Abstract
Chronic obstructive pulmonary disease (COPD) is a progressive disease, its prevalence increases with age. COPD is frequently associated with co-morbidities such as cognitive impairment, and their clinical relevance has risen in the recent past. Cognitive function may fluctuate with the variable components of COPD like hypoxaemia, hypercapnia, lung function, exacerbations or severity of the disease. The objectives of this study were to examine whether the cognitive status of COPD patients is different across clinical stages (exacerbation, at discharge and stable COPD) and also if there are cognitive areas that have more potential to change than others. Prospective observational clinical study: 62 patients admitted to hospital due to acute exacerbation of COPD were evaluated at hospital admission; 61 at discharge; and finally, 48 patients with stable COPD completed the study and were included in the analysis. Cognitive status was assessed with the Montreal Cognitive Assessment (MoCA). Our results show that all clinical variables improved from exacerbation to discharge COPD. MoCA total score, visuoconstructional, attention, language, abstraction, delayed recall and orientation subscores improved significantly from exacerbation to discharge COPD (p < 0.05). MoCA total score, visuoconstructional and naming subscores worsened significantly from discharge to stable COPD (p < 0.05). Finally, from exacerbation to stable COPD all the clinical variables improved; MoCA total score and naming, attention, language, abstraction and delayed recall subscores have shown significant differences (p < 0.05). Cognitive status of COPD patients is different across clinical stages, and there are cognitive areas with more potential to change than others.
| Abbreviations | ||
| COPD | = | Chronic Obstructive Pulmonary Disease |
| AECOPD | = | Acute Exacerbation of Chronic Obstructive Pulmonary Disease |
| GOLD | = | Global initiative for chronic Obstructive Lung Disease |
| BMI | = | Body Mass Index |
| MoCA | = | Montreal Cognitive Assessment |
| MMSE | = | Mini Mental State Examination |
| SGRQ | = | Saint George's Respiratory Questionnaire |
| MNA | = | Mini Nutritional Assessment |
| HAD | = | Hospital Anxiety and Depression Scale |
| SO2 | = | Saturation Oxygen |
| FVC | = | Forced Vital Capacity |
| FEV1 | = | Forced Expiratory Volume in 1 second |
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by airflow limitation that is not fully reversible (Citation1). It is expected to become the fifth-leading cause of disability by 2020 (Citation2), and the fourth-leading cause of mortality by 2030 (Citation3). The prevalence of COPD increases with age, with a 5-fold increased risk for patients aged over 65 years compared with those aged less than 40 years (Citation4).
The weight of co-morbidities which are frequently associated with COPD is important to consider. It is estimated that approximately two-thirds of patients with COPD have one or two co-morbidities, such as cardiovascular disease (Citation5, 6), osteoporosis (Citation7), diabetes mellitus type II (Citation8), lung cancer (Citation9) and cognitive impairment (Citation10, 11). The clinical relevance of cognitive impairment in COPD has risen in the recent past (Citation12, 13). The incidence of cognitive impairment in COPD varies in different studies (Citation12, 14), from 12% to 88%.
Hung et al. (Citation15) analyzed cognitive impairment in patients with COPD and found that such patients had a greater risk of developing cognitive impairment than patients without COPD. Factors such as hypoxaemia, hypercapnia, lung function, exacerbations or severity of the disease contribute to the deterioration of cognitive functions (Citation10). The study by Fioravanti et al. (Citation16) examined the cognitive changes associated with COPD and evaluated the relationship between the clinical characteristics of respiratory deficits with the cognitive and emotional functioning of patients.
In this line, the change in the clinical characteristics in COPD can have a repercussion on cognitive function, such as exacerbations (Citation16) and may also be affected the course of recovery. Other authors, like Pendlebury et al. (Citation17) in 2011 have reported cognitive affectations in Transient Isquemic Attack and stroke, those changes across the clinical evolution of the pathologies. In other respiratory diseases, it has been found that available treatments result in partial reversibility of cognitive dysfunction (Citation18,Citation19). We can hypothesize that the evolution of COPD patients across exacerbation and stable stages can imply changes in their cognitive profile, but no previous studies have followed subjects with COPD across their clinical evolution.
The main objective of our study was to evaluate the cognitive status of COPD patients across different clinical stages (exacerbation, at discharge and stable), and to analyze the evolution of the different cognitive areas.
Methods
Design
Prospective observational clinical study; patients admitted to hospital due to an acute exacerbation of COPD (AECOPD) were evaluated, at their hospital admission, at discharge and at stable stage. The study was approved by the Hospital Ethics Committee and all the participants gave their written consent.
Patient selection
Patients included in this study were recruited over a period of 12 months (November 2013–October 2014). The recruitment was of a consecutive sample. The selected patients were hospitalized due to AECOPD. The study was carried out at services of pulmonology of Hospitals “Virgen de Las Nieves” and “San Cecilio” in Granada.
In this study the inclusion criteria were: (Citation1) patients hospitalized due to AECOPD, (Citation2) patients admitted in the previous 24–48 hours (Citation3) age range from 50 to 90 years, (Citation4) to participate in the study voluntarily, (Citation5) to sign the informed consent designed for this study and (Citation6) to complete the assessment protocol at hospital admission. Exclusion criteria were: (Citation1) patients with cardiac decompensation, (Citation2) patients with cancer or pulmonary fibrosis, (Citation3) patients with dementia, (Citation4) patients with psychiatric illness and (Citation5) patients admitted to hospital in the previous 2 weeks.
Evaluation
Patients were assessed in three different moments of their clinical evolution: exacerbation (at hospital admission), at discharge [at hospital discharge, at 9 days mean from admission (range, 5 to 15 days)] and stable (at home, a month minimum after the last hospitalization), though personal interview.
In the first part of the personal interview patients signed the informed consent and were collected sociodemographic data. After that, we evaluated anthropometric variables [sex and body mass index (BMI)] and functional ability (Barthel index). Afterwards, we proceeded to the evaluation of cognitive status with the Montreal Cognitive Assessment (MoCA) (Citation20), respiratory function (we performed a spirometry (Citation21), saturation values and dyspnea with modified Borg scale were also evaluated) (Citation22) and other characteristics of the patients such as:
Health-related quality of life was evaluated with the Saint George Respiratory Questionnaire (SGRQ) (Citation23) that is a standardized, self-completed questionnaire for measuring impaired health and perceived health-related quality of life in patients with airway disease. It contains 50 items, divided into 3 domains: symptoms, activity, and impacts. A score is calculated for each domain, and the total score. A low score indicates better health-related quality of life.
Nutritional status was assessed with mini Nutritional Assessment (MNA) (Citation24) is a tool for nutritional screening and assessment. It is structured in 18 items grouped in 4 rubrics: anthropometry, general status, dietary habits, and self-perceived health and nutrition states. Each answer has a numerical value and contributes to the final score, which has a maximum of 30. With threshold values of ≥ 24 for well-nourished, 17–23.5 for at risk of malnutrition, and <17 for malnourished.
Levels of anxiety and depression were evaluated with the Hospital Anxiety and Depression Scale (HAD) (Citation25). The HAD scale is a 14-item self-report rating scale designed to measure both anxiety and depression. It contains two subscales, each containing seven items on a 4-point Likert scale (ranging from 0–3), each subscale scores ranging from 0 to 21. A score above 8 on either subscale indicates possible depression and anxiety, and a score above 11 indicates probable depression and anxiety. A score of < 8 on the depression scale is considered normal, 8–10 indicates mild depression, 11–14 indicates moderate depression, and ≥ 15 represents severe depression.
Cognitive status
MoCA is a cognitive screening test designed to detect mild cognitive impairment (Citation20). It assesses different cognitive domains: visuoconstructional skills, executive functions, naming, memory, attention, language, abstraction, delayed recall and orientation. These are the features and the score for each area of MoCA test ():
| • | 1 point was added for participants with 12 years of education or less on their total MoCA score (if < 30) (Citation20). | ||||
| • | Different versions of the MoCA were used throughout all the testing periods in order to eliminate the possible practice effects | ||||
| • | The time to administer MoCA is approximately 10 minutes. The total possible score is 30 points. The MoCA has been used in disease specific studies since it was initially developed for identifying mild cognitive impairment in the memory clinic population (Citation26–Citation28). | ||||
Table 1. Details on the subtests of the MoCA.
Statistical analysis
Statistical analysis was performed using SPSS statistical package (version 20.0). Normal distribution of the data was tested by Kolmogorov-Smirnov test (with Lilliefors' correction). The homogeneity of variances was approached with Fisher's F-test. If both conditions were satisfied, parametric tests (Student's t-test, paired Student's t test, and analysis of variance, followed, if necessary, by multiple comparisons using Tukey's test) were used. If any of the conditions was not satisfied, a nonparametric test (Mann–Whitney) was used instead. In all instances, α = 5%.
Results
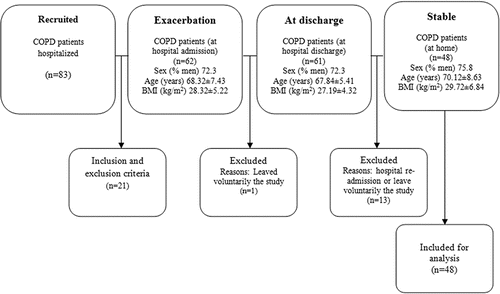
The flow diagram of the patients included in the study is described in . Eighty-three patients hospitalized due to AECOPD were recruited, 62 were included after application of the inclusion and exclusion criteria. At hospital discharge, the number of COPD patients who remained in the study was 61, because 1 patient left voluntarily the study. Finally, after have been excluded 13 due to hospital re-admission or due to leave voluntarily study, 48 patients with stable COPD completed the study and were included in the analysis.
Patients' characteristics included in every clinical stage are shown in . shows anthropometric variables, age and BMI, also Barthel Index and SGRQ. Nutritional status and, anxiety and depression symptoms shows significant differences across clinical stages (p < 0.05).
Table 2. Patients' characteristics across clinical stages.
Patients' clinical profile values across clinical stages are shown in . shows clinical variables at three different moments, exacerbation, at discharge and stable COPD. From exacerbation to discharge COPD, all clinical variables have shown improvements, showing significant differences (p < 0.05) in the case of SO2, predicted FEV1%, and dyspnea. From discharge to stable COPD, dyspnea worsened significantly (p < 0.05) without significant differences in other variables. Finally, from exacerbation to stable COPD all the variables improved, showing significant differences (p < 0.05) in SO2, and dyspnea.
Table 3. Patients' clinical profile across clinical stages.
Cognitive variables values across clinical stages included in the study are shown in . shows cognitive variables at three different moments, exacerbation, discharge and stable COPD. From exacerbation to discharge COPD, MoCA total score, visuoconstructional, attention, language, abstraction, delayed recall and orientation subscores have shown significant differences (p < 0.05). From discharge to stable COPD, MoCA total score, and only visuoconstructional and naming subscores have shown significant differences (p < 0.05). From exacerbation to stable COPD, MoCA total score and naming, attention, language, abstraction and delayed recall subscores have shown significant differences (p < 0.05).
Table 4. Cognitive variables values across clinical stages.
The percentage of COPD patients with values of cognitive impairment (MOCA < 20) in exacerbation, discharge and stable are, respectively, 48.3%, 23.6% and 36.3%.
Discussion
We hypothesized that the cognitive status of COPD patients could be different across clinical stages (exacerbation, at discharge and stable) and also there would have cognitive areas with more potential to change than others.
Our study has reflected the cognitive affectation in moderate to severe COPD patients. Our result is in line with the study of Schou et al. (Citation13), where it has been shown that there is a significant relation between the severity of COPD and cognitive impairment. In contrast, Dodd et al. (Citation10) have shown that there was no significant relationship between cognitive impairment and the severity of COPD.
Modification of the cognitive status across clinical stages
We hypothesized that a change in clinical status could produce a change in cognitive status in COPD patients. Modification of the cognitive status across clinical stages is present in diseases like chronic heart failure (Citation29) or diabetes (Citation30); also in other respiratory diseases has been found that the changes of patients' clinical status are accompanied by a modification of their cognitive status (Citation18, 19).
In another study (Citation31), Dodd et al. assessed neuropsychological performance in patients with COPD who were awaiting discharge from hospital following acute exacerbation and recovery and compared them with stable outpatients with COPD and with healthy control subjects, but they did not follow up the same patient at three different moments (exacerbation, at discharge and stable) of his disease.
In our study we show the relationship between changes in clinical status and changes in cognitive status across clinical stages in COPD patients. Nowadays, there are still no studies that followed up on the cognitive status in COPD patients across clinical stages (exacerbation, at discharge and stable COPD). The follow-up from our study has determined that changes in clinical status produce a change in cognitive status in COPD patients.
Cognitive areas evaluated in COPD
Mild cognitive impairment denotes a particular diagnosis in which categories are defined as amnestic vs non-amnestic and single vs multiple domain. There are frequent impairments in attention and executive dysfunction, followed by verbal learning and memory (Citation11).
In the present study, cognitive areas evaluated were visuoconstructional, naming, attention, language, abstraction, delayed recall and orientation. Cognitive areas with more potential to change were different across clinical stages in COPD patients.
From exacerbation to discharge COPD, cognitive areas with more potential to change were visuoconstructional, attention, language, abstraction, delayed recall and orientation. Our results are in line with those obtained by Pendlebury et al. (Citation17), which examined the patterns of change in MMSE domains between baseline and 1 month for patients with transient cognitive impairment (cerebral transient ischemic attack or minor stroke), and found that recovery was most often seen in attention/calculation, recall, drawing, writing, and orientation.
From discharge to stable COPD, cognitive areas with more potential to change were visuoconstructional and naming. And from exacerbation to stable COPD, cognitive areas with more potential to change were naming, attention, language, abstraction and delayed recall. Our study shows that cognitive function is modifiable and has a potential to improve.
Limitations of the study
The main limitation of the study has been not to use a neuropsychological assessment more complete. The high levels of fatigue presented by the patients and the large consumption of time that requires a complete neuropsychological assessment made us to choose a cognitive assessment shorter, as MoCA (Citation20). Furthermore, the alternative of a neuropsychological battery would almost have certainly resulted in a smaller and less representative patient sample. In our study, we have used MoCA because it is superior to MMSE in detecting mild cognitive impairment in patients with COPD (Citation11).
Conclusions
Cognitive status of COPD patients is different across clinical stages, and there are cognitive areas with more potential to change than others. It would be interesting to investigate more about cognitive areas with more potential for change than other across clinical stages in COPD.
Another important point to make is that acute exacerbations of COPD (as for other acute illness) impacts a patient's cognition, which has important consequences for the consideration of a patient's capacity to consent to procedures or to contribute to complex decision-making whilst hospitalised and unwell.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364(9434):613–620.
- Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA 2005; 294(10):1255–1259.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLOS Med 2006; 3(11):2011–2030.
- Postma DS, Siafakas N. Management of chronic obstructive pulmonary disease. Eur Respir Mon 1998; 7:41–73.
- Sin DD, Lacy P, York E, Man SP. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am Thorac Soc 2004; 170(7):760–765.
- Villeneuve S, Belleville S, Massoud F, Bocti C, Gauthier S. Impact of vascular risk factors and diseases on cognition in persons with mild cognitive impairment. Dement Geriatr Cogn Disord 2009; 27(4):375–381.
- Bolton CE, Ionescu AA, Shiels KM, Pettit RJ, Edwards PH, Stone MD, et al. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Resp Crit Care Med 2004; 170(12):1286–1293.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Resp J 2008; 32(4):962–969.
- Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 2005; 60(7):570–575.
- Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Resp J 2010; 35(4):913–922.
- Villeneuve S, Pepin V, Rahayel S, Bertrand JA, de Lorimier M, Rizk A, et al. Mild cognitive impairment in moderate to severe COPD: A preliminary study. Chest 2012; 142(6):1516–1523.
- Torres-Sánchez I, Rodríguez-Alzueta E, Cabrera-Martos I, López-Torres I, Moreno-Ramírez MP, Valenza MC. Cognitive impairment in COPD: a systematic review. J Bras Pneumol 2015; 41(2):182–190.
- Schou L, Østergaard B, Rasmussen LS, Rydahl-Hansen S, Phanareth K. Cognitive dysfunction in patients with chronic obstructive pulmonary disease–a systematic review. Resp Med 2012; 106(8):1071–1081.
- Cleutjens FAHM, Wouters EFM, Dijkstra JB, Spruit MA, Franssen FME, Vanfleteren LEGW. The COgnitive-Pulmonary Disease (COgnitive-PD) study: protocol of a longitudinal observational comparative study on neuropsychological functioning of patients with COPD. BMJ Open 2014; 4(3):e004495.
- Hung WW, Wisnivesky JP, Siu AL, Ross JS. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180(2):134–137.
- Fioravanti M, Nacca D, Amati S, Buckley AE, Bisetti A. Chronic obstructive pulmonary disease and associated patterns of memory decline. Dementia 1995; 6(1):39–48.
- Pendlebury ST, Wadling S, Silver LE, Mehta Z, Rothwell PM. Transient cognitive impairment in TIA and minor stroke. Stroke 2011; 42(11):3116–3121.
- Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, Cappa SF. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res Bull 2003; 61(1):87–92.
- Ferini-Strambi L, Marelli S, Galbiati A, Castronovo C. Effects of continuous positive airway pressure on cognitition and neuroimaging data in sleep apnea. Int J Psychophysiol 2013; 89(2):203–212.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCa: A brief screening tool for mild cognitive impairment. J Am Geriat Soc 2005; 53:695–699.
- Sanchís Aldás J, Casan Clará P, Castillo Gómez J, Mangado N, Palenciano Ballesteros L, Roca Torrent J. Normativa SEPAR, Espirometría, 1997.
- Segura-Méndez NH, Cortés Hernéndez R, Menez Díaz D, Espinosa Leal FD, Sosa Erosa E, Torres Salazar AB. Correlación entre la escala de Borg y la espirometría en pacientes asmáticos. Alerg Mex 2005; 52(3):127–131.
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Resp Med 1991; 85(B):25–31.
- Mini Nutritional Assessment [homepage on the internet]. Mini Nutritional Assessment: MNA. Available in: http://www.mna-elderly.com ( accessed 6 November 2014).
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiat Scand 1983; 67(6):361–370.
- Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord 2008; 23(7):1043–1046.
- Volonghi I1, Pendlebury ST, Welch SJ, Mehta Z, Rothwell PM. Cognitive outcomes after acute coronary syndrome: a population based comparison with transient ischaemic attack and minor stroke. Heart 2013; 99(20):1509–1514.
- Tiffin-Richards FE, Costa AS, Holschbach B, Frank RD, Vassiliadou A, Krüger T, Kuckuck K, Gross T, Eitner F, Floege J, Schulz JB, Reetz K. The Montreal Cognitive Assessment (MoCA)—a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One 2014; 9(10):e106700.
- Sauve MJ, Lewis WR, Blankenbiller M, Rickabaugh B, Pressler SJ. Cognitive impairments in chronic heart failure: a case controlled study. J Card Fail 2009; 15(1):1–10.
- S Roriz-Filho J, Sa-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, Moriquti JC, Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta 2009; 1792(5):432–443.
- Dodd JW, Charlton RA, Van Den Broek MD, Jones PW. Cognitive dysfunction in patients hospitalized with acute exacerbation of COPD. Chest 2013; 144(1):119–127.