Abstract
Background: Chronic Obstructive Pulmonary Disease exacerbations are associated with worsening of airway inflammation, the nature of which may be neutrophilic, eosinophilic, or both.
Objective: The primary objective was to examine the cellular nature of airway inflammation in successive COPD exacerbations in order to ascertain if they changed in individual patients. The secondary objective was to estimate the relative risk indicating the extent to which a particular type of exacerbation changed as a function of the most recent exacerbation. Design: This was a retrospective survey performed on a computerised sputum cell count database of a referral respiratory service in Hamilton, Canada. Recurrent event analyses were used to model the incidence of exacerbations and subtypes of exacerbations. Results: 359 patients and 148 patients had sputum examined during stable condition and during exacerbations, respectively. It was found 65 patients had sputum examined during both situations. The exacerbations were eosinophilic in 15.9%, neutrophilic in 18%, combined in 2.6%, of unknown clinical significance in 19.6% and normal in 19.6%. There were missing counts for 24.3% samples. In 85.2% of patients, a different subtype of bronchitis was noted in successive exacerbations. The relative risk of a subsequent neutrophilic or eosinophilic exacerbation was 6.24 (p = 0.02) and 2.8 (p = 0.24) when the previous exacerbation was neutrophilic or eosinophilic respectively. Conclusions: This non-intervention study suggests that the cellular nature of bronchitis is largely unpredictable and needs to be examined at each COPD exacerbation This has important implications in choosing the appropriate therapy. Future intervention studies would provide further evidence.
Introduction
Exacerbations in chronic obstructive pulmonary disease (COPD) cause substantial morbidity and mortality and are also responsible for increased healthcare costs associated with the disease (Citation1,Citation2). Exacerbations of COPD may be multifactorial (Citation3). They may be due to worsening of airflow limitation, acidosis, left or right ventricular dysfunction, bronchitis (airway inflammation), or one or more combinations of these. Thus all exacerbations may not be associated with worsened airway inflammation. However, those that are, may be of different types as identified by sputum quantitative assays. These are neutrophilic, eosinophilic, or combined eosinophilic and neutrophilic (Citation4). Different causes such as infections (bacterial or viral) (Citation5,Citation6), allergens (Citation7), occupational chemicals (Citation8) or inadequate corticosteroids (Citation9,Citation10) have been implicated for such worsened airway nflammation.
Identifying the specific type of airway inflammation is important as this guides treatment decisions in an individual patient. For example, an exacerbation with sputum eosinophilia (normal total cell count with eosinophils ≥3%) is associated with clinical improvement (post bronchodilator FEV1, quality of life) after treatment with corticosteroids (Citation11–Citation13), while neutrophilic inflammation (elevated total cell count with neutrophils ≥80%) due to bacterial bronchitis usually responds to antibiotics even without additional corticosteroids. When the inflammation is neutrophilic in the absence of a raised total cell count a bacterial infection is unlikely and noninfective causes such as smoker's bronchitis, ozone exposure or exposure to diesel exhaust particles, endotoxins and contaminated metal working fluids should be considered (Citation14). Isolated increase in neutrophil counts have also been considered to be an effect of corticosteroid treatment, which is known to be anti-apoptotic to neutrophils.This, however, remains to be fully understood yet.
A retrospective study on a small number of patients demonstrated that the nature of airway inflammation changes between consecutive exacerbations in approximately 50% of patients with airway diseases in general (Citation15). This has important clinical implications as this means that treating all exacerbations along similar lines may not necessarily be successful as has been suggested in global COPD guidelines. Indeed inflammometry based strategies improve quality of life in stable COPD as compared to usual care (Citation16). This strategy is cost effective in airway diseases such as asthma and is likely to be similarly cost effective in COPD too as the principles of treatment are similar (Citation17).
Considering the results of the earlier small study (Citation15), we speculated that successive exacerbations in COPD specifically may also be associated with a changing nature of airway inflammation. To assess this, we posited a null hypothesis for a statistical test in this study, that the pattern of airway inflammation during consecutive exacerbations in COPD patients is same. We then could interpret the p-value as the strength of evidence against this null hypothesis and if the null hypothesis was rejected then we could claim that there was evidence that successive exacerbations are variable in their type of airway inflammation. We examined this by analyzing data from our computerized database of induced sputum quantitative assay. The primary objective was to examine the cellular nature of airway inflammation in COPD during exacerbations in order to ascertain if they changed in successive exacerbations in individual patients. The secondary objective was to estimate the relative risk indicating the extent to which a particular type of exacerbation changed as a function of the most recent exacerbation. This was indicative of the extent to which patients tend to have exacerbations of the same inflammatory type. In this retrospective study, we did not attempt to relate the nature of bronchitis to any treatment responses or draw any conclusions regarding the modalities of therapy.
Methods
Study design
A retrospective survey was performed on a computerized database of induced sputum cell counts examined from January 1, 2004 to September 30, 2009 at the Firestone Institute for Respiratory Health. The database was used to identify the inflammatory subtypes of bronchitis in patients of COPD, their clinical characteristics including demographic characteristics, physician diagnosis and spirometry. These patients were those referred for sputum cell counts from the outpatient clinic. They were either clinically stable or had an exacerbation of physician diagnosed COPD. Only those who had two or more measurements during exacerbations at intervals ≥6 weeks were included for further analysis. Approval of the Research Ethics Board of St. Joseph's Healthcare, Hamilton was taken.
Study Definitions
Chronic obstructive pulmonary disease (COPD) was diagnosed based on a physician diagnosis, FEV1 bronchodilator reversibility of <12%, and a post-bronchodilator FEV1 / FVC ratio of <70% (Citation3). Exacerbations were regarded as synonymous with the loss of symptomatic control. An exacerbation of COPD was defined by an increase in cough, dyspnea, sputum volume or purulence that in the opinion of the physician was more than the daily variation and required an adjustment of therapy (Citation18). Patients who, according to the referring physician, had a radiological or clinical diagnosis of pneumonia or required hospitalization were excluded from this analysis.We also excluded patients who required hospitalization or ED visits as sputum induction is not feasible in these circumstances.
Sputum induction and examination for total and differential cell counts were performed by the methods used by Pizzichini et al. (Citation19). A ‘‘neutrophilic’’ exacerbation was defined as that with a total cell count of ≥10 million cells/g of sputum selected from the expectoration and proportion of neutrophils ≥80% (Citation20). An ‘‘eosinophilic’’ exacerbation, which is not usually associated with a raised total cell count, was defined as a percentage of sputum eosinophils ≥3% (Citation4). A combined ‘‘eosinophilic and neutrophilic’’ exacerbation was defined as total cell count of ≥10 million cells/g of sputum, neutrophils ≥80% and eosinophils ≥ 3%. Bronchitis of unknown signficiance (neither eosinophilic nor neutrophilic) included patients with an isolated raised total cell count (≥10 million cells/g of sputum with neutrophils <80% and eosinophils <3%) or proportion of sputum neutrophils ≥80%. A “normal” type of exacerbation was defined as a total cell count <10 million cells/g of sputum and proportion of neutrophils <80% and eosinophils <3%. Sputum induction was considered to be a failure if sputum was not obtained or was non analysable due to excessive degenerated cells.
Statistical Analysis
Data for variables including “patient ID,” demographics (gender, age), date of sputum examination, physician's diagnosis, reason of testing (exacerbation or stable), spirometry (postbronchodilator FEV1, FVC and FEV1% predicted) and induced sputum differential cell counts (total cell counts, proportion of neutrophils, eosinophils) were extracted from the computerized database. Descriptive statistics were used to summarize the types of exacerbations. Recurrent event analyses were used to model the incidence of exacerbations and sub-types of exacerbations (Citation21). The Nelson-Aalen estimate of the cumulative mean function was used to estimate the expected number of exacerbations of each type over time. Competing risk analyses were used to model the type of subsequent exacerbations according to the type of the current and past exacerbations. Specifically, estimates of the cumulative incidence functions for exacerbations of each type were obtained to estimate the proportion of patients with subsequent exacerbations of each type. Cause-specific Cox regression models were fitted to examine the effect of previous exacerbation types on risk of particular types of subsequent exacerbations. Analyses were carried out using R Version 2.14.0 with the survival and competing risks package.
Results
Of 442 patients with COPD, 276 (62.4%) males and 166 (37.6%) females, aged 22 to 89 years, were identified from the sputum database. Of these, 359 patients had sputum examination during stable condition while 148 patients had sputum examined during exacerbations ().
Table 1. Number of patients and the the number of times sputum was examined in stable state and during exacerbation
Nature of inflammation during stable disease
shows the number of patients in stable condition and the number of times they had their sputum examined.. The sputum was normal in 22.3%, neutrophilic in 21.4%, eosinophilic in 11.8%, combined in 2.5%, of unknown significance in 18.4% and differential cell counts were missing in 23.6% ().
Table 2. Frequency of subtype of bronchitis identified by sputum examinations during COPD exacerbation and stable COPD
Nature of inflammation during exacerbations
shows the number patients who had their sputum examined during exacerbations and the number of times the sputum were examined in them. The exacerbations were eosinophilic in 15.9%, neutrophilic in 18%, combined in 2.6%, of unknown significance or normal in 19.6% each and there were missing differential cell counts in 24.3% ( and ). The median FEV1% of the patients having 2 or more exacerbations was 54.7% (range 22.1 to 82.7%) predicted.
Analysis of successive inflammations
A changing pattern of airway inflammation was noted for consecutive assessments for both stable and exacerbation samples. For assessments in stable conditions, as many as 102 (93.0%) of 129 patients showed changes in successive examinations. Of the 27 patients who had at least two exacerbations, the pattern of successive inflammation was different in 23 (85.2%) patients and similar in only 4 (14.8%) patients. Further, only 4 (44.4%) neutrophilic exacerbations were subsequently followed by a neutrophilic exacerbation, while 2 (25%) eosinophilic exacerbations were subsequently followed by eosinophilic ones (). The risk of a subsequent exacerbation of any type was lowest when the previous exacerbation was of ‘unclassified” nature ().
Table 3. Frequency of type of any current exacerbations by type of previous exacerbation
The proportion of patients with exacerbations of any nature () was the lowest for individuals who had a first exacerbation with a normal sputum and greatest for those with a first exacerbation of a combined “neutrophilic and eosinophilic” type. Further, the proportion of patients with specific types of exacerbations (i.e., neutrophilic or eosinophilic or others) were not significantly different irrespective of the first exacerbation being neutrophilic or eosinophilic (). The nature of inflammation during stable state was also not predictive of the inflammation type during an exacerbation (). However, the time to exacerbation was the least when the nature of the stable assessment was neutrophilic as compared to the other types ().
Figure 1. The Kaplan-Meier estimates for the proportion of patients having future exacerbations of any type of bronchitis was lowest for patients with normal sputum and highest for patients with combined eosinophilic and neutrophilic type of bronchitis at first exacerbation. A likelihood ratio test of the null hypothesis that there is no difference between these estimates gives p = 0.4245. Note: Patients must have at least 2 assessments.
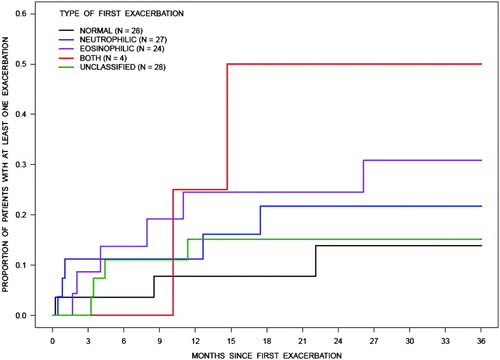
Figure 2. Among patients who had a specific (neutrophilic (N = 30) or eosinophilic (N = 26)) type of first exacerbation, the cumulative incidence functions for the proportion of patients who experienced another exacerbation by each subtype (neutrophilic, eosinophilic or other) of bronchitis up to 3 year for both groups were not significantly different.
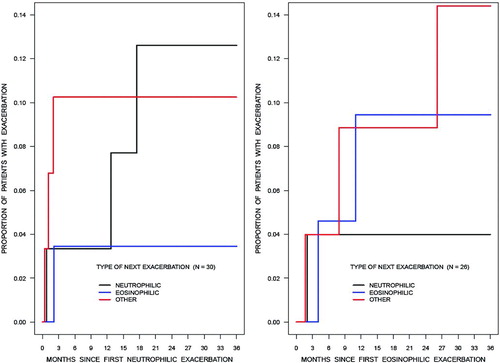
Table 4. Frequency of type of next exacerbation by type of previous assessment in clinically stable state
When the previous exacerbation was sub-typed as neutrophilic or not neutrophilic and this used as a predictor for subsequent neutrophilic exacerbations in a Cox analysis, the relative risk of a subsequent neutrophilic exacerbation was 6.24 (95% CI 1.309 to 29.797, p = 0.02; see left panel of ), whereas if the type of the previous exacerbation was neutrophilic or eosinophilic then the relative risk of the next exacerbation to be neutrophilic was 4.6 (95% CI 0.846 to 25.019, p = 0.07). Similar analysis for eosinophilic exacerbations showed a relative risk of 2.8 (95% CI 0.492 to 16.375, p = 0.24; see right panel of ) when the previous exacerbation was classifed as eosinophilic and 1.9 (95% CI 0.367 to 9.768, p = 0.45) when classified as neutrophilic or eosinophilic ().
Figure 3. Among the assessments with a specific type of exacerbation, the cumulative incidence function for the probability of having a subsequent exacerbation was greater for neutrophilic bronchitis (left panel) than for eosinophilic bronchitis (right panel). Note: Patients must have at least 2 assessments.
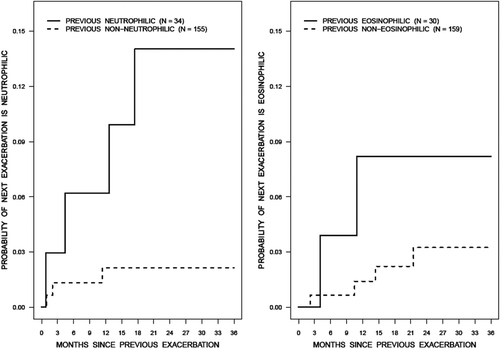
Table 5. Andersen-Gill type model for gap times between kth exacerbation to (k +1)th exacerbation
Discussion
This, perhaps, the largest outpatient clinical study of its kind, is a descriptive observational non-intervention study that demonstrates the heterogeneity of the nature of airway inflammation during the natural course of COPD exacerbations. Approximately 85% of the patients in this study showed a changing pattern of airway inflammation during successive exacerbations. This is much higher than that reported for asthma (Citation15) and is also indicative of the lack of its predictability from historical data alone. The study illustrates the limitation of cross-sectional cluster analysis of endotypes of bronchitis and the importance of measuring bronchitis at each exacerbation for appropriate treatment.
The reasons for such wide variations in the nature of airway inflammation during exacerbations are likely to be related to the diverse etiologies of COPD exacerbations. For example, bacterial infections which are considered to play a role in up to 50% of exacerbations cause a neutrophilic bronchitis (Citation6, Citation22), while allergic causes of exacerbations are associated with sputum eosinophilia. In this study, the relative risk of a subsequent exacerbation being neutrophilic was 6.24 (95% CI 1.31 to 29.8, p = 0.02) when the previous one was exclusively neutrophilic, and 2.8 (95% CI 0.49 to 16.38, p = 0.24) when the previous exacerbation was eosinophilic (). However, the confidence intervals are wide reflecting the considerable uncertainty in the association between current exacerbation type and risk of the next type of exacerbation. The confidence interval for the latter association, in particular, includes 1 and so we cannot be certain that this risk is greater when compared to other subtypes of bronchitis., In the earlier report, patients whose previous exacerbation was eosinophilic or neutrophilic, were more likely to have a subsequent eosinophilic or neutrophilic exacerbation, respectively (Citation15).
Additionally, the results of this study suggest that it is not possible to phenotype COPD patients based on their airway inflammation type as defined by a one-off sputum quantitative assay during exacerbations or in stable state. It is necessary to repeat sputum quantitative assays during every COPD exacerbation in order to guide further specific etiological investigation and appropriate treatment. This is in contrast to the view suggesting that biological clusters are repeatable and exacerbations associated with bacteria or sputum eosinophilia can be predicted from the stable state (Citation23). It is likely that COPD is a complex disease with an inherently unstable system where a delicate balance exists between Th1 and Th2 mechanisms. This balance has the potential of leaning to one of its sides depending on the precipitating factor of an exacerbation. It is critical to detect the type of bronchitis, particularly the eosinophilic bronchitis as it has important bearing on treatment outcomes.
The only randomized controlled trial showed that a strategy of using steroids to treat exacerbations guided by sputum cell counts reduced hospitalizations by 62% compared to the British Thoracic Society guideline-based approach to treating exacerbations (Citation24). Additionally, treatment decisions guided by blood eosinophil count, although a poor surrogate of airway eosinophilia, leads to better outcomes than clinical decisions (Citation23). Patients with blood eosinophil counts >0.2 seem to respond more favorably to corticosteroids than patients with normal eosinophil counts (Citation25, Citation26). More importantly, patients with normal blood eosinophil counts, who were administered corticosteroids, did poorly (Citation25).
This study is a descriptive observational non-intervention study for which sample size calculations, while desirable, are not available. The primary reason for this is that the power of the tests is driven by the frequency of the exacerbations of the different types on the first and subsequent occasions. These are the very data we are seeking to acquire in our study and so sample size calculations are difficult. In such settings readers are best informed about the uncertainty in the statements by inspection of the confidence intervals; here the wide confidence intervals convey the range of values compatible with the data.
The retrospective design did not allow us to look at the outcomes of the exacerbations. However, our objective was to simply examine the nature of airway inflammation and therefore we were unable to draw any conclusions on how the nature of exacerbations were related to treatment interventions such as antibiotics or corticosteroids that may have of course have modified the cellular response. We also did not try to relate the type of bronchitis to clinical criteria such as the severity of airflow limitation measured by spirometry. The main pitfall of this study is the small number of patients with two or more exacerbations which naturally increases the uncertainty in the estimates of relative risk.
However, we firmly believe that the low number of exacerbations is possibly a reflection of the superior nature of management of airway diseases guided by repeated sputum quantitative assays, which has been the standard of practice in Hamilton. We also did not have information on the clinical severity of the exacerbations or other comorbid conditions (GERD) and whether or not these affected the repeatability of the nature of inflammation. Information on smoking status was also not available and our inclusion criteria did not have age limitations leaving the possibility of at least some patients having other forms of obstructive lung diseases. Indeed, the diagnosis of COPD was based on physiological measurements (spirometry) and physician diagnosis.
In summary, we provide preliminary evidence in this non intervention study that the cellular nature of bronchitis during exacerbations of COPD is largely unpredictable. A large multi-centre clinical trial is necessary to evaluate the utility of sputum cell counts to guide the use of anti-inflammatory medications, particularly corticosteroids, in the treatment of exacerbations of COPD.
Declaration of Interest Statement
Parameswaran Nair is listed on a patent for a sputum filtration device (no commercial gains), has provided consultancy to a university spin-off company (Cellometrics) and has received honoraria and grants (invstigator-initiated projects, paid to university) from AZ, GSK, Novartis, BI, Sanofi, Roche and Teva. Other authors have no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
Hongyu Wang and Angira Dasgupta made equal contributions to the manuscript.
Funding
Richard J. Cook and Parameswaran Nair are supported by the Canada Research Chair Program.
References
- Wedzicha JA, Wilkinson T. Impact of chronic obstructive pulmonary disease exacerbations on patients and payers. Proc Am Thorac Soc 2006; 3:218–221.
- Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest 2004; 125:1081–1102.
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J 2006; 27:483–494.
- Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006; 173:1114–1121.
- Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J 2002; 19:68–75.
- Parameswaran K, Watson R, Gauvreau GM, Sehmi R, O'Byrne PM. The effect of pranlukast on allergen-induced bone marrow eosinophilopoiesis in subjects with asthma. Am J Respir Crit Care Med 2004; 169:915–920.
- Lemiere C, Romeo P, Chaboillez S, Tremblay C, Malo JL. Airway inflammation and functional changes after exposure to different concentrations of isocyanates. J Allergy Clin Immunol 2002; 110:641–646.
- Brightling CE, Ward R, Wardlaw AJ, Pavord ID. Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. Eur Respir J 2000; 15:682–686.
- Pizzichini MM, Pizzichini E, Clelland L, Efthimiadis A, Pavord I, Dolovich J. Prednisone-dependent asthma: inflammatory indices in induced sputum. Eur Respir J 1999; 13:15–21.
- Pizzichini E, Pizzichini MM, Gibson PG, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med 1998; 158:1511–1517.
- Brightling CD, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000; 357:1480–1485.
- Fujimoto K, Kubo K, Yamamoto H, Yagamuchi S, Matsuzawa Y. Eosinophilic inflammation in the airway is related to glucocorticoid reversibility in patients with pulmonary emphysema. Chest 1999; 115:697–702.
- Dasgupta A, Neighbour H, Nair P. Targeted therapy of bronchitis in obstructive airway diseases. Pharmacol Ther 2013; 140(3):213–222.
- D'silva L, Cook RJ, Allen CJ, Hargreave FE, Parameswaran K. Changing pattern of sputum cell counts during successive exacerbations of airway disease. Respir Med 2007; 101(10):2217–20
- McDonald VM, Higgins I, Wood LG, Gibson PG. Multidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense? Thorax 2013; 68(7):691–694.
- D'silva L, Gafni A, Thabane L, et al. Cost analysis of monitoring asthma treatment using sputum cell counts. Can Respir J 2008; 15(7):370–374.
- Vestbo J1, Hurd SS, Agustí AG, Jones PW, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- Pizzichini E, Pizzichini MM, Efthimiadis A, Hargreave FE, DolovichJ. Measurement of inflammatory indices in induced sputum: effects of selection of sputum to minimize salivary contamination. Eur Respir J 1996; 9:1174–1180.
- Berlyne GS, Efthimiadis A, Hussack P, Groves D, Dolovich J, Hargreave FE. Sputum in asthma: Color versus cell counts. J Allergy Clin Immunol 2000; 105:182–183.
- Lawless JF. Statistical Models and Methods for Lifetime Data, 2nd edition. Hoboken, NJ: John Wiley and Sons, Inc., 2003.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Eng J Med 2008; 359:2355–2365.
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184(6):662–671.
- Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir 2007; 29:906–913.
- Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid 424 treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186:48–55.
- Aaron SD, Vandemheen KL, Maltais F, et al. TNFα antagonists for acute exacerbations of COPD: a randomised double-blind controlled trial. Thorax 2013; 68:142–148.
