Abstract
Over the last 20 years smoking has become the most common method of heroin use and increasing numbers of heroin smokers are presenting to local medical services, before the age of 40 years, with severe airway disease. To determine COPD prevalence we recruited 129 subjects from two local community drug services, of whom 107 were heroin smokers. We collected demographic, medical and treatment data, smoking history (including cannabis and opiates) and details of symptoms including MRC dyspnoea. Subjects completed the COPD Assessment Tool and spirometry. Thirty heroin smokers were identified as having COPD resulting in a COPD prevalence of 28%. Mean age was 43 Citation(4) years and FEV1 was 2.71 (0.98) L; 70 Citation(23) %predicted. Breathlessness and wheeze were more common in subjects with COPD (p < 0.04 and p < 0.05) but symptoms were common in all heroin smokers. MRC score was higher (3 vs. 2.4; p < 0.04) in those with COPD and health status appeared poorer (CAT 20.4 vs. 15.8; p < 0.07). Only 4 (11%) had previously been diagnosed with COPD and only 16 (53%) received any inhaled medication. Asthma prevalence was also high at 33% and asthmatic subjects had similar symptoms and health status compared with the COPD subjects, and were also significantly undertreated. COPD and asthma are common in current and former heroin smokers. They are often present at a young age and are underdiagnosed and undertreated. Awareness of this issue should be highlighted within drug services and in particular to heroin smokers. Screening this high-risk population with spirometry should be considered.
Introduction
Since the early 1990s, there has been a gradual change in the method of heroin use from injecting to smoking using foil, and worldwide this has become the most common method (Citation1,Citation2). One of the drivers of this change was the perception that smoking heroin was safer, due to the well-documented risks of intravenous drug use, including the transmission of blood-borne infections such as HIV and viral hepatitis, and thromboembolic disease Citation(1). However, this may have led to an increase in the incidence of respiratory disease in this population.
There is a recognised association between heroin use and asthma exacerbations (Citation3–6) and bronchial hyper-responsiveness is more common in smokers of heroin and cocaine Citation(7). This latter is the only study to estimate asthma prevalence. Little is known about the association between heroin smoking and COPD. Buster et al. obtained spirometry and information on dyspnoea in 100 subjects attending a methadone treatment centre Citation(8), and found an association between heroin smoking and a reduction in FEV1, as well as a non-significant trend towards increased breathlessness in individuals with greater heroin exposure. However, this study did not define airflow obstruction using an FEV1/FVC ratio, and the incidence of asthma was not stated. A more recent case series of heroin smokers with severe, early onset emphysema did not assess COPD prevalence Citation(9) but described severe emphysema occurring at a very young age, with individuals dying of respiratory failure before the age of 40 years. This study was designed to establish the prevalence and severity of COPD and asthma amongst current and former heroin smokers, selected because they were opiate smokers and not because they were symptomatic, and to assess the frequency of respiratory symptoms and the impact these have on health status.
Methods
Crime Reduction Initiative (CRI) is a community-based service aimed at improving the lives of families and individuals affected by drugs, alcohol, crime, homelessness, domestic abuse and antisocial behaviour across England and Wales. CRI has a team of volunteers, peer mentors and key workers dedicated to promote recovery and improve the quality of life of the service users. There are three CRI centres in Sefton, Merseyside, two in the south of the borough (Bootle) and one in the north (Southport). The centres have 980 service users, many of who are current or former drug users; however, the service configuration means that only a minority would attend the CRI centres during any year. All study volunteers were recruited from these centres between October 2012 and March 2013. Support for the study was obtained from the Service Users Forum, and the study was approved by Sefton REC – number 10/H1015/68.
The study was advertised throughout the service in advance of recruitment and key workers informed their clients. The Research Assistant approached all clients who presented to CRI and any current or former heroin smoker willing to take part was recruited. Clients who had never smoked heroin but had injected heroin or smoked tobacco or cannabis were also recruited to form a control group. Each individual provided written informed consent and then underwent the following assessments.
Questionnaires
The subject completed 3 questionnaires:
The first recorded demographic data, occupational history, past medical history (including exacerbations of or admissions with chest disease), treatment and symptoms.
The second recorded the participant's tobacco smoking history, including childhood exposure within the home, and any history of smoking cannabis, heroin or crack cocaine. This included age at which substance abuse started, years and amount smoked and equipment used including inhalation of plastic and other possible toxins. Finally, any history of intravenous opiate and methadone use was recorded.
The third contained the COPD assessment test™ (CAT) Citation(10) and MRC dyspnoea score Citation(11).
Spirometry
Volunteers performed spirometry consistent with American Thoracic Society/European Respiratory Society Citation(12) recommendations using a MicroMedical Microloop 3300 spirometer (CareFusion, San Diego, USA). They were then offered reversibility testing by inhalation of 400 mcg salbutamol administered via a Volumatic™ spacer (Allen & Hanburys, Uxbridge, UK) and 20 minutes after inhalation, spirometry was repeated. The subject was given a copy of his or her spirometry result, and if the results were abnormal, permission was sought to inform their primary care physician.
Defining COPD and asthma
COPD was defined as a post-bronchodilator FEV1/FVC ratio below the lower limit of normal (LLN) using GLI-2012 equations Citation(13). Airflow obstruction was defined as a standard residual z-score of below -1.64. In the few subjects with airway obstruction who declined reversibility testing, a diagnosis of COPD was also made in those with pre-bronchodilator airflow obstruction if they had smoked heroin for at least 7 years and had not been diagnosed with asthma before the age of 25 years and/or before smoking heroin for at least 7 years. The latter criterion is based on a large case series of heroin smokers with early onset emphysema, all whom had at least 7 years of heroin smoking Citation(9).
Asthma was defined in subjects with either 1) airflow obstruction that normalised with bronchodilation or 2) airflow obstruction where there was a FEV1 increase of 9% of predicted value with bronchodilation Citation(14), or 3) an asthma diagnosis before the age of 25 years or where asthma was diagnosed before the subject had smoked heroin for 7 years.
Statistics
Statistical analysis was performed using SPSS Version 19.0 (IBM, USA). Continuous data are presented as mean and standard deviation and categorical data as a percentage of the population. Normal distribution was confirmed using the Shapiro–Wilk test. Unpaired t-tests were used for 2 group comparisons and ANOVA and Tukey's Honest Significant Difference test were used for 3 group comparisons and post-hoc analysis. Pearson's coefficient was used for correlation. A p-value of 0.05 was considered significant.
Results
Study flow
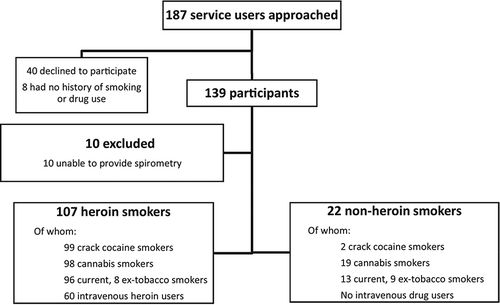
A total of 187 people were approached. Of these 40 had a history of drug or cigarette smoking but declined to participate and 8 had no history of tobacco smoking or drug use, leaving 139 people who were recruited (a recruitment rate of 74%). Ten were excluded as they were unable to provide adequate spirometry, which left 129 people, of whom 107 were heroin smokers and a further 22 who smoked crack cocaine, cannabis and/or cigarettes. Of the 107 heroin smokers, 99 had also smoked crack cocaine, 98 had used cannabis and all but 3 were current or ex-cigarette smokers. All participants used foil to smoke heroin (“chasing the dragon”). A variety of methods were used to smoke crack cocaine, including foil, a bong, plastic, glass, and metal pipes, plus a variety of homemade devices, frequently made from plastic bottles. Sixty of the participants recruited had used intravenous heroin, all of whom had also smoked heroin. The study flow is shown in
Subject demographics
Details are shown in . This was a young, predominantly male (90/129; 70%) population, with a mean age of 43 Citation(4) years.
Table 1. Demographics, lung function, symptoms, treatment and history of drug use in subjects with COPD, with asthma, with a history of heroin smoking but no evidence of airway disease, and subjects who have never smoked heroin or crack cocaine.
Spirometry and COPD prevalence
Of the 129 subjects completing spirometry 14 (11%) declined reversibility testing; of these 5 had pre-bronchodilator airflow obstruction. Of the 107 heroin smokers, 30 were identified as having COPD; hence, the prevalence of COPD amongst heroin smokers was 28%. None of the 22 non-heroin smokers had COPD. According to ATS/ERS classification (15), of the 30 who had COPD, most had mild-to moderate disease (13 mild, 10 moderate, 4 severe, and 3 very severe) and the mean age of this group was 41.5 (6.9) years.
A further 35 heroin smokers were identified as having asthma based on the pre-bronchodilator airflow obstruction with significant reversibility or normalisation of spirometry Citation(7) or a prior diagnosis of asthma before the age of 25 years or before smoking heroin for 7 years Citation(28). Forty-two people had normal lung function and no evidence of airway disease. The demographics, lung function, symptoms and drug use of these groups are shown in
Comparison of heroin smokers with COPD and other subjects
We compared the heroin smokers with COPD to the heroin smokers without COPD and the control participants who had not smoked heroin or crack cocaine (). There was no difference in age, gender, cigarette pack-years smoked, years of heroin, crack cocaine or cannabis smoking, or years of intravenous drug use. There was no significant correlation between duration of heroin smoking and FEV1, MRC score or CAT score.
Cough, sputum, wheeze, and dyspnoea were common in all groups who smoked heroin. However, more heroin smokers with COPD reported breathlessness (87% vs. 64%; p < 0.04) and wheezing (90% vs. 71%; p < 0.05) than heroin smokers without COPD. The COPD group also reported a higher MRC dyspnoea score (3 vs. 2.3, p < 0.04) and a poorer CAT score (20.4 vs. 15.8; p = 0.07) though the latter was not statistically significant.
There was no significant difference in lung function when comparing the heroin smokers without COPD and the group who had never smoked heroin but the former group had a higher MRC dyspnoea score (2.3 vs. 1.3, p-value < 0.01).
Prior diagnosis and treatment
Forty of the 129 participants had received a prior diagnosis of asthma of whom 8 actually had COPD (27% of the 30 diagnosed with COPD) and 32 were presumed correctly diagnosed with asthma. In contrast of the 11 subjects previously diagnosed with COPD only 4 had COPD, 4 had asthma and 3 had neither. The other 18 subjects with COPD had no prior diagnosis of airway disease. Overall only 4/30 (13%) were correctly diagnosed with COPD.
The majority of subjects with COPD were aged 36–45 years with a COPD prevalence of 23% in those aged 36–40 years and 45% in those aged 41–45 years ().
Table 2. Proportion of people who agreed to screening who were diagnosed with COPD.
Of the 30 participants with COPD only 16 (53%) were receiving any inhaled therapy and only 8 (27%) were prescribed a long-acting bronchodilator. Of the 35 participants with asthma only 11 (31%) were receiving inhaled steroids ().
Table 3. Treatment at time of study participation in the 30 heroin smokers were found to have COPD and the 35 with asthma.
Discussion
We have shown that at least 28% of current or former heroin smokers have airflow obstruction consistent with COPD, and this is present at a very young age. Despite this high COPD prevalence these individuals are frequently underdiagnosed or misdiagnosed and significantly undertreated. In addition up to a third of heroin smokers appear to have asthma and this is also often undertreated. The overall prevalence of airway disease in this population was 61%.
In contrast the prevalence of COPD in the general population is estimated at between 2 and 4% Citation(16), although this varies widely depending on which diagnostic criteria are used, and a higher prevalence is associated with a lower socio-economic status Citation(17) and increasing age. The closest direct comparator group, The Health Survey for England Citation(18) revealed a 17% prevalence of COPD in current and former smokers, diagnosed on the basis on spirometry criteria alone, with a mean age of 63 years. Targeted spirometric screening of a local population of smokers with symptoms showed a prevalence of 25% Citation(19) and a mean age of 61 years. This contrasts with our prevalence of 28% and a mean age of 43 years in this study with a prevalence of 30% in subjects younger than 46 years. Hence, the prevalence of COPD in our study was higher than previous studies in the same country and city in a dramatically younger population not selected because of symptoms.
Only 13% of our population had a correct diagnosis of COPD, while 27% had been diagnosed with asthma. This misdiagnosis has been shown previously to be common in primary care even in a typical “COPD-age” population (Citation19,Citation20) and is understandable in this population where two-thirds of people were aged 45 years or younger. This underlines the importance of spirometry in the diagnosis of lung disease in heroin smokers but emphasises the need to consider the diagnosis of COPD in heroin smokers rather than making a clinical diagnosis of asthma. However, this may present a practical problem in some UK areas where provision of primary care spirometry is often only commissioned to accept referrals for people aged 40 or 45 years of age or older. As a cohort, the COPD group was significantly undertreated despite a high level of symptoms and poor health status. In addition to underdiagnosis, this is also likely to reflect the drug-users’ chaotic social circumstances and poor engagement with medical services which may be due to a degree of nihilism and acceptance of symptoms, by both the individual and healthcare professionals, due to an altered expectation of health.
In our study, symptoms were a poor discriminator of a diagnosis of COPD. Symptom scores were higher generally in heroin smokers than in those who did not smoke heroin although 60–75% of heroin smokers without COPD or asthma reported breathlessness, wheeze, cough and phlegm. Breathlessness and wheeze were even more common in heroin smokers with COPD but with all symptoms being so common spirometry is essential to identify individuals with COPD. The other statistically significant difference in symptoms between the heroin smokers with and without COPD was the MRC dyspnoea score. However, the lack of difference in other measures is likely to relate, in part, to subject numbers in each group. The health status of the heroin smokers, measured by the CAT score, was generally poor with the mean CAT score of 20.4 in the COPD group being similar to an unselected group of COPD patients prior to commencing pulmonary rehabilitation Citation(21).
We based a diagnosis of COPD in our study on LLN to avoid under diagnosis of COPD in this young cohort, especially in women. Not all of our participants were prepared to undergo bronchodilator reversibility testing; therefore, we elected to use pre-bronchodilator spirometry for classification of these individuals. However, we did include post-bronchodilator spirometry where results were available.
There have been previous case reports documenting the precipitation of asthma symptoms by heroin smoking and snorting (Citation3–6) and Boto de los Bueis et al. Citation(7) showed a 44% prevalence of bronchial hyper-reactivity and 22% prevalence of asthma (defined as wheeze and bronchial hyper-responsiveness) in subjects inhaling a mixture of heroin and cocaine. A previous American, inner city study of 166 patients admitted with asthma exacerbations revealed a 43% prevalence of heroin or cocaine use in hospital admissions with asthma, with those who had used heroin or cocaine significantly more likely to be intubated for their asthma exacerbation Citation(22). A more recent study showed that near fatal asthma was more common in heroin and cocaine users Citation(23). Our 33% prevalence of asthma was high and similar to Boto de los Bueis albeit based on different diagnostic criteria. Twice as many of our asthmatic subjects complained of wheeze (66% vs. 33%) but our group were older (41 vs. 30 years) and smoked heroin for longer (15 vs. 6 years).
In our study the presence of symptoms, particularly wheeze, did not allow differentiation between heroin smokers with asthma, COPD or neither. We accepted a prior primary care diagnosis of asthma if made at a young age or before prolonged heroin smoking and our higher prevalence of asthma may relate to inaccuracies with this diagnosis; however, we were unable to confirm this by reviewing primary care records. In this context COPD prevalence may be as high as 36% as 6 individuals with airflow obstruction were labelled asthmatic because they received a diagnosis of asthma aged 6–18 years before they started to smoke heroin aged 16–25 years and 2 further individuals with airflow obstruction were diagnosed with asthma 2–3 years after starting to smoke heroin. One or more of these individuals may have COPD or ‘asthma-COPD overlap syndrome’ which has been diagnosed in 27% of primary care asthmatics with a moderate cigarette smoke exposure Citation(24). Notwithstanding, the prevalence of airway disease is very high and a focus on respiratory diagnosis in such populations could have a major impact on quality of life and healthcare utilisation. As with the COPD cohort the asthmatic subjects were significantly undertreated.
One major challenge when assessing the impact of drug use on COPD prevalence is use of multiple drugs by an individual. The majority of the heroin smokers included in our study had also smoked crack cocaine and this has been shown previously to produce acute bronchospasm Citation(25). However, the only long-term effect shown on lung function is a mild reduction in DLCO after long-term heavy use Citation(25). The majority of participants also smoked cannabis which has been linked with large airway obstruction and hyperinflation (Citation25,Citation26) and possibly emphysema (Citation26,Citation27) but the effect on spirometry is very modest; however, in our population the heroin smokers with and without COPD had a similar duration of tobacco, heroin, crack cocaine, cannabis and intravenous opiate use albeit subject numbers in each group are small and may be inadequate to detect small differences.
One of our key messages is that there are likely to be a large number of individuals potentially affected with COPD due to heroin smoking. This has a significant impact on healthcare utilisation evidenced by another study from Liverpool which described 13% of hospitalisations for COPD exacerbations were in drug smokers with higher readmission rates and higher need for non-invasive ventilation Citation(28). In the Liverpool local authority, there are 5350 individuals attending drug treatment centres Citation(29); albeit not all will have extensive heroin smoking exposure. However, if the overall population is similar to that in our study there may be up to 1500 opiate users in the city affected by COPD and up to 70,000 nationally Citation(30) with a prevalence of 2/1000 population. Therefore, COPD in heroin smokers represents a significant public health problem, with significant potential costs to the health service. These figures present a compelling justification for early identification of this high-risk population.
One weakness is that prevalence could be overestimated because symptomatic individuals are more likely to have volunteered. However, the high acceptance rate of 74%, in this difficult to access population, combined with the high number of symptomatic individuals with normal lung function suggests any overestimation would be modest. Another problem we encountered was how to quantify the degree of heroin exposure in contrast to pack years tobacco smoking and joint years Citation(26) of cannabis smoking. Previous attempts to quantify heroin exposure all have weaknesses (Citation2,Citation7), so pragmatically we used the number of years of use accepting this is an inexact measure. A consensus view on how to quantify heroin smoking exposure would aid further studies.
Conclusions
COPD is common in heroin smokers, occurs at a young age, and represents a significant public health problem. It is frequently underdiagnosed or misdiagnosed, and is undertreated. Asthma appears to be equally as common and is also undertreated. Spirometry is essential to diagnosis as symptoms are common in all heroin smokers and should be considered in any opiate user complaining of breathlessness, regardless of age. Screening of this high-risk population should be considered.
Declaration of interest
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This research was funded by Sefton Primary Care Trust.
References
- Strang J, Griffiths P, Gossop M. Heroin smoking by “chasing the dragon”: origins and history. Addiction 1997; 92:673–683; discussion 685–695.
- Strang J, Griffiths P, Powis B, Gossop M. Heroin chasers and heroin injectors: differences observed in a community sample in London, UK. Am J Addict 1999; 8:148–160.
- Hughes S, Calverley PMA. Heroin inhalation and asthma. BMJ 1988; 297:1511–1512.
- Cygan J, Trunsky M, Corbridge T. Inhaled heroin-induced status Asthmaticus: Five cases and a review of the literature. Chest 2000; 117:272–275.
- Krantz AJ, Hershow RC, Prachand N, Hayden DM, Franklin C, Hryhorczuk DO. Heroin insufflation as a trigger for patients with life-threatening asthma. Chest 2003; 123:510–517.
- Doshi V, Shenoy S, Ganesh A, Rishi MA, Molnar J, Henkle J. Profile of acute asthma exacerbation in drug users. Am J Ther 2014 Oct 10. ( Epub ahead of print)
- Boto de los Bueis A, Pereira Vega A, Sánchez Ramos JL, Maldonado Pérez JA, Ayerbe Garcia R, García Jiménez D, Pujol de la Llave E. Bronchial hyperreactivity in patients who inhale heroin mixed with cocaine vaporized on aluminum foil. Chest 2002; 121:1223–1230.
- Buster M, Rook L, van Brussel GH, van Ree J, van den Brink W. Chasing the dragon, related to the impaired lung function among heroin users. Drug Alcohol Depend 2002; 68:221–228.
- Walker PP, Thwaite E, Amin S, Curtis J, Calverley PM. The association between heroin smoking and early onset emphysema. Chest 2015 May 28. doi: 10.1378/chest.15-0236. [ Epub ahead of print]
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34:648–654.
- Bestall JC, Paul E, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581–586.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respir J 2012; 40(6):1324–1343.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16:5–40.
- American Thoracic Society and European Respiratory Society. Standards for the Diagnosis and Treatment of Patients with Chronic Obstructive Pulmonary Disease, 2004. Available from: https://www.thoracic.org/copd-guidelines/resources/copddoc.pdf ( accessed 04 November, 2015)
- Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. London: National Clinical Guideline Centre, London; 2010. Available from: http://www.nice.org.uk/guidance/cg101 ( accessed 04 November, 2015).
- Bischoff EWMA, Schermer TRJ, Bor H, Brown P, van Weel C, van den Bosch WJ. Trends in COPD prevalence and exacerbation rates in Dutch primary care. Br J Gen Pract 2009; 59:927–933.
- Jordan RE, Miller MR, Lam K-BH, Cheng KK, Marsh J, Adab P. Sex, susceptibility to smoking and chronic obstructive pulmonary disease: the effect of different diagnostic criteria. Analysis of the Health Survey for England. Thorax 2012; 67:600–605.
- Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J 2006; 28:945–952.
- Griffiths C, Feder G, Wedzicha J, Foster G, Livingstone A, Marlowe GS. Feasibility of spirometry and reversibility testing for the identification of patients with chronic obstructive pulmonary disease on asthma registers in general practice. Respir Med 1999; 93:903–908.
- Dodd JW, Hogg L, Nolan J, Jefford H, Grant A, Lord VM, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011; 66:425–429.
- Levine M, Iliescu ME, Margellos-Anast H, Estarziau M, Ansell DA. The effects of cocaine and heroin use on intubation rates and hospital utilization in patients with acute asthma exacerbations. Chest 2005; 128:1951–1957.
- Doshi V, Shenoy S, Ganesh A, Lankala S, Henkle J. Near fatal asthma in an inner city population. Am J Ther 2014; Oct 3. [ Epub ahead of print].
- Kiljander T, Helin T, Venho K, Jaakkola A, Lehtimäki L. Prevalence of asthma-COPD overlap syndrome among primary care asthmatics with a smoking history: a cross-sectional study. NPJ Prim Care Respir Med 2015; 25:15047.
- Tashkin DP. Airway effects of marijuana, cocaine, and other inhaled illicit agents. Curr Opin Pulm Med 2001; 7:43–61.
- Aldington S, Williams M, Nowitz M, Weatherall M, Pritchard A, McNaughton A, et al. Effects of cannabis on pulmonary structure, function and symptoms. Thorax 2007; 62:1058–1063.
- Johnson MK, Smith RP, Morrison D, Laszlo G, White RJ. Large lung bullae in marijuana smokers. Thorax 2000; 55:340–342.
- Yadavilli R, Collins A, Ding WY, Garner N, Williams J, Burhan H. Hospital readmissions with exacerbation of obstructive pulmonary disease in illicit drug smokers. Lung 2014; 192(5):669–673.
- Whitfield M, Reed H, Harrison R, Davies C, McVeigh J. Drug and Alcohol Treatment in Cheshire and Merseyside 2012/13. Centre Public Heal Liverpool John Moores University. 2013. Available from: http://cph.org.uk/wp-content/uploads/2014/03/Drug-and-Alcohol-Treatment-in-Cheshire-and-Merseyside-12-13.pdf (accessed 04 November, 2015).
- Hay G, dos Santos AR, Worsley J. Estimates of the Prevalence of Opiate Use and/or Crack Cocaine Use, 2011/12: Sweep 8 Summary Report. Natl Treat Agency Subst Misuse, now part Public Heal Engl. 2012. Available from http://cph.org.uk/wp-content/uploads/2014/03/Drug-and-Alcohol-Treatment-in-Cheshire-and-Merseyside-12-13.pdf (accessed 04 November 2015).