Abstract
Proper use of inhaler devices may be problematic in elderly patients due to age-related difficulties. A survey was administered to elderly patients to investigate the usability of the Genuair® device and patients' subjective viewpoint on the device. A representative sample of the Italian population aged ≥ 65 years was completed with a pre-defined sample of 89 patients with hand arthritis/arthrosis. Of 526 respondents, 88 were not self-sufficient. Only the replies of the 438 self-sufficient respondents were analyzed. A total of 107 participants (24%) reported having respiratory diseases, and 81 of these (76%) were users of inhaler devices. After the first test, the device was considered “practical/handy” by 90% of patients and “easy to use” by 89%. After the second test, in which patients received a demonstration of the correct inhalation maneuver, the percentage of patients scoring ≥ 7 increased to 93% for the first characteristic and was confirmed for the second, with no differences between the groups in terms of age, educational level, use of devices, and presence of arthritis/arthrosis. The mean time to explain the inhaler technique and to perform a correct inhalation was 1'38"± 1'37", and 70% of the respondents required less than 2 minutes, with no differences between the groups in terms of age, education level, use of devices, and presence of arthritis/arthrosis. In conclusion, Genuair® was well accepted and easy to use in a representative sample of the Italian population aged ≥ 65 years. These characteristics make it a valid choice in the elderly, thus enabling patients to better cope with the problems and difficulties that are common to this age group.
Keywords:
Introduction
Inhalers are the mainstay of pharmacologic treatment of asthma (Citation1) and chronic obstructive pulmonary disease (COPD) (Citation2). They are essential for administering appropriate medication, and correct use thereof enables favorable clinical outcomes and successful management of both diseases. Several devices are now available, and each has specific characteristics in terms of how to prepare the dose and deliver the drug to the airways. However, inhalation techniques (Citation3, Citation4) and adherence to treatment (Citation5, Citation6) are suboptimal, leading to poor response to treatment, uncontrolled disease, and increased direct and indirect costs (Citation6). The effectiveness of inhaled medications depends on a correct inhalation maneuver. Guidelines highlight the importance of teaching patients and checking inhaler technique to improve the efficiency of drug delivery (Citation1, Citation2).
Moreover, the patient's opinions, feelings, and preferences with respect to the device have been shown to affect adherence to treatment and the likelihood of correct use (Citation7–Citation10). The issues of adherence and proper use of inhalers are of particular relevance in the elderly. In fact, the physical and cognitive problems common to this age group may influence disease management and the ability to correctly use a handheld device (Citation11). The availability of a device that is easy to use can facilitate administration and thus improve adherence to treatment, especially when patients face specific difficulties.
Genuair® is a novel, breath-activated, multidose dry-powder inhaler (DPI) (Citation12, Citation13) with high lung deposition (Citation14) and built-in safety measures such as a dose indicator, empty inhaler warning, and overdosing prevention system. Previous small-scale trials have assessed the success rate of inhalation, user satisfaction, and overall patient preference in comparison with other devices (Citation15, Citation16) in patients with respiratory diseases. The aim of this practical public survey was to investigate the usability of the Genuair® device and the subjective viewpoint of elderly patients who were prescribed the device. To explore usability independently of previous experience with inhaled therapy, which could influence the patient's subjective judgment (Citation8), we recruited a representative sample of elderly Italian population.
Methods
The sample of individuals who participated in this ad hoc survey was selected as being representative of the Italian population aged ≥65 years (12.3 million people). As we hypothesized that gender and geographic range were not discriminating factors for the variables evaluated, we decided to balance the sample by including the same number of men and women and recruiting participants equally from throughout Italy (North West, North East, Center, South/Islands). We also recruited (according to disease prevalence) a pre-defined sample of 89 patients with hand arthritis/arthrosis in order to better explore usability in terms of movement difficulties that interfere with the use of a device. The maximum margin of error was taken into account when establishing sample size. With a sample of 526 respondents from the overall population aged ≥65 years, a sampling error of ± 4.29% would ensure a confidence level of 95% in the worst-case scenario of parameter estimation (p = 50%).
The usability test was performed at the respondents' homes. All of the respondents were self-sufficient in taking drugs. The test included the same steps that the patients follow when using an inhaler device (reading the package insert and receiving instructions and a demonstration of the correct inhalation maneuver). The usability test was followed by a survey based on personal interviews held at the respondents' homes using a structured questionnaire developed by a panel of experts from the Italian Society of Respiratory Medicine (SIMeR) and the Italian Society of Gerontology and Geriatrics (SIGG). The questionnaire took approximately 15 minutes to complete with the help of a laptop computer (CAPI system, Computer-Assisted Personal Interviewing).
Each question was answered on a 10-point scale. Scores between 1 and 5 indicate that the factor evaluated is “insufficient,” a score of 6 is “sufficient”, 7 and 8 are “good,” and 9 and 10 are “excellent.” Scores ≥ 7 are considered indicative of satisfactory judgment and high awareness on the part of the patient. A total of 51 fully trained interviewers from the national network of DOXA who were specialized in face-to-face interviews conducted the survey between March 20 and April 14, 2014. The interviewers were trained to perform a correct inhalation maneuver, to demonstrate the maneuver to the patient, and to recognize correct performance according to the instructions in the package insert.
Statistical analysis
Demographic and clinical data and patients' survey responses were expressed as descriptive statistics. ANOVA was used to compare within-group differences for selected study variables (age, education level, presence of respiratory disease, use of inhaler devices, presence of hand arthritis/arthrosis). Scores ≥ 7 were adopted for subgroup analysis.
Results
Out of 526 respondents, 88 were not self-sufficient. Only the answers of the 438 self-sufficient respondents were analyzed. The respondents' demographic data are provided in . A total of 107 patients (24%) reported having respiratory diseases; of these 81 (76%) were current users of inhaler devices. Taking into account the instructions they read, 88% of participants attributed a score ≥7 to the questions “Is it easy to understand how to use the device?” (mean, 7.8 ± 1.4) and “Is it easy to learn to use the device” (mean, 8 ± 1.3). Significant differences in mean scores were detected for age, educational level, and previous use of devices (). Only 4% of the sample reported doubts associated with using the device.
Table 1. Demographic data (N = 438).
Table 2. Opinions on the instructions (whole sample and differences between groups).
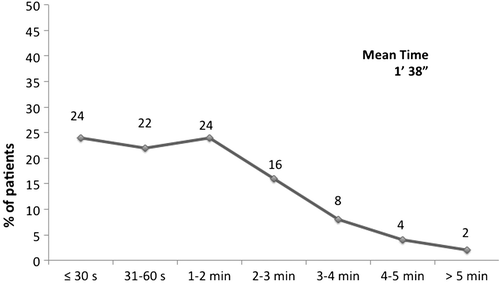
After a first test, the device was considered “practical/handy” and “easy to use,” by 90% and 89% of interviewers, respectively. After the second test and receiving instructions and a demonstration of the correct inhalation maneuver, the percentage of patients with a score ≥ 7 increased to 93% for the first characteristic and was confirmed for the second. In both cases, no differences in mean scores were detected between the groups according to age, educational level, previous use of devices, and presence of arthritis/arthrosis. The mean time necessary to explain the inhaler technique and to perform a correct inhalation was 1'38" ± 1'37" (median 1'08"), and 70% of the interviewees required less than 2 minutes, as shown in
Six individuals (1.4%) were not able to perform a correct inhalation maneuver. No significant differences in mean time required were detected between patients with a high/low educational level, users/nonusers of inhaler devices, and respondents with/without hand arthritis/arthrosis. On the contrary, significant differences emerged between patients with respiratory disease and subjects who did not have respiratory disease (1'57" vs 1'32" p = 0.012) and between subjects aged >74 years and subjects aged 65–74 years (1'57" vs 1'26", p = 0.008). Table 3 provides data on the usability of the device and the differences between the groups. In 87% of cases, the respondents reported that they would be very likely to use Genuair® if they needed inhaled therapy (score, ≥7; mean 8.3 ± 1.3). No significant differences in mean scores were detected between groups, except for the comparison between users and nonusers of inhaler devices (8.8 ± 1.2 vs 8.2 ± 1.8, p = 0.0005).
Table 3. Usability of the device (whole sample and differences between groups).
Discussion
Both adherence and inhalation technique are complicated issues in patients with respiratory disease, especially in elderly patients (Citation11). To maximize the effect of treatment, the instructions should be simple and the inhalers should be efficient and easy to use, even for patients with objective limits and difficulties. Although a wide range of devices is available, the patient's viewpoint and ability to learn and correctly use the inhaler should be taken into account in order to ensure that the appropriate device is chosen. Genuair®, a novel, multidose, breath-activated DPI is a novel option for patients with respiratory diseases. It is preferred over another widely used DPI, HandiHaler, and was associated with higher preference and satisfaction and fewer critical errors in a cross-over study of 130 COPD patients aged ≥40 (Citation15).
The results of our practical survey of a representative sample of the Italian population of elderly patients highlight how Genuair® is considered a simple and well-accepted tool in patients at high risk of nonadherence and of incorrect inhaler use. The interviewers perceived that the instructions in the package insert were easy to understand and that it was simple to learn how to use the device: nearly 90% of patients attributed a score ≥7 to both characteristics. Mean scores were also high in patients aged >74 years, in those with a low educational level, and in those who had never used a device, and differences were significant after comparison, respectively, with younger patients, patients with a high educational level, and patients who regularly used a device.
The device was considered practical/handy (90%) and easy to use after the first test (89%). Following a demonstration of the correct inhalation maneuver, this percentage was even higher for the first characteristic (93%) and stable for the second. No differences were found for age, educational level, previous experience with devices, or presence of arthritis/arthrosis. The mean time necessary to learn to use the device correctly was 1'38", and 70% of the patients were able to reach this objective in less than 2 minutes. The time necessary to achieve a correct maneuver was quite moderate. Data on the mean time for learning how to use a device after reading the package insert and a demonstration are not available in the literature.
However, a study of inhaler competence and patient satisfaction with Easyhaler® reports that correct performance was recorded after just 1 demonstration in 92–98% of adults, 87–99% of elderly patients, 81–96% of children, and 83–99% of adolescents (Citation17); therefore, it seems that the time to learn to perform a correct inhalation differs little from the values recorded in our study. In addition, the high percentage of individuals who are able to perform a correct maneuver (99.6%) was similar to available data (Citation17). Although no differences in mean time were detected according to educational level, previous use of inhaler devices, or presence of arthritis/arthrosis, older patients and patients with respiratory diseases took significantly longer to learn how to perform a correct maneuver. The cognitive and motor problems of people aged >74 years justify the need for more time to learn and perform the inhalation correctly. The ergonomic characteristics of Genuair® were considered positive by most of the sample.
Although the scores were high in each of the groups evaluated, patients aged >74 years, patients with a low educational level, and patients with arthritis/arthrosis reported significantly lower scores. These data indicate that special attention should be paid to patients with comorbid conditions and difficulties related to age and educational level. The acceptability of Genuair® is further confirmed by participants' willingness to use it in the future (87% of the responders and a significantly higher percentage in patients who already use the device). Comparison of Genuair® with other inhalers will confirm the characteristics of Genuair® and provide sufficient information to enable patients to make the appropriate choice of inhaler.
Conclusions
Genuair® proved to be a well-accepted, easy-to-use device in a representative sample of the Italian population aged ≥ 65 years. These characteristics make Genuair® a valid choice in elderly patients, enabling them to better cope with the problems and difficulties that are common to this age group.
Acknowledgments
The authors take full responsibility for the views expressed in this article, which may not be shared by the sponsor.
Declaration of interest statement
F. Blasi has received research grants from Chiesi, Zambon, and Pfizer, congress lecture fees from Guidotti, Menarini, GSK, Chiesi, Pfizer, and Novartis, and consultancy fees from AstraZeneca, Menarini, Mundipharma, Novartis, GSK, and Pfizer.
G.W. Canonica has been a scientific consultant, researcher in clinical trials, speaker at scientific meetings, seminars, and educational activities for which he has been totally or partially supported by Alk-Abelló, Allergopharma, Allergy Therapeutics, Lofarma, Stallergenes, Thermo Fisher, GSK, Novartis, Astra Zeneca, Mundipharma, Alimirall, and Chiesi Farmaceutici.
S. Centanni has received fees from Novartis and Pfizer for research and from Astra Zeneca, Boehringer Ingelheim, GSK, Novartis, Almirall, Mundipharma, and Teva for participating in advisory boards and providing scientific consultancy services. C. Mereu has no conflicts of interest to declare. R. Bernabei has no conflicts of interest to declare. G. Paolisso has no conflicts of interest to declare. R. Antonelli Incalzi has no conflicts of interest to declare.
A. C. Corsico has received payment from Astra Zeneca, Boehringer Ingelheim, GSK, Novartis Nycomed for participation in advisory boards.
F. Di Marco has received honoraria for lectures at national and international meetings from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompé, Guidotti/Malesci, GSK, Menarini, Novartis, and Zambon. He has served as a consultant for Astra Zeneca, Chiesi Farmaceutici, Novartis, and Zambon. He has received financial support for research from Novartis.
M. Milanese has no conflicts of interest to declare. F. Pagano has no conflicts of interest to declare.
P. Santus has received financial support for research and for attending scientific meetings from Pfizer, Boehringer Ingelheim, Novartis, Chiesi Farmaceutici, Glaxo Smith Kline, Menarini, and Air Liquide. He has received honoraria from Chiesi for lectures at national meetings.
N. Scichilone has received lecture fees and payment for participating in advisory boards from Chiesi Farmaceutici, Mundipharma, Novartis, GSK, and Astra Zeneca.
M. Sumberesi has no conflicts of interest to declare.
F. Braido has received conference lecture fees from Guidotti, Malesci, Menarini, GSK, Chiesi, Novartis, AstraZeneca, Mundipharma, Zambon, Boheringer Ingheleim, Almirall, and Biofutura.
I. Baiardini has no conflicts of interest to declare.
The authors alone are responsible for the content and writing of the paper.
Funding
This study was funded by Almirall Spa.
References
- Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention 2008. Available from: www.ginasthma.com (accessed 1 December, 2014).
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014, Available from: http://www.goldcopd.org (accessed 16 November, 2014).
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011; 105:930–938.
- Price D, Bosnic-Anticevich S, Briggs A, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med 2013; 107:37–46.
- Jung E, Pickard AS, Salmon JW, et al. Medication adherence and persistence in the last year of life in COPD patients. Respir Med 2009; 103:525–534.
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med 2013; 107:1481–490.
- Gupta VK, Bahia JS, Maheshwari A, et al. To study the attitudes, beliefs and perceptions regarding the use of inhalers among patients of obstructive pulmonary diseases and in the general population in Punjab. J Clin Diagn Res 2011; 5:434–439.
- Chorão P, Pereira AM, Fonseca JA. Inhaler devices in asthma and COPD—An assessment of inhaler technique and patient preferences. Respir Med 2014; 108:968–975.
- De Blaquiere P, Christensen DB, Carter WB, Martin TR. Use and misuse of metered-dose inhalers by patients with chronic lung disease. A controlled, randomized trial of two instruction methods. Am Rev Respir Dis 1989; 140:910–916.
- Welch MJ, Nelson HS, Shapiro G, et al. Comparison of patient preference and ease of teaching inhaler technique for Pulmicort Turbuhaler versus pressurized metered-dose inhalers. J Aerosol Med 2004; 17:129–139.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging 2014; 9:23–30.
- Chrystyn H, Nierderlaender C. The Genuair inhaler. A novel, multidose dry powder inhaler. Int J Clin Pract 2012; 66:309–317.
- Magnussen H, Watz H, Zimmermann I, et al. Peak inspiratory flow through the Genuair® inhaler in patients with moderate or severe COPD. Respir Med 2009; 103:1832–1837.
- Newman SP, Sutton DJ, Segarra R, et al. Lung deposition of aclidinium bromide from Genuair®, a multidose dry powder inhaler. Respiration 2009; 78:322–328.
- van der Palen J, Ginko T, Kroker A, et al. Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opin Drug Deliv 2013; 10:1023–1031.
- Hass C, Engdahl K, Albert W, et al. Patient preferences and perceived ease of use in inhaler features: Genuair® vs other inhalers. Poster presented at the American College of Chest Physicians Annual Congress, Vancouver, BC, Canada, 30 October–4 November, 2010.
- Gálffy G, Mezei G, Németh G, et al. Inhaler competence and patient satisfaction with Easyhaler®: results of two real-life multicentre studies in asthma and COPD. Drugs R D 2013; 13:215–222.