRNA3 gene and causes a synonymous nucleotide substitution Citation(6). It is in complete LD with another widely studied SNP, rs16969968 (in HapMap3 samples of European ancestry r2 = 1.0) (Citation7–9). This SNP is located in the CHRNA5 gene and causes an amino acid substitution (D398N). The amino acid change is located at a highly conserved site of the protein and potentially causes functional changes in the receptor.
Using either of these tagging SNPs the CHRNA5/CHRNA3 locus was first associated with nicotine dependency (OR 1.3–1.4) (Citation6, Citation9) and with the number of cigarettes smoked per day (CPD). The genetic effect was additive, each risk allele increased the number of smoked cigarettes per day by one (Citation4, Citation10). Then consistent associations were found with smoking related diseases such as lung cancer (OR 1.2-5.7) (Citation6, Citation11–13) and COPD (OR 1.3–1.4) (Citation14–16) in multiple population cohorts of European ancestry. The locus has been also associated with poorer outcome of smoking cessation attempts among pregnant women (OR 1.2–1.3) (Citation17, Citation18). Two studies have reported an independent association with the CHRNA5/CHRNA3 locus and severity of smoking related emphysema and accelerated lung function decline (Citation15, Citation19). In the most recent studies the locus has been associated with schizophrenia Citation(20), alcohol and substance abuse Citation(21) and furthermore, non-disease outcomes such as lower cognitive performance and high interest in novelty seeking personality (Citation22, Citation23).
So far, the majority of the associations have been established in population cohorts and fewer in patient populations such as COPD patients. COPD patients usually are both heavy and early-onset smokers and they display several co-morbidities such as atherosclerosis, diabetes, osteoporosis, cachexia, depression and anxiety which can dramatically impair the prognosis of the disease. In the present candidate gene study of smoking related COPD, we aimed to estimate first the prevalence of rs1051730 among COPD patients compared with the general adult Finnish population. Then we further studied the association of rs1051730 with pack-years, cancer prevalence, and all-cause mortality in the COPD patient cohort. We also replicated the analyses in another cohort of Finnish male smokers.
Materials and methods
COPD cohort
COPD patients (N = 575) were a randomly selected subcohort of the Finnish Chronic Airway Disease (CAD) cohort. CAD cohort was identified from the Discharge Registries of the Helsinki and Turku University Hospitals (ICD10 J44.8) collected during years 2005–2007 Citation(24). DNA extraction and genotyping was done in collaboration with DeCode Genetics Inc. Citation(6).
At recruitment patients gave their permission to collect their medical records from which data were drawn on spirometry, weight, height, smoking status (current/former smoker), smoking history (number of pack-years), and history of chronic co-morbidities such as coronary disease, cerebrovascular disease and peripheral artery occlusive disease, cancer, alcohol abuse (including chronic alcoholism and alcohol use related disorders), psychiatric conditions needing regular medication, and type I and II diabetes Citation(24). If smoking status and pack-years could not be found from medical records, the data was acquired from a follow-up questionnaire. 348 (60.5%) patients were former smokers (Citation25). One hundred forty-one (24.5%) patients died during the seven-year follow-up. Data on death was acquired from Helsinki and Turku University Hospitals’ archives. At recruitment 5% of the deceased had FEV1 > 80%, 15% had FEV1 80–65%, 43% had FEV165–40%, and 37% FEV1< 40% of predicted.
Population control group
Health2000 is a nationally representative sample of adult Finns aged > 30 Citation(26). A random subsample of 1730 subjects with normal lung function (FEV1 >80% of predicted) was selected and their rs1051730 genotyped. Forty percent (N = 811) of them were men and the mean age was 50 years (SD 11 years).
Male smokers
The main analyses were repeated in the placebo arm of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC-study) Citation(27) (). The cohort consisted only of men, 50 to 69 years of age, who smoked at least 5 cigarettes per day and had no history of cancer at enrollment in 1985–1988. Based on self-reported data, alcohol use >40 g per day was considered as alcohol abuse Citation(28). A whole blood sample was collected 3 to 7 years after the enrollment (years 1991–1992) and rs1051730 was genotyped for a random subsample of 1911 men Citation(29). During the 18-year follow-up after (blood) sampling, 1485 men (77.7%) of the cohort deceased. Data on death were derived from Statistics Finland.
Table 1. Background characteristics of the COPD patients and male smokers.
Statistical analysis
The association between COPD and rs1051730 was analyzed using PLINK (version 1.07). All the regression models were done using the statistical software package SPSS (version 20.0). The association of rs1051730 with pack-years and prevalence of cancer were analyzed with logistic regression. Cox regression was used to analyze all-cause mortality. The multivariate models were adjusted for age, gender, smoking cessation, pack-years, BMI, spirometry, and prevalence of cancer, cardiovascular disease, diabetes, and psychiatric disease for the COPD cohort; and for age, smoking cessation, pack-years, alcohol abuse, and BMI for the male smoker cohort. Kaplan–Meier analysis with log rank tests was used to analyze survival. Likelihood ratio test was used to test interactions between the genotype and background characteristics. Level of statistical significance was p < 0.05 in all analyses.
The study design was approved by the Coordinating Ethics Committee of Helsinki and Uusimaa Hospital District.
Results
COPD patients
The COPD cohort consisted of 575 men and women (mean age 64 years) who all had heavy smoking history (mean 41 pack-years). They represented all the stages of the disease as well as different common co-morbidities (). rs1051730 G>A allele was significantly more common among COPD patients (MAF 0.38) when compared to the general adult Finnish population with normal lung function (MAF 0.35, OR 1.4 (95%CI 1.20–1.65), p = 3.2 × 10-5).
Heavy smoking history was not associated with rs1051730 G>A allele among the COPD patients: smoking over 40 pack-years had an OR of 0.92 for GA heterozygotes and 0.86 for AA homozygotes compared with the major GG homozygotes (Table S1).But alcohol abuse, advanced COPD, current smoking, and age associated significantly with the pack-years >40 (Table S1). Of the background characteristics, the prevalence of any type of cancer associated significantly with rs1051730. Compared to the wild-type (GG), the risk of cancer of the GA heterozygotes was 1.8-fold (95% CI 0.9–3.3) and that of the AA homozygotes 2.3 fold (95% CI 1.0-5.1) (Table S2, supplementary information). The associations remained similar after adjustment for age, gender, BMI, severity of airway obstruction, pack-years, and alcohol abuse.
When all-cause mortality was studied among the COPD patients, rs1051730 associated with the risk of death. Compared to the wild-type, AA homozygotes had significantly elevated risk of death (HR 2.2, 95% CI 1.2–3.8) that increased a little after adjustment for severity of airway obstruction, smoking status, BMI and co-morbidities (HR 2.6, 95%CI 1.4–4.9) (). The risk of death was also slightly elevated among heterozygotes, though not statistically significant (HR 1.2 95% CI 0.7-1.9). In addition, severe (FEV1 < 40% of predicted) airway obstruction (HR 3.38, 95% CI 1.3–8.6), alcohol abuse (HR 2.8, 95% CI 1.9-4.2) and psychiatric diseases (HR 1.6, 95% CI 1.1–2.3) increased the risk of death, whereas former smoking associated with decreased mortality (HR 0.4, 95% CI 0.3–0.6).
Table 2. Risk factors for increased mortality in the study cohorts.
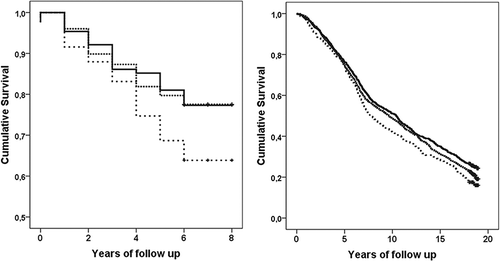
Kaplan–Meier analysis confirmed the poorer survival of the AA homozygotes compared with wild-type or GA heterozygote (). Effect modification of background characteristics on the association between rs1051730 and mortality was also analyzed and no statistically significant interactions were found. However, the association between rs1051730 and mortality seemed to be stronger among those COPD patients who were current smokers (HR 1.6, 95% CI 1.2–2.2) compared to that of former smokers (HR 1.1, 95% CI 0.7–1.5; p for interaction 0.14) and among those who had smoked at least 40 pack-years (HR 1.6, 95% CI 1.2–2.3) compared with less than 40 pack-years (HR 1.2, 95% CI 0.8–1.7; p for interaction 0.22).
Figure 1. Kaplan-Meier curves of overall survival during the follow up according to rs1051730 genotype (GG wild-type: solid line, GA heterozygote: dashed line, and AA homozygote: dotted line) in the COPD (left) and male smokers (right) cohorts. In COPD cohort the difference between the groups by log rank analysis is p = 0.03, in male smokers p < 0.01.
Male smokers
To further explore the role of rs1051730 in mortality, we repeated the analysis in the ATBC male smoker cohort (N = 1911 men) (). These men were recruited at about the same age, 63.5 years, and they had a similar heavy smoking history, 41 pack-years as COPD patients (). During the 18-year follow-up, 78% of the men deceased at a mean age of 73 years.
Similar association between rs1051730 and all-cause mortality as among COPD patients was observed among male smokers (). Compared to wild-type, both GA heterozygosity (HR 1.2, 95% CI 1.0–1.3) and AA homozygosity (HR 1.3, 95% CI 1.1–1.5) were significantly associated with increased risk of death. The associations changed only marginally after adjusting for smoking status, alcohol abuse, and BMI. In Kaplan-Meier analysis, AA homozygosity seemed to associate with poorer survival after seven year of follow-up (). Neither smoking cessation nor pack-years modified the association between rs1051730 and mortality. Among male smokers, unlike in COPD patients, both GA heterozygosity (OR 1.3, 95%CI 1.1–1.6) and AA homozygosity (OR 1.4, 95%CI 1.1–1.8) were associated with pack-years >40. The associations remained unchanged when the model was adjusted for age, alcohol abuse and former/current smoking.
Discussion
The present candidate gene study confirmed several previous findings on the association between the CHRNA5/CHRNA3 locus and smoking related traits and further expands the data on its role in mortality among heavy smokers. Even though the COPD cohort was rather small, rs1051730 was significantly associated with COPD when compared to the Finnish population at large. rs1051730 G>A allele frequency was approximately the same among Finns as among other European populations (MAF 0.35 vs. 0.33) (Citation14, Citation30).
From the clinical point of view the most remarkable finding of our study was the association between the CHRNA5/CHRNA3 locus and all-cause mortality both among COPD patients and long-term male smokers. The mean age of death in the COPD cohort was 69 years which is substantially less than in Finland in general (men 77 years, women 83 years Citation(31) or in the male smoker cohort 73 years). COPD patients suffer from multi-morbidity. We chose to study all-cause mortality since the contributing role of COPD in death is not always recognized. Among the hospitalized COPD patients, the main causes of death are acute respiratory or cardiac failure due to lung infections, malignancies, and ischemic cardiovascular diseases Citation(32). Sudden cardiac deaths are common among patients who die at home Citation(33). In the present cohort, 77% of deceased patients had moderate or severe airway obstruction suggesting that COPD is a significant contributing factor in their deaths. rs1051730 remained an independent risk factor for death in both study cohorts when the models were adjusted for other background factors suggesting that rs1051730 has a direct association with all-cause deaths. None of the variables modified significantly the association between rs1051730 and mortality. However, the association was stronger among the COPD patients who were current smokers or had smoked at least 40 pack-years suggesting that also smoking is involved in the association between rs1051730 and increased all-cause mortality.
The underlying mechanisms for the observed genetic associations are poorly understood. In most studies the effect of the locus on outcomes such as COPD, lung cancer, coronary disease, and peripheral arterial disease have been assumed to be indirect - resulting through heavy smoking, nicotine dependence being the underlying cause Citation(34). In alcohol abuse, cognition deficiencies, and different psychiatric and addiction disorders the effect has been assumed to be direct - through nicotine mediated pathways in central nervous system and changes in brain metabolism Citation(35). Both mechanisms could be involved in the increased mortality among smokers: heavy smoking due to nicotine dependence and difficulties to stop smoking due to accumulation of certain addictions, psychiatric diseases, and personality-related phenotypes lead to multi-morbidity and increased risk of death. There are also other genes, such as IREB2, that have been associated with COPD pathogenesis and are in LD with the CHRNA5/CHRNA3 locus Citation(36). Interestingly, recent studies have shown that there might be other direct molecular genetic mechanisms yet unknown.
Nicotinic receptors are expressed in bronchial smooth muscle and epithelium which could explain the genetic association to airway obstruction reported also among never-smokers Citation(30). An association with CHRNA5/CHRNA3 gene cluster and lung cancer has been found in large genome wide association studies (Citation37, Citation38) and the association has been significant in some studies among never-smokers (Citation5, Citation15, Citation19, Citation36, Citation39). In a study by VanderWeele et al the association between rs1051730 and lung cancer was analyzed and their main finding was that although a small proportion of the association was mediated through smoking, mainly the effect of rs1051370 was mediated through other, presumably direct, pathways Citation(40). Similar pattern could be observed in our study. Other studies have not found a direct association and suggest the association to be mediated through smoking behavior (Citation41, Citation42).
In most of the population based studies rs1051730 has been associated with cigarettes per day or pack-years (Citation4, Citation10, Citation34). This is in accordance with our findings among the long-term male smokers. However, when COPD populations have been studied, no association has been found (Citation5, Citation15, Citation19, Citation36). Obviously these study designs differ greatly from each other. First, COPD population may represent smokers who are especially sensitive to harmful effects of cigarette smoke. Second, the sample size of these data sets is often smaller than in population studies, which diminishes further the statistical power. Another possible confounding factor is the underreporting of pack-years after the COPD diagnosis (Citation8, Citation43, Citation44). It is also evident that pack-years are only an estimate of the lifetime smoking exposure. Other variables such as serum cotinine levels and nicotine dependency test results have been used as more accurate measures Citation(43). However, these methods provide information only on current smoking and thus do not replace pack-years.
rs1051730 is widely studied and reported to be one of the most informative markers in the CHRNA5/CHRNA3 gene cluster, consistently associating with lung functions, lung cancer, smoking variables, addiction diseases, schizophrenia and personality related phenotypes. Our results suggest that the locus may have independent role in increasing the risk of all-cause mortality. The mechanisms behind this association can be indirect resulting from heavy smoking or there might be also some direct mechanisms which are at the moment poorly understood.
Conclusions
CHRNA5/CHRNA3 locus has been previously associated with several smoking related traits. Like in other Caucasian populations the locus was associated with the increased risk of COPD also among the Finns. Among the COPD patients the locus also increased the risk of all type of cancer. In another cohort of long-term Finnish male smokers the locus was associated with pack-years and in both cohorts the locus was shown to be associated also with increased all-cause mortality emphasising the clinical importance of the finding.
Appendix A
Table A1. Risk factors for having >40 pack-years
Table A2. Risk factors for having any type of cancer
Acknowledgments
The authors would like to thank Professor Ingileif Jonsdottir and the personnel of deCODE Genetics, Iceland for DNA extraction and genotyping, clinical research nurses Ms. Kerstin Ahlskog, Kirsi Sariola, and Päivi Laakso for their skillful patient recruitment, and Ms. Tuula Lahtinen for monitoring of the project.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 2000; 343(4):269–280.
- Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis 2003; 46(1):91–111.
- Lee PN, Forey BA, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 2012; 12:385,2407-12-385.
- Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 2010; 42(5):441–447.
- Siedlinski M, Cho MH, Bakke P, Gulsvik A, Lomas DA, Anderson W, et al. Genome-wide association study of smoking behaviours in patients with COPD. Thorax 2011; 66(10):894–902.
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 2008; 452(7187):638–642.
- The HapMap project [Internet]. Available from: http://hapmap.ncbi.nlm.nih.gov/ ( accessed 2 February, 2015 ).
- Munafo MR, Timofeeva MN, Morris RW, Prieto-Merino D, Sattar N, Brennan P, et al. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. J Natl Cancer Inst 2012; 104(10):740–748.
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007; 16(1):36–49.
- Ware JJ, van den Bree MB, Munafo MR. Association of the CHRNA5-A3-B4 gene cluster with heaviness of smoking: a meta-analysis. Nicotine Tob Res 2011; 13(12):1167–1175.
- Gu M, Dong X, Zhang X, Wang X, Qi Y, Yu J, et al. Strong association between two polymorphisms on 15q25.1 and lung cancer risk: a meta-analysis. PLoS One 2012; 7(6):e37970.
- Liu P, Vikis HG, Wang D, Lu Y, Wang Y, Schwartz AG, et al. Familial aggregation of common sequence variants on 15q24-25.1 in lung cancer. J Natl Cancer Inst 2008; 100(18):1326–1330.
- Sakoda LC, Loomis MM, Doherty JA, Neuhouser ML, Barnett MJ, Thornquist MD, et al. Chromosome 15q24-25.1 variants, diet, and lung cancer susceptibility in cigarette smokers. Cancer Causes Control 2011; 22(3):449–461.
- Kaur-Knudsen D, Nordestgaard BG, Bojesen SE. CHRNA3 genotype, nicotine dependence, lung function and disease in the general population. Eur Respir J 2012; 40(6):1538–1544.
- Lambrechts D, Buysschaert I, Zanen P, Coolen J, Lays N, Cuppens H, et al. The 15q24/25 susceptibility variant for lung cancer and chronic obstructive pulmonary disease is associated with emphysema. Am J Respir Crit Care Med 2010; 181(5):486–493.
- Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 2009; 5(3):e1000421.
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet 2009; 18(15):2922–2927.
- Thorgeirsson TE, Stefansson K. Commentary: gene-environment interactions and smoking-related cancers. Int J Epidemiol 2010; 39(2):577–579.
- Mohamed Hoesein FA, Wauters E, Janssens W, Groen HJ, Smolonska J, Wijmenga C, et al. Variants in the 15q24/25 locus associate with lung function decline in active smokers. PLoS One 2013; 8(1):e53219.
- Hong LE, Yang X, Wonodi I, Hodgkinson CA, Goldman D, Stine OC, et al. A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes Brain Behav 2011; 10(5):530–535.
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psych 2009; 14(5):501–510.
- Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, et al. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet B Neuropsychiatr Genet 2010; 153B(8):1448–1458.
- Etter JF, Hoda JC, Perroud N, Munafo M, Buresi C, Duret C, et al. Association of genes coding for the alpha-4, alpha-5, beta-2 and beta-3 subunits of nicotinic receptors with cigarette smoking and nicotine dependence. Addict Behav 2009; 34(9):772–775.
- Laitinen T, Hodgson U, Kupiainen H, Tammilehto L, Haahtela T, Kilpelainen M, et al. Real-world clinical data identifies gender-related profiles in chronic obstructive pulmonary disease. COPD 2009; 6(4):256–262.
- Kupiainen H, Kinnula VL, Lindqvist A, Postma DS, Boezen HM, Laitinen T, et al. Successful smoking cessation in COPD: Association with comorbidities and mortality. Pulm Med 2012; 2012:725024.
- Health and functional capacity in Finland Baseline results of the Health2000 examination survey [Internet]: National Institute of Health and Welfare 2004. Available from: www.terveys2000.fi/julkaisut/baseline.pdf (accessed 20 January, 2015).
- The ATBC Cancer Prevention Study Group. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: design, methods, participant characteristics and compliance.. 1994;4(Ann Epidemiol):1–10.
- Salaspuro M, Kiianmaa K, Seppä K. Alkoholiongelman varhaistoteaminen. In: Seppä K, editor. Päihdelääketiede. Helsinki, Finland: Kustannus Oy Duodecim; 2003; 58.
- Evans A, Salomaa V, Kulathinal S, Asplund K, Cambien F, Ferrario M, et al. MORGAM (an international pooling of cardiovascular cohorts). Int J Epidemiol 2005; 34(1):21–27.
- Wilk JB, Shrine NR, Loehr LR, Zhao JH, Manichaikul A, Lopez LM, et al. Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. Am J Respir Crit Care Med 2012; 186(7):622–632.
- Statistics Finland [Internet]. 2012. Available from: http://www.stat.fi/til/vaenn/2012/vaenn_2012_2012-09-28_tau_001_fi.html( accessed 15 February, 2015).
- Soto-Campos JG, Plaza V, Soriano JB, Cabrera-Lopez C, Almonacid-Sanchez C, Vazquezoliva RM, et al. Causes of death in asthma, COPD and non-respiratory hospitalized patients: a multicentric study. BMC Pulm Med 2013; 13(1):73.
- McGarvey LP, Magder S, Burkhart D, Kesten S, Liu D, Manuel RC, et al. Cause-specific mortality adjudication in the UPLIFT(R) COPD trial: findings and recommendations. Respir Med 2012; 106(4):515–521.
- Budulac SE, Vonk JM, Postma DS, Siedlinski M, Timens W, Boezen MH. Nicotinic acetylcholine receptor variants are related to smoking habits, but not directly to COPD. PLoS One 2012; 7(3):e33386.
- Ware JJ, van den Bree M, Munafo MR. From men to mice: CHRNA5/CHRNA3, smoking behavior and disease. Nicotine Tob Res 2012; 14(11):1291–1299.
- Siedlinski M, Tingley D, Lipman PJ, Cho MH, Litonjua AA, Sparrow D, et al. Dissecting direct and indirect genetic effects on chronic obstructive pulmonary disease (COPD) susceptibility. Hum Genet. 2013 Apr;132(4):431–41.
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 2008; 452(7187):633–637.
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 2008; 40(5):616–622.
- Kaur-Knudsen D, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Nicotinic acetylcholine receptor polymorphism, smoking behavior, and tobacco-related cancer and lung and cardiovascular diseases: a cohort study. J Clin Oncol 2011; 29(21):2875–2882.
- VanderWeele TJ, Asomaning K, Tchetgen Tchetgen EJ, Han Y, Spitz MR, Shete S, et al. Genetic variants on 15q25.1, smoking, and lung cancer: an assessment of mediation and interaction. Am J Epidemiol 2012; 175(10):1013–1020.
- Wang Y, Broderick P, Matakidou A, Eisen T, Houlston RS. Role of 5p15.33 (TERT-CLPTM1L), 6p21.33 and 15q25.1 (CHRNA5-CHRNA3) variation and lung cancer risk in never-smokers. Carcinogenesis 2010; 31(2):234–238.
- Truong T, Hung RJ, Amos CI, Wu X, Bickeboller H, Rosenberger A, et al. Replication of lung cancer susceptibility loci at chromosomes 15q25, 5p15, and 6p21: a pooled analysis from the International Lung Cancer Consortium. J Natl Cancer Inst 2010; 102(13):959–971.
- Keskitalo K, Broms U, Heliovaara M, Ripatti S, Surakka I, Perola M, et al. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum Mol Genet 2009; 18(20):4007–4012.
- Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. Br Med J 2009; 339:b4347.
