Abstract
The concurrent diagnosis of chronic obstructive pulmonary disease (COPD) and sleep apnoea–hypopnoea syndrome (SAHS) (overlap syndrome), can contribute to worsening respiratory symptoms, but whether the severity of COPD is associated with co-morbid SAHS is unknown. We investigated whether the severity of COPD is associated with the complication of SAHS by examination of nocturnal oximetry as an alternative to polysomnography. Patients with COPD concurrently completed nocturnal oximetry, pulmonary function tests, a COPD assessment test, an Epworth sleepiness scale and a hospital anxiety and depression scale to evaluate the severity of COPD and possible concurrent presence of SAHS. We retrospectively analysed the data to assess correlation between the oxygen desaturation index (ODI) and each clinical variables and evaluated the predictors of ODI ≥ 15. This study included 103 patients (91 males, 88%) with a mean age of 72 ± 8 years and body mass index of 22 ± 3 kg/m2. ODI was positively correlated with FEV1, FEV1/FVC and FEV1% predicted, which meant that ODI was inversely correlated with airflow limitation. Univariate logistic regression analysis revealed that FEV1% predicted and FEV1/FVC were predictors of ODI ≥ 15. ODI is inversely correlated with airflow limitation and milder COPD patients may have co-morbid SAHS.
Abbreviations
| AHI | = | Apnoea–hypopnoea index |
| BMI | = | Body mass index |
| CAT | = | Chronic obstructive pulmonary disease assessment test |
| COPD | = | Chronic obstructive pulmonary disease |
| ESS | = | Epworth sleepiness scale |
| FEV 1 | = | Forced expiratory volume in one second |
| FVC | = | Forced vital capacity |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| HADS | = | Hospital anxiety and depression scale |
| ODI | = | Oxygen desaturation index |
| PSG | = | Polysomnography |
| REM | = | Rapid eye movement |
| SAHS | = | Sleep apnoea–hypopnoea syndrome |
Introduction
Chronic obstructive pulmonary disease (COPD) is a common condition characterized by persistent airflow limitation that is usually progressive and associated with systemic co-morbid conditions (Citation1, Citation2). Sleep apnoea–hypopnoea syndrome (SAHS) is a common disorder, although often unrecognized, and is associated with considerable functional impairment (Citation3). The concurrent diagnosis of COPD and SAHS was first described as the “overlap syndrome” in 1985 (Citation4). These patients have particularly severe disease, with a lower partial pressure of oxygen in arterial blood (PaO2), higher partial pressure of carbon dioxide in arterial blood (PaCO2) and higher pulmonary artery pressures than patients with SAHS or COPD alone (Citation5–Citation7). Patients with overlap syndrome also have increased mortality compared with patients with SAHS alone (Citation8, Citation9), and continuous positive airway pressure treatment improves survival in them (Citation10, Citation11).
Polysomnography (PSG) is currently the gold standard test in the diagnosis of SAHS. However, PSG is costly and time-consuming, and therefore difficult to perform for the majority of patients suspected of having SAHS. Thus, nocturnal oximetry has been proposed as a simpler alternative to PSG in the diagnosis of SAHS because it is readily available, relatively inexpensive, and easily administered. The oxygen desaturation index (ODI) obtained by nocturnal oximetry correlates well with the apnoea–hypopnoea index (AHI) and performs well in the diagnosis of SAHS (Citation12–Citation15); hence, nocturnal oximetry might meet the demand for screening of SAHS in the community, and is a possible diagnostic tool.
We hypothesized that patients with severe COPD suffer from more severe SAHS. Therefore, we conducted this retrospective study to investigate whether the severity of COPD is associated with the complication of SAHS by examination of nocturnal oximetry as an alternative to PSG.
Methods
Subjects
We conducted this retrospective study in a 400-bed hospital that has a respiratory referral centre. The subjects had been referred to or were being treated at our hospital between July 2014 and April 2015. We performed pulmonary function tests and nocturnal oximetry to evaluate the severity of COPD and to screen for the co-morbidity SAHS for stable COPD outpatients. The COPD assessment test (CAT), Epworth sleepiness scale (ESS) and hospital anxiety and depression scale (HADS) were completed concurrently. We included patients with a diagnosis of COPD supported by a history of past or current smoking and obstructive lung disease with a forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) < 70%. However, patients who suffered from acute illness, dementia and poor performance status were excluded because it was difficult for them to perform the tests. Patients who received domiciliary oxygen therapy were also excluded.
Data collection
All clinical data were collected from medical records. Exacerbation of COPD was defined as “chest problems requiring treatment with antibiotics and/or oral corticosteroids” based on a previous report (Citation16). We counted exacerbations of COPD within a year of the examinations, including nocturnal oximetry, pulmonary function tests and questionnaires. We used a de-identified database for the analysis in order to account for confidentiality; therefore, this study did not pose any risk to patients. The Institutional Review Board of Shinko hospital approved this study (approval number: 1431).
Questionnaires
We evaluated CAT, ESS and HADS concurrently with nocturnal oximetry and pulmonary function tests. CAT is a questionnaire for people with COPD that is designed to measure the impact of COPD on a person's life (Citation17). The CAT scale ranges from 0 to 40, with higher scores indicating worse symptoms and control. ESS is an instrument used to measure average daytime sleepiness (Citation18). A score of 11 or more reflects greater than normal daytime sleepiness. HADS is a screening tool for assessing the severity of symptoms of depression and anxiety (Citation19). The HADS consists of two subscales assessing depression and anxiety. A score of 0–7 denotes the absence of depression/anxiety, 8–10 indicates possible depression/anxiety, and 11 or higher indicates probable depression/anxiety. If it was difficult for patients, especially for the elderly, to answer the questions, trained staff interviewed the patients and completed the questionnaires.
Pulmonary function tests
Pulmonary function tests were conducted by trained operators, in accordance with American Thoracic Society/European Respiratory Society guideline (Citation20), using a CHESTAC-8800 system (Chest, Tokyo, Japan).
Nocturnal oximetry
Patients underwent home nocturnal oximetry using a pulse oximeter (PULSOX-Me300, Konica Minolta Sensing Inc., Osaka, Japan). A technician visually checked the oximetry recordings and obvious artefacts were deleted, followed by analysis of the recordings using a computer program (DS-Me, Konica Minolta Sensing Inc., Osaka, Japan). Desaturation events are defined as at least a 4% decrease in the percutaneous oxygen saturation (SpO2) from the mean level lasting for 4–120 seconds. The oximetry tracings show a sawtooth pattern and the dips of ≥ 4% are detected as desaturation events. The total number of desaturations was divided by the total time sleeping in hours as reported by the patient, and calculated as the median ODI. The cut-off point of ODI was ≥ 15. The total time of SpO2 < 90% was divided by the hours of sleep to give the percentage of time with SpO2 < 90% (%T90).
Statistics
Continuous variables were expressed as numbers (%), means (standard deviation [SD]) or medians (interquartile range [IQR]). Correlations between ODI and other clinical parameters were assessed using the Spearman correlation coefficient. The Kruskal–Wallis test was used to compare the value of ODI among the four stages of the GOLD (Global Initiative for Obstructive Lung Disease) classification. Univariate logistic regression analysis was used to identify significant variables predicting ODI≥15. A P value < 0.05 was deemed statistically significant. Statistical analysis was performed using JMP 9 software (SAS Institute Inc., Cary, NC, USA).
Results
Patients
This study included 103 patients with a mean age of 72 ± 8 years and 91 males (88%). shows baseline characteristics of the patients who participated in this study. The BMI was 22 ± 3 kg/m2. All patients received some inhaled medication; long-acting methacholine antagonist was the most frequent choice. shows medical conditions associated with COPD. Twenty-nine patients (28%) suffered from exacerbations of COPD within a year. The median (IQR) value of CAT, ESS, HADS anxiety and HADS depression were 13 (Citation7–Citation18), 5 (Citation3–Citation8), 3 (Citation1–Citation7) and 6 (Citation2–Citation9), respectively. Pulmonary function tests showed FEV1/FVC of 51±12% and FEV1% predicted of 61±21%.
Table 1. Baseline characteristics of subjects
Table 2. Medical conditions associated with COPD
Nocturnal oximetry
shows results of nocturnal oximetry. The mean (SD) value of nocturnal SpO2 was 93 (Citation4)%. The median (IQR) value of ODI was 4 (Citation2–Citation7). Thirty-one patients (30%) showed ODI ≥ 5, 15 patients (15%) showed ODI ≥ 10 and 9 patients (9%) showed ODI ≥ 15.
Table 3. Nocturnal oximetry
Correlations between results of ODI and clinical variables
shows the correlation between ODI and clinical variables. ODI was positively correlated with FEV1, FEV1/FVC and FEV1% predicted, which meant that ODI was inversely correlated with airflow limitation. The mean (SD) values of ODI of GOLD 1, GOLD 2, GOLD 3 and GOLD 4 were 9.9 (10.7), 4.4 (4.3), 4.8 (6.2) and 2.8 (3.6), respectively, and ODI was significantly different among GOLD classifications (). Univariate logistic regression analysis showed that ODI≥15 was predicted by FEV1% predicted and FEV1/FVC. It was revealed age was the positive predictor of ODI≥15 and CAT was the negative predictor of ODI ≥ 15 ().
Table 4. Correlations between ODI and clinical variables
Figure 1. Oxygen desaturation index (ODI) by the GOLD (Global Initiative for Chronic Obstructive Lung Disease) classification. ODI was significantly different among GOLD classifications and tended to be higher in the milder GOLD classification groups.
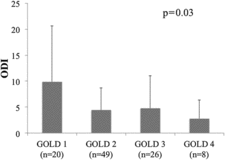
Table 5. Logistic regression analysis for predictors of ODI ≥ 15
Discussion
The principal results of this study are (Citation1) the ODI is inversely correlated with airflow limitation, and ODI is higher in the milder GOLD classification groups; (Citation2) FEV1% predicted and FEV1/FVC are significant predictors of ODI ≥ 15. We hypothesized that severe COPD patients would tend to have the complication of SAHS, but precisely the opposite result was obtained: milder COPD patients tended to have a higher ODI.
This paradoxical correlation between airflow limitation and ODI is a surprising observation. In a large epidemiological study conducted in the United States with 5,954 participants, the relationship between AHI and FEV1/FVC was evaluated by analysis of variance after adjusting for age, race, sex, BMI and smoking status (Citation21). The multivariate models revealed no significant interaction between BMI and FEV1/FVC in their relationship with AHI, but there was a significant positive relationship between AHI and FEV1/FVC in that study. It is unknown whether airflow limitation itself is associated with AHI, or why severe COPD patients show lower AHI. In a similar way, it is challenging to explain why patients with severe airflow limitation tend to show a lower ODI, but several possible causes may contribute to the finding.
The first assumption is that diminished fat and soft tissue around the neck after losing weight during COPD progression may result in upper airway patency and reduce the possibility of nocturnal desaturation. It has been reported neck circumference is a positive independent risk factor for high AHI regardless of BMI (Citation22). The second assumption is that diminished inspiratory force resulting from muscle weakness and a flattened diaphragm during COPD progression may have an effect on upper airway patency and may lead to a lower ODI, because negative intrathoracic pressure mainly derived from contraction of the diaphragm during inspiration may collapse the upper airway. Finally, diminished rapid eye movement (REM) sleep, which is observed with progression of COPD, could reduce the risk of SAHS (Citation23), and therefore severe COPD patients may also have a lower ODI. These assumptions are largely speculative and there is also a need to determine whether age and CAT score predict the ODI levels or not.
PSG is the gold standard test in the diagnosis of SAHS. However, PSG is costly and time consuming, so nocturnal oximetry can be used as an alternative or the screening test (Citation12). ODI obtained by nocturnal oximetry correlates well with AHI (Citation13, Citation14). Nakano et al. reported a diagnostic sensitivity and specificity for ODI calculated at a 4% threshold for SAHS defined as AHI ≥ 15 (Citation15). The sensitivity/specificity of ODI ≥ 15 for patients with a BMI < 25 was 54%/100%. Moreover, Chiner et al. reported a sensitivity/specificity for ODI calculated at a 4% threshold for SAHS defined as AHI ≥ 15. The sensitivity/specificity of ODI ≥ 15 were 62%/93% (Citation12). These studies suggested that nocturnal oximetry performed well in screening for SAHS. However, a final diagnosis of SAHS by oximetry alone is not an established method, therefore we suggest more extensive diagnostic tools such as polygraphy or polysomnography for quantification of severity before deciding on therapy.
The major limitations of the study were its retrospective design and small size. The differential diagnosis of obstructive SAHS or central SAHS could provide important information, but was not possible by nocturnal oximetry. In addition, we could not evaluate important factors such as neck circumference and cephalometric abnormalities, which are associated with the pathophysiology of SAHS (Citation22, Citation24). Because the study was retrospective, we were unable to evaluate PaO2 and PaCO2.
In conclusion, ODI is inversely correlated with airflow limitation, and FEV1% predicted and FEV1/FVC are significant predictors of ODI ≥ 15. Milder COPD patients may be likely to have the co-morbidity of SAHS.
Declaration of Interest Statement
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fabbri LM, Luppi F, Beghé B, et al. Complex chronic comorbidities of COPD. Eur Respir J 2008; 31:204–212.
- Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32:962–969.
- Malhotra A, White DP. Obstructive sleep apnoea. Lancet 2002; 360:237–245.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Weitzenblum E, Chaouat A. Sleep and chronic obstructive pulmonary disease. Sleep Med Rev 2004; 8:281–294.
- Chaouat A, Weitzenblum E, Krieger J, et al. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 1995; 151:82–86.
- Bhullar S, Phillips B. Sleep in COPD patients. COPD 2005; 2:355–361.
- Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep 1995; 18:149–157.
- Lavie P, Herer P, Lavie L. Mortality risk factors in sleep apnoea: a matched case-control study. J Sleep Res 2007; 16:128–134.
- Machado M-CL, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J 2010; 35:132–137.
- Stanchina ML, Welicky LM, Donat W, et al. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med 2013; 9:767–772.
- Chiner E, Signes-Costa J, Arriero JM, et al. Nocturnal oximetry for the diagnosis of the sleep apnoea hypopnoea syndrome: a method to reduce the number of polysomnographies? Thorax 1999; 54:968–971.
- Lin C-L, Yeh C, Yen C-W, et al. Comparison of the indices of oxyhemoglobin saturation by pulse oximetry in obstructive sleep apnea hypopnea syndrome. Chest 2009; 135:86–93.
- Chung F, Liao P, Elsaid H, et al. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 2012; 114: 993–1000.
- Nakano H, Ikeda T, Hayashi M, et al. Effect of body mass index on overnight oximetry for the diagnosis of sleep apnea. Respir Med 2004; 98:421–427.
- Spencer S, Calverley PMA, Burge PS, et al. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J 2004; 23:698–702.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34:648–654.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14:540–545.
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52:69–77.
- Miller MR, Hankinson J, Brusasco V, et al., ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 2005; 26:319–338.
- Sanders MH, Newman AB, Haggerty CL, et al. Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am. J Respir Crit Care Med 2003; 167: 7–14.
- Young T, Shahar E, Nieto FJ, et al. Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162:893–900.
- McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med 2009; 180:692–700.
- Sakakibara H, Tong M, Matsushita K, et al. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J 1999; 13:403–410.
