Abstract
Introduction: Information regarding cost-effectiveness of community-based exercise programmes in COPD is scarce. Therefore, we have investigated whether a community-based exercise programme is a cost-effective component of self-management for patients with COPD after 2 years of follow-up.
Methods: All included COPD patients participated in four self-management sessions. Additionally, patients in the COPE-active group participated in an 11-month community-based exercise programme led by physiotherapists. Patients trained 3 times/week for 6 months and two times/week during the subsequent 5 months. In both periods, one of these weekly training sessions was home-based (unsupervised). No formal physiotherapy sessions were offered to COPE-active patients in the second year. A decision analytical model with a 24-month perspective was used to evaluate cost-effectiveness. Incremental cost-effectiveness ratios (ICER) were calculated and cost-effectiveness planes were created.
Results: Data of 77 patients participating in the exercise programme and 76 patients in the control group were analysed. The ICER for an additional patient prevented from deteriorating at least 47.5 meters on the ISWT was €6257. The ICER for an additional patient with a clinically relevant improvement (≥ 500 steps/day) in physical activity was €1564, and the ICER for an additional quality-adjusted life year (QALY) was €10 950.
Conclusion: Due to a lack of maintenance of beneficial effects on our primary outcome exercise capacity after 2 years of follow-up and higher costs of the programme, the community-based exercise programme cannot be considered cost-effective compared to self-management programmes only. Nevertheless, the ICERs for the secondary outcomes physical activity and QALY are generally considered acceptable.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by persistent and chronic airflow limitation, and its major symptoms are chronic and progressive breathlessness, cough, and sputum production (Citation1). Patients also experience a reduced exercise capacity, and have a lower daily physical activity level compared to healthy persons (Citation2–Citation5). Therefore, the COPE-II study was designed to compare the effects of an 11-month community-based exercise programme within a self-management programme to a self-management programme only (Citation6).
After 1 year of follow-up, maximal exercise capacity was better in the COPE-active group compared to the control group. This benefit was, however, not maintained at 2 years' follow-up. In contrast with this, the exercise programme did achieve a sustained increase in daily physical activity level over 2 years of follow-up. Besides the effectiveness of the programme on health outcomes, another important aspect is the cost of the programme. COPD poses a large economic burden on society, and costs are expected to increase in the upcoming years. The total costs for medication, outpatient and inpatient care attributable to COPD in Europe are estimated to be 23.3 billion euro per year (Citation7). Indirect costs for absence from work and early retirement account for an additional 25.1 billion euro per year (Citation7). In the Netherlands, after medication, hospitalisations, and medical care, physiotherapy accounts for most of the direct costs of COPD (Citation8). With limited health care budgets, it is thus important to offer programmes that are as cost-effective as possible.
Several studies on self-management reported a cost-effectiveness analysis, but exercise was not a major component of the programmes in these studies (Citation9–Citation12). The programmes that did include an exercise programme showed conflicting results regarding cost-effectiveness. Additionally, the recently updated ATS guideline on pulmonary rehabilitation stated that only four studies adequately assessed cost-effectiveness of pulmonary rehabilitation (Citation3). The estimates of these studies were hard to compare since the programmes varied in intensity, duration, and outcomes measured. As we did in the current study, one study (Citation13) also assessed cost-effectiveness of a community-based programme with a follow-up of 2 years. The authors concluded that mean costs were higher for patients in the intervention group than patients in the control group that resulted in a moderate, but acceptable, cost to quality-adjusted life year (QALY) ratio (Citation13).
The information regarding cost-effectiveness of self-management in general, and community-based exercise programmes within a self-management programme is thus scarce. Therefore, we investigated whether a community-based exercise programme (the COPE-active programme) is a cost-effective component of a self-management programme after 2 years of follow-up.
Methods
Study design
This cost-effectiveness analysis (CEA) was conducted as part of the COPE-II study. The detailed design and clinical effectiveness of this randomised controlled trial (RCT) were published previously (Citation6,Citation14,Citation15). In short, a 2×2 factorial design was used, meaning that two independent interventions were evaluated in one design. In this CEA, a community based exercise programme within a self-management programme was compared to a self-management programme only. Patients were assessed at baseline, after 7, 12, 18 and 24 months. The study protocol was approved by the medical-ethical review committee of Medisch Spectrum Twente hospital and written informed consent was obtained from all participants. The COPE-II study was registered in the ISRCTN register (ISRCTN81447311).
Patients
Participants were recruited from the outpatient department of pulmonary medicine from November 2004 through July 2006. The major inclusion criteria were age between 40 and 75 years, a clinical diagnosis of COPD according to GOLD criteria (Citation1), and at least three exacerbations or one hospitalisation for respiratory problems in the 2 years preceding study entry. Serious co-morbidities, including disorders or diseases that seriously influenced walking ability, were the major reasons for exclusion.
Interventions
Patients in both the COPE-active and control group participated in four weekly two hour self-management sessions, with telephone reinforcement calls at 4, 13, 26, 52, and 78 weeks after the last session. All patients received a booklet with the content of the course.
Patients in the COPE-active group additionally participated in a community-based exercise programme (COPE-active) under supervision of a physiotherapist, of which details were published earlier (Citation6). The programme was divided into two parts: a ‘compulsory’ 6-month, and a subsequent optional but recommended 5-month training period. In the first period, patients trained three times per week, and in the second period patients trained two times per week. In both periods, one of these weekly training sessions was performed at home to encourage the patients to exercise in their own environment. Besides improvement of exercise capacity, the main goal of COPE-active was a behavioural change towards exercise. In the second year, the patients were instructed not to follow any formal physiotherapeutic exercise training. Instead, the patients were encouraged to participate in other forms of community- or home-based exercise.
Health outcomes
Primary outcome for the cost-effectiveness analysis was the net proportion of patients with at least a clinically relevant improvement of 47.5 meters on the incremental shuttle walk test (ISWT) over a period of 24 months (Citation16,Citation17). The ISWT is an externally paced walking test on a 10-meter course (Citation16,Citation17). As secondary outcome, we assessed the net proportion of patients who improved their mean daily step count with at least 500 steps over 24 months of follow-up. Daily physical activity was assessed with a pedometer (Yamax Digi-Walker SW-200; Tokyo, Japan) during a 7-day period.
No minimal clinically important distance for daily physical activity is known (Citation3), but 500 steps/day is approximately 10% of baseline step count in our population (), and it is approximately equal to the mean change from baseline in both groups over 24 months. In both outcomes, the net proportion of patients with a clinically relevant change (either improvement or deterioration) was calculated as the proportion of patients with a clinically relevant improvement minus the proportion of patients with a clinically relevant deterioration. Also, the number of QALYs as calculated from EuroQol-5D (EQ-5D) utilities was included in the cost-effectiveness analysis (Citation18,Citation19).
Table 1. Baseline characteristics
Health care utilisation and unit costs
Health care utilisation was prospectively recorded during study follow-up. Data on hospital admissions, emergency department visits, (telephone) consults with the physician were collected by actively following the patients' records. Information on consultations with the general practitioner and the use of respiratory medication was regularly requested from the patients' general practitioner office and pharmacy. Also, attendance at physiotherapy sessions was registered by the supervising physiotherapist. Health care utilisation was valued by 2009 unit prices and as recommended by Dutch guidelines (Citation20).
Cost-effectiveness
The cost-effectiveness of a self-management programme with and without a community-based exercise programme was evaluated with a decision analytic model with a time perspective of 24 months. Analyses were performed from the healthcare payer perspective, meaning that indirect costs related to for example loss in productivity or reduced daily physical activities were not taken into account. Cost-effectiveness was assessed by calculating incremental cost-effectiveness ratios (ICER). ICERs were calculated by dividing the mean difference in cost per person between the COPE-active and control group by the difference in net change in health outcome or mean difference in QALY between the COPE-active and control group. We calculated ICERs for the cost per additional patient with a clinical relevant improvement in distance walked at the ISWT, the cost per additional patient with 500 steps/day improvement and the cost per additional QALY. Our primary analysis focused on the costs and effects after 2 years of follow-up.
Also, a sensitivity analysis was performed on this data by executing a Monte Carlo simulation with 1000 iterations. Cost-effectiveness planes of these simulations were created, to express the uncertainty around the ICERs. In these cost-effectiveness planes the difference in health outcome was plotted against the difference in costs between the COPE-active and control group. Second, we have calculated ICERs for the costs and effects after 1 year of follow-up, and the hypothetical scenario that patients in the COPE-active group continued supervised exercise once a week under the supervision of a physiotherapist. In this scenario it was assumed that 1-year effects were maintained, and that patients in the control group participated in the same number of physiotherapy sessions as they did in the first year.
Results
Patients
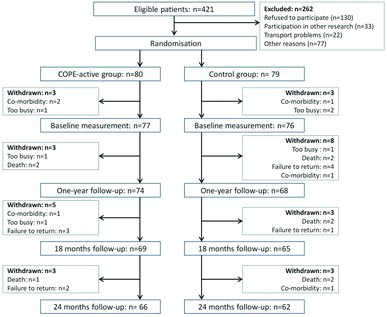
Self-management including a community-based exercise programme was randomly assigned to 80 patients (COPE-active group), while self-management only was assigned to 79 patients (control group). Three patients in each group dropped out before the baseline measurements, and were not included in the analysis. Baseline characteristics of the remaining 153 patients were described in . During 2 years of follow-up, an additional 11 patients dropped out in the COPE-active group, and 14 patients in the control group. Reasons for drop out were comparable between the groups ().
Health outcomes
After 24 months of follow-up, a non-significant between-group difference of 12.2 meters (95%CI: −16.6 to 41.0) in mean change from baseline in ISWT walking distance in favour of the COPE-active group was found. This was also reflected in a larger proportion of patients deteriorating at least the minimal clinical important difference of 47.5 meters, compared to the proportion of patients improving this distance in both the COPE-active and control group (). The difference in net change between the two groups was 7%, and should be interpreted as a difference in net deterioration since in both groups the net change was negative after 2 years of follow-up. In this case, the difference of 7% indicates that the proportion of patients improving at least 47.5 m is 6% higher, and the proportion of patients deteriorating is 1% lower in the COPE-active group compared to the control group ().
Table 2. Net changes in health outcomes and incremental cost effectiveness ratios of a self-management programme with vs. without a community-based exercise programme after 2 years of follow-up, 1 year of follow-up, and in the case of continuing exercise in the second year (hypothetical)
In daily physical activity, a statistically significant difference in mean change from baseline of 1193 steps/day (95%CI: 203 to 2182) in favour of the COPE-active group was found after 24 months of follow-up. In the COPE-active group, the proportion of patients increasing at least 500 steps/day compared to baseline, was larger than the proportion of patients decreasing the same amount of steps. In the control group the opposite occurred. The net improvement was 8.6% in the COPE-active group and the net deterioration was −19.1% in the control group, resulting in a difference in net change between the two groups of 27.7% (). This was in favour of the COPE-active group in which 13% more patients improved, and 15% less patients deteriorated 500 steps/day over 24 months ().
The mean number of QALYs per patient after 2 years of follow-up was 1.53 (95%CI: 1.43 to 1.63) in the COPE-active group and 1.49 (95%CI: 1.39 to 1.59) in the control group. The between-group difference was 0.04 (95%CI: −0.10 to 0.18), which was not statistically significant.
Costs and resource use
The decision analytic model is presented in . The costs of the self-management programme were equal for both study groups (€123). The physiotherapy costs were substantially higher in the COPE-active group (€1648) than in the control group (€469), which was due to a higher mean number of visits to the physiotherapist in the COPE-active group. Smaller differences in favour of the COPE-active group were detected in healthcare contacts (€-12) and hospital admissions (€-812). Also, small differences were found for medication use, both general respiratory medication and medication for exacerbation, but these were in favour of the control group. Total direct medical costs per patient over 2 years were €6949 in the COPE-active group and €6511 in the control group. The additional cost per patient participating in the COPE-active programme was €438 ().
Figure 2. Decision analytic model of a self-management programme including a community-based exercise programme (COPE-active) vs. self-management only (control).
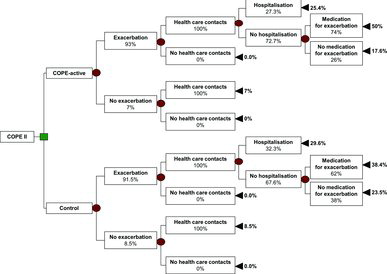
Table 3. Mean costs (€) per patient over 2 years in patients participating in a self-management programme with and without community-based exercise
Cost-effectiveness
Since the difference in net improvement in ISWT walking distance should be interpreted as a net deterioration, the ICER of €6257 presents the cost per additional patient prevented deteriorating at least 47.5 m on the ISWT. The cost per additional patient improving the mean number of steps with at least 500 steps/day was €1564. The incremental cost for an additional QALY was €10 950.
Sensitivity analysis
The cost-effectiveness plane of the difference in costs against the difference in net ISWT deterioration reveals that in the majority of the iterations of the sensitivity analysis (68.9%) the difference in net deterioration was in favour of the COPE-active group (). However, only in 29.9% of the iterations this was accompanied by lower costs for this group. The cost-effectiveness plane of the difference in costs against the difference in net improvement in steps/day (), shows that 54.6% of the iterations were in the upper-right quadrant of the plane. This indicates higher costs, but also a higher number of patients with a clinically relevant improvement in physical activity in the COPE-active group. In 41.1% of the iterations, a beneficial effect of the COPE-active programme was combined with lower costs in this group.
Figure 3. (a) Cost-effectiveness plane of the difference in costs against the difference in net deterioration of distance walked on the incremental shuttle walk test (ISWT, m). A positive difference in net deterioration in steps/day and a negative difference in costs are in favour of the COPE-active group. (b) Cost-effectiveness plane of the difference in costs against the difference in net improvement of physical activity (steps/day). A positive difference in net improvement of steps/day and a negative difference in costs are in favour of the COPE-active group. (c) Cost-effectiveness plane of the difference in costs against the difference in QALYs. A positive difference in QALYs and a negative difference in costs are in favour of the COPE-active group.
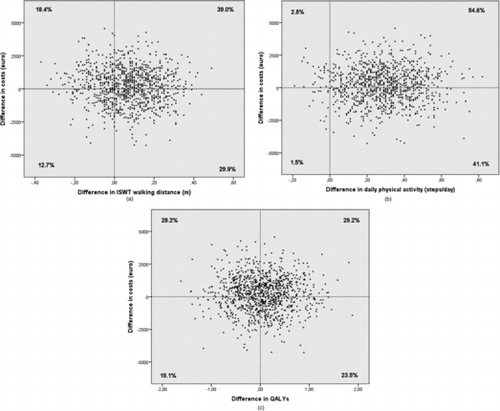
The iterations in the cost-effectiveness plane of the difference in costs against the difference in QALYs () were approximately equally divided over the four quadrants, so no dominant strategy with respect to this outcome could be depicted.
Secondary analyses
In contrast to the data after 2 years of follow-up, after 1 year of follow-up, there was a statistically significant difference in mean change from baseline on ISWT walking distance of 35.1 meters (95%CI: 8.4 to 61.8) (Citation6). The difference in net improvement between the COPE-active and control group was 0.27 in favour of the COPE-active group (). The between-group difference in mean change in steps/day after the first year was also statistically significant with 1190.4 steps/day (95%CI: 256 to 2125) (Citation6). The difference in net improvement was 0.22 in favour of the COPE-active group. Mainly due to the more comparable hospitalisation costs per patient in the first year, the overall difference in direct medical costs was larger after 1 year than after 2 years: €983. This resulted in ICERs for an additional patient with a clinically relevant improvement in ISWT walking distance and steps/day of €3641 and €4468 over 1 year, respectively. The costs for an additional QALY were high (€98 300) due to a minimal between-group difference in QALYs.
We have also assessed the hypothetical scenario that patients in the COPE-active group continued to train once a week under supervision of a physiotherapist in the second year of follow-up leading to maintenance of 1-year effects on exercise capacity in the second year (). Under the assumption that patients in the COPE-active group continued to exercise at the frequency that they exercised in the second half of the first year (once per week at the physiotherapist and once a week at home), the costs of physiotherapy increased to €2625 in this group. In the control group, it was assumed that the number of sessions in the second year equalled the number of sessions in the first year, so costs doubled to €938 over 2 years. This led to a larger difference in physiotherapy costs in favour of the control group, but this was partly compensated by the larger difference in hospitalisation costs in favour of the COPE-active group over 2 years. The overall difference in direct medical costs in this scenario was €946, resulting in an ICER of €3504 over 2 years per additional patient with a clinical relevant improvement in ISWT walking distance. The ICER for steps/day based on a difference in net improvement of 28% was €3379, and the cost per additional QALY was €23 650.
Discussion
This cost-effectiveness analysis showed that self-management including a community-based exercise programme led to increased costs compared to self-management alone after 2 years of follow-up. Due to the lack of between-group difference in exercise capacity measured with the ISWT after 2 years of follow-up, only the costs per additional patient prevented from a clinical relevant deterioration could be calculated, which was €6257. Costs per additional patient with a meaningful improvement in physical activity could however be calculated and was €1564. The gain of an additional QALY came with higher costs: €10 950.
Only a few studies on exercise programmes for patients with COPD have investigated the cost-effectiveness of their intervention. The study of van Wetering et al. most closely resembles our study (Citation13,Citation21). In this study, the cost-effectiveness of a community-based COPD management programme was compared to usual care. As in our study, community-based physiotherapy was a significant component of their self-management programme and patients were followed up for 2 years. Their primary outcome was health-related quality of life measured with the SGRQ and therefore not comparable to ours, but they also incorporated QALYs in their analysis. Comparable to our results, direct medical costs were higher in the intervention group. They neither found a statistically significant difference in QALYs between their study groups.
Although the between-group difference in QALYs seemed somewhat larger than in our study, due to the higher difference in costs, the ICER of €32 425 in the study of van Wetering et al. was considerably larger than the ICER of €10 950 in our study (Citation13). This indicates that the gain of an additional QALY was less costly with our intervention than theirs. Moreover, they performed their primary cost-effectiveness analysis from a societal perspective, whereas we used the healthcare payer perspective implying that we excluded costs for lost productivity from our analysis. We have chosen to use the healthcare payer perspective since in the context of current Dutch healthcare policy, healthcare insurances decide which costs are reimbursed and costs due to productivity loss are of less importance to them.
Since the main goals of the COPE-active programme were improvement of exercise capacity and a behavioural change towards exercise, the primary outcome of the COPE-II study was exercise capacity. In the cost-effectiveness analysis we had therefore planned to calculate the cost per additional patient with a clinically relevant improvement in walking distance on the ISWT. However, the between-group difference in ISWT walking distance after 2 years of follow-up was only minimal and not statistically significant, and in both study groups the proportion of patients deteriorating at least 47.5 meters was larger than the proportion of patients improving that same distance. The cost of €6257 was therefore for an additional patient prevented from a clinically relevant deterioration in walking distance. Prevention of a clinically relevant deterioration in exercise capacity was not a pre-defined goal of the COPE-active programme, however, it should be considered relevant since COPD is a progressive disease, with a worsening health status over the years (Citation1). The inclusion of only frequently exacerbating patients in this study and the steady declining exercise capacity in the control group during the 2 years of follow-up underlines this (Citation6).
Directly after the end of the community-based exercise programme (after 1 year of follow-up) the patients who participated in the COPE-active programme had an improved exercise capacity compared to the control group (Citation6). As anticipated, the improvement in exercise capacity in the COPE-active group was mainly gained in the first half of the first year of follow-up, and was maintained in the second half of that year with a lower training frequency of one supervised and one unsupervised home-based session per week (Citation6). Despite the higher between-group difference in direct medical costs, calculation of an ICER with a time horizon of 1 year led to lower cost per patient with a clinically improvement of walking distance. It is therefore important to critically appraise the time horizon of cost-effectiveness analysis conducted alongside clinical trials. Numerous studies on exercise programmes for patients with COPD still only report short-term effectiveness (Citation22). This can lead to acceptable cost-effectiveness ratios, which in fact might be less favourable on the long-term.
Patients in the COPE-active group were encouraged to participate in sport activities in the second year of follow-up, but they were not allowed to participate in a formal physiotherapeutic training programme. Whereas exercise capacity was maintained during the second half of the first year (with one supervised physiotherapy session a week), a clear deterioration in exercise capacity was observed during the second year (with no supervised physiotherapy sessions) in the COPE-active group. By continuing supervised exercise for at least once a week in the second year of follow-up, the current exercise programme might have the potential to become cost-effective after 2 years of follow-up with respect to its primary outcome. As was shown in our secondary analysis, an additional physiotherapy session will lead to an additional increase in costs, but this might be offset by the lower hospitalisation costs in the COPE-active group and the assumed maintenance of initial beneficial effects on exercise capacity. Maintenance of improvement in exercise capacity after an initial exercise programme is however a challenge in patients with COPD. Maintenance programmes are suggested to contribute to the preservation of gains in exercise capacity, but are not yet uniformly successful (Citation23,Citation24), and further research on the optimal structure of these follow-up programmes is warranted.
Patients with COPD often have a reduced physical activity level (Citation2,Citation4), and this is associated with negative health outcomes such as an accelerated decline in lung function (Citation25), an increased risk for COPD-related hospitalisations and mortality (Citation26). It was even stated that the level of physical activity is the strongest predictor for all-cause mortality in patients with COPD (Citation27). In contrast to the loss of beneficial effects of the exercise programme on exercise capacity in our study, the increase in daily physical activity level was maintained after 24 months of follow-up.
Although physical activity was not our primary outcome, the COPE-active programme was particularly designed to target physical activity. A relatively long training period of 11 months was chosen to facilitate behavioural change toward exercise and improvement of physical activity level. Moreover, one unsupervised home-based training session per week was deliberately incorporated to make patients feel comfortable exercising in their own environment. Physiotherapists weekly discussed this home-based session with the patient and provided feedback. We consider the maintained improvement in physical activity level therefore as a relevant finding. Whether a mean improvement in physical activity level of 500 steps/day can also be considered as clinically relevant is debatable since no minimally clinically important difference of steps/day is known. However, 500 steps/day is approximately 10% of the baseline number of steps, and approximately equal to the mean change from baseline after 24 months of follow-up, so we believe 500 steps/day is a reasonable choice. The additional costs of €1564 for an additional patient with an improvement of 500 steps/day is acceptable with respect to the potential health benefits associated with an increased physical activity level.
The number of QALYs was not statistically significantly different between the COPE-active and control group. Based on the 1-year results, this was expected on forehand (Citation6). Eighteen percent of the patients already had a utility of one at baseline based on EQ-5d values, and 39% had a utility higher than 0.80. This points out that baseline values were already relatively high and the opportunity for improvement low. The small between-group difference in QALYs led to relatively high additional costs (€10 950) for each QALY gained. Since QALYs are the golden standard in cost-effectiveness research, there is information available concerning the acceptability of costs per QALY. The United Kingdom is the only European country where cost-effectiveness data are used above other factors to make clinical decisions.
The National Institute for Health and Care Excellence (NICE) advises local healthcare services on reimbursements, and has defined a threshold between approximately €25 000 and €40 000 per QALY gained (Citation28). Above this threshold, reimbursement will become more unlikely. The healthcare system in the United Kingdom is considerably different from that in the Netherlands, and the threshold as defined by NICE cannot simply be translated to the Dutch situation. Aiming to curb the continuing increase in healthcare costs, the Dutch government is planning to incorporate cost-effectiveness of interventions as a factor in medical decision making, but this is not systematically done yet (Citation29). Nevertheless, in Dutch scientific literature and policy papers, several cost-effectiveness thresholds are mentioned ranging from €20 000 to € 100 000 (Citation30).
The National Healthcare Institute in the Netherlands stated that it is unclear what the value of a QALY is, the only threshold that was used with respect to pharmacological interventions was €80 000. This is a threshold for maximum burden of disease; for COPD this amount has to be multiplied by 0.61 (indicating a lower burden of disease) resulting in €48 000 per QALY (Citation29,Citation31). From a societal perspective, another study states that the Dutch are willing to pay approximately €50 000 for a QALY of a random other person (Citation30). In this context, the cost-effectiveness ratio for an additional QALY that we found is considered to be acceptable.
Although the COPE-active programme is effective in achieving a sustained behavioural change toward physical activity, the long-term effect on exercise capacity is small and not distinguishable from chance. In combination with higher costs of the programme, the community-based exercise programme cannot be considered cost-effective regarding exercise capacity compared to a self-management programme only after 2 years of follow-up. However, the costs per patient with a relevant improvement in daily physical activity, and the cost per QALY were acceptable. When patients continue supervised exercise for at least once a week after the end of the programme, it is expected that the programme will also be cost-effective regarding improvement of exercise capacity.
Funding
This work was supported by the Lung Foundation Netherlands, Grant Number 3.4.02.12.
Declaration of Interest Statement
There are no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2014. Available from http://www.goldcopd.org [Accessed 2014 June 16]
- Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171(9):972–977.
- Spruit MA, Singh SJ, Garvey C, et al. An official american thoracic society/european respiratory society statement: key concepts and advances in pulmonary rehabilitation. Amer J Respir Crit Care Med 2013; 188(8):e13–e64.
- Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med 2010; 104(7):1005–1011.
- Watz H, Waschki B, Meyer T, et al. Physical activity in patients with COPD. Eur Respir.J 2009; 33(2):262–272.
- Effing T, Zielhuis G, Kerstjens H, et al. Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med 2011; 105(3):418–426.
- Gibson GJ, Loddenkemper R, Sibille Y, Lundbäck B. The European Lung White Book. Sheffield, UK: European Respiratory Society, 2013.
- Suijkerbuijk AW, de Wit GA, Wijga AH, et al. [Societal costs of asthma, COPD and respiratory allergy]. Ned Tijdschr Geneeskd 2013; 57(46):A6562.
- Bourbeau J, Collet JP, Schwartzman K, et al. Economic benefits of self-management education in COPD. Chest 2006; 130(6):1704–1711.
- Gallefoss F, Bakke PS. Cost-benefit and cost-effectiveness analysis of self-management in patients with COPD–a 1-year follow-up randomized, controlled trial. Respir.Med 2002; 96(6):424–431.
- Khdour MR, Agus AM, Kidney JC, et al. Cost-utility analysis of a pharmacy-led self-management programme for patients with COPD. Int .J Clin Pharm 2011; 33(4):665–673.
- Monninkhof E, van der Valk P, Schermer T, et al. Economic evaluation of a comprehensive self-management programme in patients with moderate to severe chronic obstructive pulmonary disease. Chron Respir Dis 2004; 1(1):7–16.
- Hoogendoorn M, van Wetering CR, Schols AM, et al. Is INTERdisciplinary COMmunity-based COPD management (INTERCOM) cost-effective? Eur Respir J 2010; 35(1):79–87.
- Effing T, Kerstjens H, van der Valk P, et al. (Cost)-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: the COPE II study. Thorax 2009; 64(11):956–962.
- Zwerink M, van der Palen J, Kerstjens HA, et al. A community-based exercise programme in COPD self-management: two years follow-up of the COPE-II study. Respir Med 2014Oct;108(10):1481–1490.
- Singh SJ, Morgan MD, Scott S, et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992; 47(12):1019–1024.
- Singh SJ, Jones PW, Evans R, et al. Minimum clinically important improvement for the incremental shuttle walking test. Thorax 2008; 63(9):775–777.
- Brooks R. EuroQol: The current state of play. Health Pol 1996; 37(1):53–72.
- Dolan P. Modeling valuations for EuroQol health states. Med.Care 1997; 35(11):1095–1108.
- Hakkaart-van Roijen L, Tan, SS, Bouwmans CAM. Handleiding voor kostenonderzoek. College voor Zorgverzekeringen, 2010.
- van Wetering CR, Hoogendoorn M, Mol SJ, et al. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax 2010; 65(1):7–13.
- Lacasse Y, Martin S, Lasserson TJ, et al. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A Cochrane systematic review. Eura.Medicophys. 2007; 43(4):475–485.
- Beauchamp MK, Evans R, Janaudis-Ferreira T, et al. Systematic review of supervised exercise programs after pulmonary rehabilitation in individuals with COPD. Chest 2013Oct;144(4):1124–33.
- Spruit MA, Singh SJ. Maintenance programs after pulmonary rehabilitation: how may we advance this field? Chest 2013; 144(4):1091–1093.
- Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am.J.Respir.Crit Care Med. 2007; 175(5):458–463.
- Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61(9):772–778.
- Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140(2):331–342.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008; 26(9):733–744.
- Ligtenberg G, Staal PC, Goettsch WG, Knies S. Kosteneffectiviteit in de zorg. College voor Zorgverzekeringen; 2013. Available from https://www.rijksoverheid.nl/binaries/rijksoverheid/documenten/rapporten/2013/09/30/kosteneffectiviteit-in-de-zorg/kosteneffectiviteit-in-de-zorg.pdf, [cited 2014 July 1]
- van Gils PF, Schoemaker CG, Polder JJ. [How much should a gained life-year cost? Study on the assessment of a QALY]. Ned Tijdschr Geneeskd 2013; 157(52):A6507.
- Raad voor de Volksgezondheid en Zorg. Zinnige en duurzame zorg. 2006. Available from: http://www.raadrvs.nl/uploads/docs/Achtergrondstudie_Zicht_op_zinnige_en_duurzame_zorg.pdf, [cited 2014 July 1]