Abstract
Background: Bronchodilators are commonly used to manage patients with stable chronic obstructive pulmonary disease (COPD). This meta-analysis assessed the efficacy of aclidinium bromide with respect to clinical events, health-related quality of life and symptom scales, pulmonary function, and safety among patients with stable COPD. Methods: A comprehensive search of MEDLINE, EMBASE, CINAHL and the Cochrane Library databases was undertaken to identify randomized controlled trials (RCTs) that compared aclidinium with placebo, treatment over at least 12 weeks. Trials were measured using either odds ratios (OR) or mean differences (MD) with 95% confidence intervals (CI). Results: Seven studies, containing 7001 patients, were included in this meta-analysis. Compared with placebo, aclidinium bromide reduced the incidence of exacerbation-related hospitalizations (OR 0.64; 95% CI 0.47 to 0.89) and improved quality of life as measured by a lower total George's Respiratory Questionnaire [SGRQ] score (MD −2.34; 95% CI −3.18 to −1.51) and attenuated dyspnea symptom as assessed by changes in the Transitional Dyspnea Index [TDI] (MD 0.76; 95% CI 0.43 to 1.10). Similar changes were observed with regard to trough FEV1 and FVC and peak FEV1 and FVC. No significant differences were observed with regard to all-cause mortality, COPD exacerbations, non-fatal serious adverse events or cardiac adverse events. Conclusions: Aclidinium reduced the incidence of exacerbation-related hospitalizations and improved quality of life, COPD symptoms and pulmonary function. In addition, aclidinium did not increase the incidence of non-fatal serious adverse events, cardiac adverse events, or COPD exacerbations and was not associated with increased mortality.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent and irreversible airflow limitations usually caused by a mixture of chronic bronchitis and emphysema (Citation1). World Health Organization (WHO) estimates that COPD will become the third-leading cause of death by 2030 worldwide because of the increasing elderly population. This demographic will also be associated with global increases in morbidity, mortality and health-care costs worldwide (Citation2,Citation3). The main goals for treating stable COPD are controlling symptoms, improving lung function, preventing and controlling exacerbations, improving quality of life and decreasing mortality (Citation4).
At present, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend using inhaled long-acting bronchodilators as the first-line therapy for patients with stable, moderate-to-severe COPD (Citation4). Based on the results of recent studies, long-acting muscarinic antagonists (LAMAs) are considered as a more favorable type of bronchodilator in the management of COPD (Citation5–Citation7), because an important underlying mechanism of COPD is an increase in cholinergic tone, which might be the sole reversible element of the disease (Citation5). However, traditional muscarinic receptor antagonists, including ipratropium bromide and tiotropium bromide, are associated with several adverse side effects, particularly adverse cardiac events (Citation8,Citation9). Therefore, novel LAMAs have been developed and confirmed as effective with regard to controlling symptoms in patients with COPD, without increasing the incidence of adverse cardiac events (Citation10,Citation11).
Aclidinium bromide is a novel LAMA, approved by the U.S. Food and Drug Administration for use among patients with stable, moderate-to-severe COPD (Citation12). Its primary mechanism of action involves the blockade of muscarinic acetylcholine receptors, particularly M3 receptors with an affinity approximately 6-fold that involving the M2 receptor subtype in terms of kinetic selectivity (Citation13,Citation14). This action is the primary reason for the aforementioned decreased incidence of cardiac side effects. Compared with tiotropium, aclidinium has a faster onset but a shorter duration (t1/2 = 29 hours). Therefore, aclidinium bromide may be prescribed either once or twice daily. Because butyrylcholinesterase quickly hydrolyzed aclidinium into either alcohol metabolites or carboxylic acid, it has a short plasma half-life and fewer drug-drug interactions, the primary reasons for the decreased incidence of the systemic side effects associated with aclidinium compared with LAMAs, such as tiotropium (Citation13,Citation15,Citation16).
Several randomized controlled trials (RCTs) have described both the efficacy and safety of aclidinium in the treatment of patients with stable COPD; however, controversies exist regarding these patients' clinical outcomes. To perform a high-quality meta-analysis, we did not consider either crossover studies or studies with brief follow-up period. The current meta-analysis might provide clinicians with a clearer picture of the true effects of aclidinium bromide treatment compared with placebo.
Methods
Data sources
We comprehensively searched the MEDLINE, EMBASE, CINAHL and Cochrane Libraryn databases to identify published studies using the following medical subject headings and keyword terms: “COPD” AND “Aclidinium” OR “Aclidinium bromide” OR “LAS34273” OR “Eklira” OR “Genuair.” We also performed searches using drug manufacturer's database and Clinical Trials.gov; furthermore, we manually searched the respiratory journals without restrictions pertaining to language. All trials in this meta-analysis were published prior to March 1, 2015. Moreover, we did not included clinical studies published only in abstract form because of the unavailability of the data related to outcomes and methodological quality.
Study selection
The clinical trials were required to meet the following criteria for inclusion in our analysis: (Citation1) Target population: patients with stable, moderate-to-severe COPD consistent with the diagnostic criteria of the GOLD (Citation4), American Thoracic Society (ATS) and European Respiratory Society (ERS) (Citation17); (Citation2) Intervention: clinical trials comparing aclidinium bromide with placebo; (Citation3) Study design: RCTs employing a parallel-group, placebo-controlled, double-blinded design that followed patients for at least 12 weeks after randomization.
Two primary reviewers (Yong Zou and Jian Xiao) independently identified trials after reading the titles, abstracts and full texts when necessary using the above inclusion criteria. All disagreements were resolved via consensus.
Data extraction and methodological quality assessment
Two reviewers (Yong Zou and Jun Li) independently extracted data using a standardized data extraction form and agreed on all of the items. The primary outcomes included COPD exacerbations, hospitalizations for COPD exacerbations, all-cause mortality and a quality of life scale (St George's Respiratory Questionnaire, SGRQ) (Citation18). The secondary outcomes included symptom scores (Transitional Dyspnea Index, TDI) (Citation19), trough FEV1 and FVC values, peak FEV1 and FVC values, non-fatal serious adverse events and cardiac adverse events. Based on the Cochrane Collaboration guidelines, we assessed the methodological quality of all of the included trials using a risk of bias graph and summary.
Statistical analyses
RevMan 5.3 was used to perform the meta-analysis. Odds ratios (OR) or mean differences (MD) with 95% confidence intervals (CI) were calculated for each trial to assess the dichotomous variables and continuous outcomes, respectively. The Cochran Q test and I2 test were used to determine data heterogeneity, which was considered significant when the Cochran Q test p-value ≤ 0.10 and the I2 value ≥ 50%. A random effects model was used when heterogeneity was observed; otherwise a fixed-effects model was employed for the data synthesis.
Trials were combined into categories related to the frequency of the aclidinium bromide administration (once or twice daily) for each outcome. Potential publication biases would have been examined using a funnel plot if the outcomes had included enough studies. A sensitivity analysis would have been used if any of the subgroups had included a low-quality trial.
Table 1. Main characteristic of seven trials included in this meta-analysis.
Results
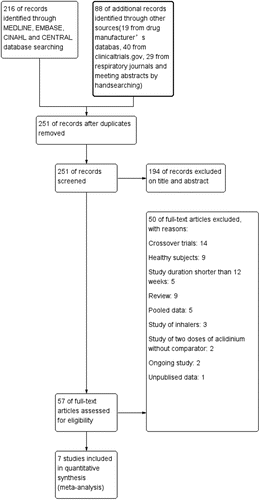
Of the 251 potentially relevant studies identified (after duplicates removed), 194 records were excluded based on the title or abstract and 50 records were excluded based on the full text. For detailed search results, please see . Thus, 7 RCTs (Citation20–Citation25), including 7001 participants, were kept in the meta-analysis. The largest study (Citation23) included 1729 participants, whereas the smallest trial (Citation22) contained 544 patients. The main characteristics of the seven included trials are listed in
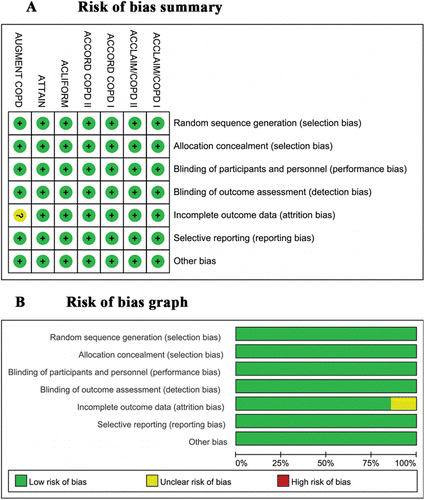
Each of the seven trials compared aclidinium bromide with placebo; in two trials (Citation20), aclidinium was prescribed once daily, whereas the remaining five trials prescribed it twice daily (Citation21–Citation25). Each RCT employed a double-blinded, placebo-controlled, parallel design, and lasted for at least 12 weeks. Each study was of acceptable methodological quality; detailed assessments of each trial are presented as percentage bar plots for each quality item and as a risk of bias summary (). The baseline characteristics, including gender, age, smoking history, disease severity were generally balanced between the two groups. The inclusion criteria and concomitant medications were also similar between groups.
Clinical events
COPD exacerbations
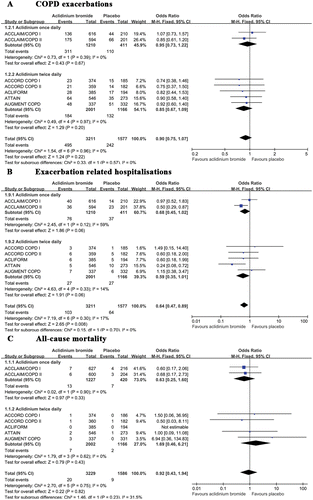
Seven articles reported the number of patients who suffered from at least one COPD exacerbation and were treated with antibiotics, short course of oral steroids, or both (Citation20–Citation25). The pooled results demonstrated that the cumulative rates of COPD exacerbation were 15.4% and 15.3% in the aclidinium and placebo groups, respectively. Fewer COPD exacerbations were observed in the aclidinium group than the placebo group, although the difference was small and not significant (OR 0.90; 95% CI 0.75 to 1.07; p = 0.22). A subgroup analysis did not reveal significant differences (p = 0.57) between once daily (OR 0.95; 95% CI 0.73 to 1.22) and twice daily (OR 0.85; 95% CI 0.67 to 1.09) administrations of aclidinium .
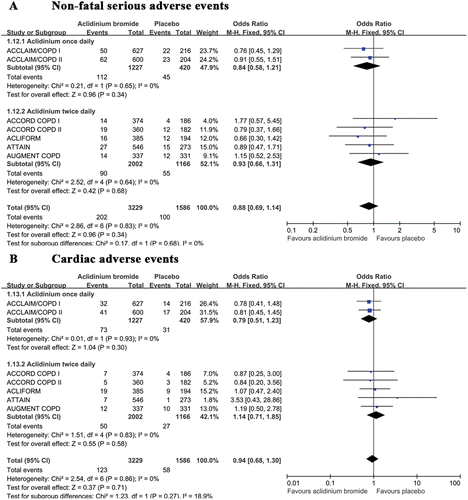
Figure 3. A summary of the effects of aclidinium on (A) COPD exacerbations (B) exacerbation-related hospitalizations and (C) all-cause mortality.
Hospital admission due to exacerbation
Seven trials reported data regarding hospitalizations due to exacerbations; aclidinium was administered once daily in 2 trials (Citation20) and twice daily in 5 trials (Citation21–Citation24). The cumulative rates of exacerbation-related hospitalizations between the aclidinium and placebo group were 3.2% and 4.1%, respectively. Compared with placebo, aclidinium significantly reduced the rate of hospital admission due to exacerbation (OR 0.64; 95% CI 0.47 to 0.89; p = 0.008). A subgroup analysis did not reveal significant differences (p = 0.7) between once daily (OR 0.68; 95% CI 0.45 to 1.02) and twice daily (OR 0.59; 95% CI 0.35 to 1.01; ).
All-cause mortality
Seven trials reported the number of deaths in the two groups (Citation20–Citation25). In general, no significant between-group differences were observed with regard to death (OR 0.92; 95% CI 0.43 to 1.94; p = 0.82). No data heterogeneity was noted among the studies (I2 = 0%). A subgroup analysis between once daily (OR 0.63; 95% CI 0.25 to 1.60) and twice daily (OR 1.69; 95% CI 0.46 to 6.21) aclidinium administrations did not indicate any significant difference (p = 0.23; ).
Health-related quality of life and symptom scales
SGRQ
SGRQ was used to evaluate health-related quality of life in seven trials (Citation20–25). Compared with placebo, treatment with aclidinium significantly reduced the total SGRQ score (). More patients achieved the threshold of 4 units in the aclidinium group than did those in the placebo group (). However, significant heterogeneity was noted among the studies (I2 = 58%). A subgroup analysis revealed that both once and twice daily aclidinium administration groups exhibited significantly reduced total SGRQ scores and increased numbers of patients who achieved ≥ 4 units of improvement in their total SGRQ score despite the absence of a significant difference between group differences (p = 0.56 and p = 0.25, respectively).
Figure 4. A summary of the effects of aclidinium on the (A) changes in total SGRQ scores and (B) number of patients who achieved ≥ 4 units of improvement in their total SGRQ scores.
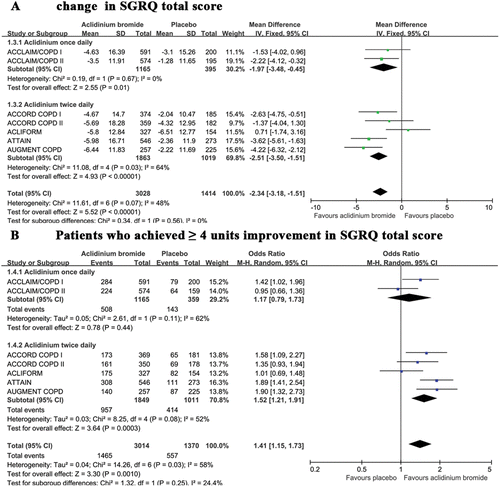
Figure 5. A summary of the effects of aclidinium on the (A) change in the TDI focal score and the (B) patients who achieved ≥ 1 unit of improvement in their TDI focal score.
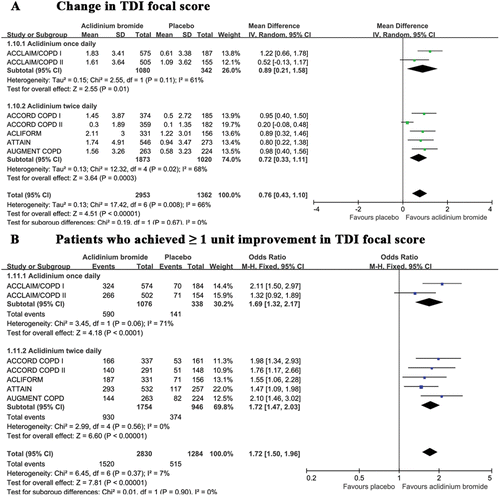
TDI
Both change in the TDI and the number of patients who meet the threshold of at least 1 unit of improvement on the TDI were suitable evaluations of dyspnea. The pooled results demonstrated that the change in the TDI was greater among patients treated with aclidinium compared with those receiving placebo (). A random effects model was employed because of the significant heterogeneity (I2 = 66%). To determine the source of the heterogeneity, we performed sensitivity analyses by excluding one study (Citation23) in which the baseline FEV1 was lower among patients treated with aclidinium than those receiving placebo and observed no heterogeneity (MD 0.95; 95% CI 0.72 to 1.19; I2 = 0%). A similar result was found regarding the number of patients who experienced a clinically significant change in their TDI (). A subgroup analysis between the once and twice daily administration groups did not reveal significant differences in the above indices (p = 0.67 and p = 0.90, respectively).
Spirometric indices
Change in trough FEV1 and FVC (ml)
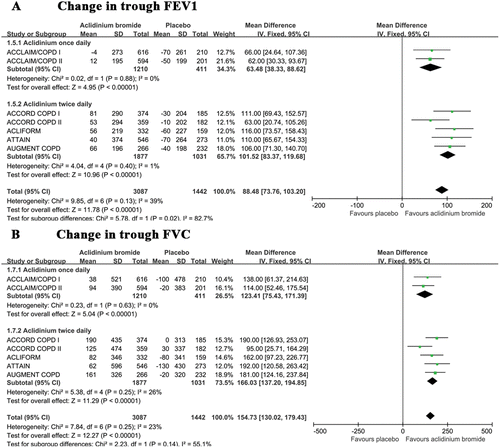
The pooled results from the seven included trials (Citation20–Citation25) showed that the mean improvement in the trough FEV1 related to aclidinium was greater than that associated with placebo from baseline to the end of the study (). Some data heterogeneity was observed among the studies (I2 = 39%). A subgroup analysis determined that twice daily aclidinium treatments (MD 101.52; 95% CI 83.37 to 119.68) elicited greater improvement than once daily treatments (MD 63.48; 95% CI 38.33 to 88.62) (p = 0.02). A similar result was observed with regard to the trough FVC between the aclidinium and placebo groups (). A small amount of data heterogeneity was noted among the trials (I2 = 23%). A subgroup analysis did not reveal a significant difference between the once daily and twice daily groups (p = 0.14).
Change in peak FEV1 and FVC (ml)
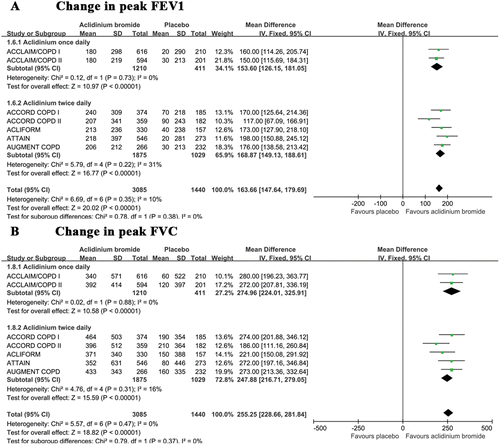
Pooling the data of all of the included trials indicated a greater improvement in peak FEV1 () and FVC () in the aclidinium group versus the placebo group from baseline to the end of the trial. No data heterogeneity was noted in the above two indices. A subgroup analysis did not indicate any significant difference between the once and twice daily groups with respect to peak FEV1 and FVC (p = 0.38 and p = 0.37, respectively).
Safety
Non-fatal serious adverse events
Seven trials included detailed information regarding the rates of non-fatal serious adverse events and cardiac adverse events during the trials. The overall cumulative incidence of non-fatal adverse events was approximately the same for both groups (6.3%). We did not observe significant between-group differences in the rates of non-fatal serious adverse events (OR 0.88; 95% CI 0.69 to 1.14; p = 0.34; ). No statistical heterogeneity was observed across the studies. An additional subgroup analysis of the two aclidinium treatment frequencies did not uncover significant differences (p = 0.68).
Cardiac adverse events
All seven of the included trials reported the rates of cardiac adverse events. Aclidinium was taken once daily in two trials (Citation20) and twice daily in five trials (Citation21–Citation24). The cumulative incidence of cardiac events was lower in the aclidinium group than the placebo group although this difference was not significant (OR 0.94; 95% CI 0.68 to 1.30; p = 0.71; ). No statistical heterogeneity was noted among the studies. An additional subgroup analysis did not reveal significant differences between the once daily and twice daily groups (p = 0.27).
Discussion
This systematic review of seven double-blinded, placebo-controlled, parallel, long-term RCTs demonstrated that aclidinium, compared with placebo, reduced the incidence of exacerbation-related hospitalizations and improved quality of life among patients with moderate to severe, stable COPD. Decreases were noted regarding the number of COPD exacerbations and the number of total deaths among participants treated with aclidinium compared with those receiving a placebo, although no significant differences were observed. In term of secondary outcomes, clinically significant improvements in both symptom scales and spirometric indices were noted among the patients treated with aclidinium bromide, compared with those receiving a placebo. Compared with the placebo, both the change in the TDI focal score and the number of patients who achieved the threshold of 1 unit were larger among patients treated with aclidinium. Improvements in both trough and peak FEV1 as well as trough and peak FVC were significantly greater in the aclidinium group than the placebo group. In terms of safety, decreases were observed in the rates of non-fatal serious adverse events and adverse cardiac events among participants treated with aclidinium compared with those receiving a placebo, although the decreases were not significant.
The results of this meta-analysis were somewhat inconsistent with those of previously published reviews. The combined results of two RCTs (Citation21,Citation24) inlcuded in a previous review (Citation26) shown significant improvements in both spirometric indices (trough FEV1 and peak FEV1) and quality of life among patients treated with aclidinium compared with those receiving a placebo, which is consistent with our findings. However, aclidinium significantly reduced the rates of COPD exacerbations according to the pooled analysis, which differs from the findings of our review. Results similar to those found in our review was reported with regard to both trough and peak FEV1 in a meeting abstract (Citation27) including the results of three RCTs (Citation21, Citation22, Citation24) in the comparison aclidinium versus placebo.
Ten trials included in another review (Citation28) involving both parallel-group and cross-over studies reported the data regarding FEV1, exercise capacity and hyperinflation, rescue medication use, health status and symptom relief. However, this review did not perform a meta-analysis, and its conclusions were only based on the strengths of the individual studies with respect to particular outcomes. Therefore, the results reported by that review were not comparable with the current results. Two reviews (Citation29,Citation30) analyzed the rates cardiovascular death, non-fatal myocardial infarction and stroke; no significant between-group differences were observed, which supported our results.
Seven trials were of excellent methodological quality and met all of the inclusion criteria. The clinical characteristics and disease severity values of the samples recruited in these studies were similar across all but one RCT (Citation22) in which the participants treated with aclidinium exhibited lower FEV1 values than did those receiving a placebo. Therefore, we repeated our analysis of all outcomes by excluding this trial to detect any changes. However, the corresponding results indicated that alterations were not present in the heterogeneity observed among the outcome parameters with the exception of the mean change in the TDI focal score.
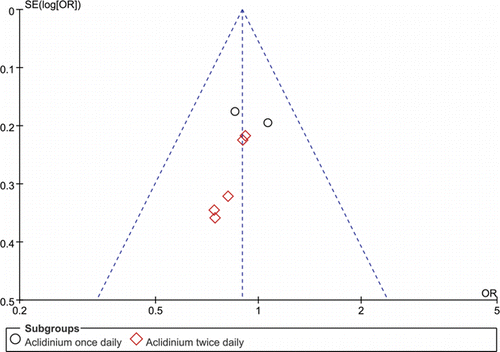
Another potential restriction of this meta-analysis was the double counting of participants included in multiple publications and we successfully averted this bias by extracting only the data pertaining to the first period of the primary study (Citation25) rather than the results at the end of the extension study (Citation31) because the extension period was not a randomized comparison and patient withdrawals affected the results. We undertook systematic searches to determine all relevant studies, emailed the authors of the trials that included incomplete data and independently evaluated the included trials by two reviewers, avoiding selection bias adequately. A funnel plot of the COPD exacerbation data did not exhibit any publication bias.
The current meta-analysis did not consider RCTs with a crossover design and restricted the duration to at least 12 weeks in order to increase the quality of the included trials. Moreover, this meta-analysis was the first to evaluate the benefits and risks of aclidinium treatment among patients with moderate-to-severe COPD using sub-group analysis that compared the results of once and twice daily administrations. The results indicated that aclidinium dosing did not significantly affect the outcome measures with the exception of the mean change in the trough FEV1 value; specifically two daily dose elicited greater improvements than one daily dose.
Our meta-analysis has several limitations and shortcomings. First, only seven trials were selected for this meta-analysis and a ongoing study (Citation32) was not included because its data was not available. Second, we did not compare aclidinium with either LABAs or LAMAs because they lack the trials needed to fulfill the review's inclusion criteria. Finally, we did not perform a sub-group analysis of aclidinium dosages between 200 μg and 400 μg twice daily because of the lack of eligible studies.
Taken together, this meta-analysis demonstrates that inhaled aclidinium bromide reduces the incidence of exacerbation-related hospitalizations and improves quality of life over placebo. Aclidinium also significantly improves symptoms and pulmonary function but did not significantly decrease the rates of COPD exacerbations or mortality. A decreased rather than increased trend toward cardiac adverse events, non-fatal serious adverse events was noted among participants treated with aclidinium compared to placebo in spite of no statistical difference. Consequently, aclidinium was of good efficacy and safety profile and recommended as a reasonable first-line choice of managing the patients with moderate-to-severe stable COPD. Because of the limited number of eligible trials included in this paper, clinicians should be cautious when applying these results to clinical decision-making; additional long-term trials should be performed to evaluate the risks and benefits of aclidinium bromide compared with other LAMAs or LABAs.
Declaration of interest statement
The authors have no conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
We express our gratitude to the authors of the included studies and we thank Almirall for providing additional data and information.
References
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- World Health Organization (WHO). COPD predicted to be third leading cause of death in 2030. World Health Organization 2012. Available from: http://www.who.int/respiratory/copd/WorldHealthStatistics˙2008/en/index.html# ( accessed 25 December 2012).
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765–773.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD updated 2014: Global Initiative for Chronic Obstructive Lung disease, Inc. Available from: http://www.goldcopd.org; 2014 ( accessed 18 May 2014).
- Barnes PJ. The role of anticholinergics in chronic obstructive pulmonary disease. Am J Med 2004; 117 Suppl 12A:24S–32S.
- Celli BR, MacNee, W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932–946.
- Hodder R. Tiotropium is superior to salmeterol in reducing frequency of exacerbations: but the effect of adding tiotropium to the combination of inhaled corticosteroid and long-acting beta(2)-agonist remains unclear. Evid Based Med 2012; 17(3):93–95.
- Moulton BC, Fryer AD. Muscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPD. Br J Pharmacol 2011; 163(1):44–52.
- Jacoby DB, Fryer AD. Anticholinergic therapy for airway diseases. Life Sci 2001; 68(22–23):2565–2572.
- Sharafkhaneh A, Majid H, Gross NJ. Safety and tolerability of inhalational anticholinergics in COPD. Drug Healthc Patient Saf 2013; 5:49–55.
- Reid DJ, Pham NT. Emerging therapeutic options for the management of COPD. Clin Med Insights Circ Respir Pulm Med 2013; 7:7–15.
- U.S. Federal Drug Administration (FDA). Drug approval package; Tudorza Pressair (aclidinium bromide) inhalation powder. United States Food, Drug Administration 2012. Available from: http://www.accessdata.fda.gov/drugsatfdadocs/nda/2012/202450Orig1s000TOC.cfm ( accessed 25 December 2012).
- Gavalda A, Miralpeix M, Ramos I, et al. Characterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profie. J Pharmacol Exp Ther 2009; 331(2):740–751.
- Casarosa P, Bouyssou T, Germeyer S, Schnapp A, Gantner F, Pieper M. Preclinical evaluation of long-acting muscarinic antagonists: comparison of tiotropium and investigational drugs. J Pharmacol Exp Ther 2009; 330(2):660–668.
- Sentellas S, Ramos I, Alberti J, et al. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci 2010; 39(5):283–290.
- Alberti J, Martinet A, Sentellas S, Salva M. Identification of the human enzymes responsible for the enzymatic hydrolysis of aclidinium bromide. Drug Metab Dispos 2010; 38(7):1202–1210.
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155(3):179–191 .
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145(6):1321–1327.
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984; 85(6):751–758.
- Jones PW, Rennard SI, Agusti A, et al. Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res 2011; 12:55.
- Kerwin EM, D'Urzo AD, Gelb AF, et al. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012; 9(2):90–101.
- Rennard SI, Scanlon PD, Ferguson GT, et al. ACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clin Drug Investig 2013; 33(12):893–904.
- Singh D JP, Bateman E, Korn S, et al. Evaluation of the efficacy and safety of two doses of aclidinium and formoterol in fixed-dose combination in patients with COPD: the ACLIFORM study. Chest 2014; 145:375A.
- Jones PW SD, Bateman ED, Agusti A, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J 2012; 40(4):830–836.
- D'Urzo A, Rennard S, Mergel V, Garcia Gil E, Leselbaum A, Caracta C. The AUGMENT COPD trial: efficacy and safety of a fixed-dose combination of aclidinium bromide and formoterol fumarate in COPD patients. Chest 2014; 145:426A .
- Jones P. Aclidinium bromide twice daily for the treatment of chronic obstructive pulmonary disease: a review. Adv Ther 2013; 30(4):354–368.
- D'Urzo A, Jones P, Ferguson GT, Rekeda L, Garcia Gil E, Caracta C. Aclidinium bromide improves lung function in a wide range of patients with moderate to severe COPD: Pooled subgroup analysis of the ACCORD COPD I and II and ATTAIN trials. Chest 2013; 144(4˙Meeting abstracts):746A .
- Suppli Ulrik C. Aclidinium bromide: Clinical benefit in patients with moderate to severe COPD. Open Respir Med J 2012, 6:150–154.
- Donohue J, Tashkin D, Ferguson GT, Kowey P, Rekeda L, Shrestha P. Long-term cardiovascular safety of aclidinium bromide in patients with COPD. Chest 2013; 144(4˙Meeting abstracts):716A .
- Ferguson GT, Kerwin E, Singh D, Kowey P, Rekeda L, Shrestha P. Cardiovascular safety of aclidinium bromide in COPD: pooled results from three placebo-controlled studies. Chest 2013; 144(4˙Meeting abstracts):715A .
- Almirall, S. A. Efficacy, safety and tolerability of two fixed dose combinations of aclidinium bromide/formoterol fumarate, aclidinium bromide, formoterol fumarate and placebo for 28-weeks treatment in patients with moderate to severe stable chronic obstructive pulmonary disease (COPD). Available from: http://clinicaltrials.gov/ct2/show/NCT01572792 ( accessed 1 May 2014) .
- Evaluate the effect of aclidinium bromide on long-term cardiovascular safety and COPD exacerbations in patients with moderate to very severe COPD (ASCENT COPD). Available from: http://clinicaltrials.gov/ct2/show/NCT01966107 ( accessed 1 May 2014) .