Abstract
In COPD, body composition studies have focused primarily on low BMI. We examined obesity (BMI ≥ 30 kg/m2) as a risk factor for poor function and longitudinal functional decline. Data from a longitudinal cohort of adults with COPD (n = 1096) and an age- and sex-matched comparison group collected in two in-person visits ∼49 months apart were analyzed. Two measures of functioning were examined: six-minute walk distance (6MWD) and Short Physical Performance Battery (SPPB). Multivariate regression analyses examined relationships of obesity with functioning. Secondary analyses stratified by GOLD classification (GOLD-0/1, GOLD-2, GOLD-3/4). Obesity (53% of COPD cohort) was associated cross-sectionally with 6MWD and SPPB in COPD, and only with 6MWD in the comparison group. Obesity predicted significant functional decline in 6MWD for individuals with COPD (odds ratio (OR) for decline [95% CI] 1.8 [1.1, 2.9]), but not the comparison group. Secondary analyses revealed that the risk of decline was significant only in those with more severe COPD (GOLD 3/4, OR = 2.3 [1.0, 5.4]). Obesity was highly prevalent and was associated with poor function concurrently and with subsequent decline in 6MWD in COPD. Obesity in COPD should be considered a risk not only for more co-morbidities and greater health care use, but also for functional decline.
Introduction
In the general population of individuals without chronic obstructive pulmonary disease (COPD), obesity is associated with poor health outcomes, including increased functional limitations (both self-reported and performance-based) and greater disability Citation(1). In COPD, low BMI (especially as a proxy for low muscle mass) has been the primary focus of body composition studies instead of obesity, and has been associated with mortality Citation(2). Indeed, obesity actually appears to be somewhat protective for COPD mortality Citation(3).
Obesity clearly appears to be more prevalent in individuals with COPD than in the general population (Citation3,Citation4). Obesity can lead to expiratory flow limitations and increase both the work and oxygen cost of breathing at rest and during exercise, both of which may be linked to worsened dyspnea (Citation3,Citation5,Citation6), which may in turn lead to compromised function. Obesity in COPD has been linked to higher risk for co-morbidities and increased hospitalizations, re-admission rates, and health care use Citation(6). Yet, because studies of the impact of obesity on functioning in COPD have yielded mixed results (Citation7–9), obesity's role in physical functioning in COPD remains an open question (Citation9,Citation10).
The goal of this analysis was to examine prospectively the role of obesity as a risk factor for poor function and longitudinal functional decline among individuals with COPD by studying a well-characterized patient cohort and comparing them to an age- and sex-matched comparison group. Additional analyses examined whether the role of obesity was different among those with mild-to-moderate COPD (GOLD 0, 1, or 2) compared to those with more severe disease (GOLD 3 and 4).
Methods
Study cohort and timeline
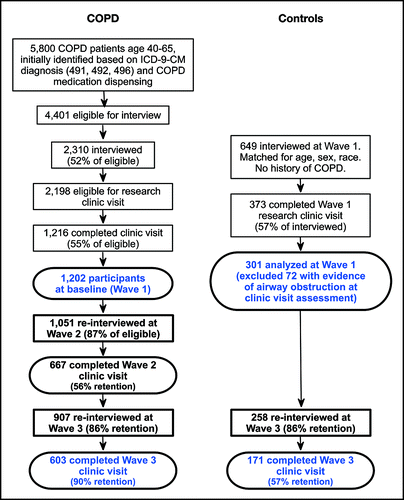
We used data from the Function, Living, Outcomes, and Work (FLOW) study, a prospective longitudinal cohort study of working-aged adults (40–65 years at baseline) recruited from an integrated health care delivery system. The FLOW cohort at baseline consisted of 1,202 Kaiser Permanente Medical Care Program (KPMCP) members with COPD recruited using a validated algorithm based both on recent health-care utilization linked to COPD, pharmacy dispensing for COPD-related medications, and a physician's diagnosis of COPD. Recruitment methods have been described previously (Citation4,Citation11).
At baseline (Wave 1), we conducted structured telephone interviews that ascertained sociodemographic characteristics, COPD clinical history, and health status. A subset of interviewees (n = 1202) participated in a research clinic visit in which spirometry and other physical assessments were performed. Of those examined at baseline, 667 (55%) completed the first follow-up research clinic visit (Wave 2) an average of 24 (±2.5) months later, and 603 completed the second follow-up research clinic visit (Wave 3) an average of 49 (±4.9) months later (). Age-, sex-, and race-matched controls with no history of COPD were also recruited (n = 649), with 171 completing the Wave 3 research clinic visit (57% retention). Although controls could self-report a history of asthma, any potential control subjects with airflow obstruction at baseline were excluded from enrollment. Sixteen subjects in the COPD cohort with BMI ≤21 were excluded because low BMI has previously been linked to poor outcomes in COPD Citation(12), thus these could not be treated as part of the “normal” weight referent group. Observations for an additional 4 individuals were deleted because of missing data for the six-minute walk distance (6MWD), yielding a final analysis sample of 358.
The study was approved by the UC San Francisco Committee on Human Research and the Kaiser Foundation Research Institute institutional review board.
Variables
Obesity
Height was measured with a wall-mounted stadiometer. Weight was measured with subjects wearing light indoor clothing and no shoes. Body mass index (BMI) was calculated as weight (Kg) divided by height (meters2), and obesity defined as BMI ≥ 30 kg/m2 Citation(13).
Functioning
Two measures, which assess different aspects of functioning, were examined.
Six-minute walk distance (6MWD)
We used a standardized flat, straight course of 30 meters in accordance with American Thoracic Society guidelines Citation(14). Every 2 minutes, a technician used standardized phrases to encourage effort. Distance is reported in meters. Five individuals (1 in GOLD 2 and 4 in GOLD 3/4) did not attempt the 6MWD and were excluded from analyses of 6MWD.
Short physical performance battery (SPPB) Citation(15)
This battery includes 3 performance measures of balance, chair stands and a 4-meter walk, each scored from 0 to 4 points. A summary score can range from 0 to 12. Poorer performance on the SPPB is predictive of incident disability, institutionalization, and mortality in older persons, independent of co-morbidity or socioeconomic factors Citation(15). The SPPB has been validated for use in COPD and linked to functional impairment and low muscle mass and strength (Citation16,Citation17).
Other variables
Pulmonary function testing was conducted using the EasyOne™ Frontline spirometer (ndd Medical Technologies, Chelmsford, MA), which meets American Thoracic Society (ATS) criteria. Spirometry was performed according to ATS guidelines Citation(18). Predictive equations derived from NHANESIII were used to calculate percent predicted pulmonary function values, including the LLN for FEV1/FVC Citation(19). For the COPD cohort, pulmonary function was categorized according to GOLD classification using lower-limit of normal (LLN) criteria for the FEV1/FVC ratio.
Age, sex, and smoking history were collected by structured telephone interview. Smoking history was classified as current, former, or never. Dsypnea symptoms were classified using the Medical Research Council (MRC) scale Citation(20). Participants were asked whether a physician had diagnosed any of the following co-morbid conditions: high blood pressure, heart disease, diabetes, arthritis, cancer, stroke, kidney disease, or obstructive sleep apnea (OSA). For analysis, the number of co-morbid conditions was categorized as two indicator variables representing 1 or ≥ 2 co-morbidities. No information was available on prior pulmonary rehabilitation or exercise training programs used by participants.
Data analysis
Differences in baseline characteristics between obese and non-obese subjects were assessed with chi-square analyses (test for trend for ordinal categorical variables) and t-tests. Bivariate differences between obese and non-obese in baseline functioning based on each of the two measures described above were also assessed with t-tests. Differences in baseline characteristics between subjects who remained for Wave 3 and those who were lost to follow-up were assessed in a similar manner.
Cross-sectional multivariate analyses were conducted to identify independent relationships between obesity and functioning at baseline, controlling for age, sex, current smoking, baseline FEV1 percent predicted, presence of OSA and other co-morbid conditions. Analyses were conducted for the control and COPD cohorts separately. In secondary analyses, the COPD cohort was stratified into three severity groups: GOLD 0 and 1; GOLD 2; and GOLD 3 and 4.
Declines in functioning
In longitudinal analyses of changes from baseline to follow-up, a decline in functioning for a given measure was defined dichotomously as a decline exceeding an established cut-point. That cut-point was set as the median change observed for the entire analysis sample plus either the published minimal clinically important differences (MCID) for the measure in question, or, if there was no published MCID, a decrease of 0.5 standard deviation (SD), which has been found to approximate a minimum clinically important difference (Citation21,Citation22).
For the SPPB, a decline was defined as the median change for the entire cohort plus the published minimal clinically important differences (MCID). The median change in SPPB was 0, and the published MCID is 1 point Citation(23); therefore, a decline in SPPB was defined as a decrease in SPPB score of 1 point. The median observed change in 6MWD for the cohort was -30 meters. A number of MCIDs have been published, ranging from 25 to 80 meters (Citation22–25). Because there is no consensus, we used a decrease of 0.5 SD in our cohort (53 meters), which falls in the mid-range of these values. Therefore, a decline was defined as -83 meters.
Differences between obese and non-obese subjects in the occurrence of functional decline were tested initially using chi-square analyses. Multivariate logistic regression analyses were carried out for each measure of functional decline, separately for control and COPD subjects, controlling for time to follow-up, age, sex, baseline smoking, baseline FEV1 predicted, baseline value of the measure of functioning being analyzed, presence of OSA and the number of other co-morbid conditions at baseline (as categorized in the cross-sectional analyses), change in FEV1% predicted, and change in body weight from baseline to follow-up. These analyses were repeated, stratifying by GOLD classification (GOLD 0/1, GOLD 2, and GOLD 3/4). Using data from the final multivariate model, we calculated the population attributable fraction (PAF) for function decline associated with obesity.
All analyses were conducted with SAS 9.3 (SAS, Cary NC).
Results
Subject characteristics
COPD cohort
The mean age was 58.5 (±6.2) years; 58.2% were female (). Approximately one in three (31.5%) was a current smoker. The mean FEV1 predicted was 63.3% (±23.4), and 28.2% of the group was classified as GOLD ≥3. Use of supplemental oxygen was reported by 8.9%, and 41.5% reported severe breathlessness (MRC dyspnea grade 3+; “stop for breath after walking a few minutes on level ground”). Sleep apnea was reported by 22.8%, and the mean number of other co-morbid conditions was 2.2 (±1.3; median number = 2).
Table 1. Baseline characteristics by obesity status for adults with COPD and controls.
Just over half (52.9%) of the COPD sample was classified as obese. Compared to all others, those who were obese were twice as likely to use supplemental oxygen (11.6% vs. 5.9%, p =.001), had more severe breathlessness (51.1% vs. 31.0% with highest rating of breathlessness, p < .0001), were more likely to report sleep apnea (34.0% vs. 10.2%, p < .0001), and had a higher number of other co-morbid conditions (2.6 vs. 1.7, p < .0001).
Controls
Significantly fewer of the control subjects were obese (42.4%, p = .001). Significant differences were seen between obese and non-obese control subjects in FEV1% predicted, breathlessness, presence of sleep apnea, and number of co-morbid conditions ().
Cross-sectional relationship between obesity of functioning
In the COPD cohort as a whole, obese subjects had significantly worse performance on both measures of functioning, which were somewhat attenuated but remained significant after adjusting for covariates. In analyses stratified by GOLD (0/1, 2, 3/4), obese subjects in each stratum had significantly worse function by both 6MWD and SPPB in both unadjusted and adjusted analyses.
At baseline in the control group, obese subjects had significantly worse performance on the 6MWD, but not SPPB (). The difference in 6MWD remained after adjustment for covariates.
Table 2. Baseline functioning defined by the Short Physical Performance Battery and 6-Minute Walk Distance by obesity status.
Longitudinal analyses
Lost to follow-up comparison
There was no significant difference in the proportion of individuals in the COPD and control cohorts who were lost to follow-up (45.0% vs. 43.1%, p = 0.55). Individuals in the COPD cohort who were lost to follow-up prior to Wave 3 were younger at baseline, more likely to be smokers, reported more severe breathlessness, and were less likely to have concurrent sleep apnea (). They also had worse scores on both measures of functioning. In contrast, there were no significant differences in baseline characteristics between those who remained for Wave 3 and those who were lost to follow-up in the control cohort.
Table 3. Comparison of baseline characteristics of individuals who remained in the FLOW cohorts with those who dropped out prior to Wave 3.
Functional decline
Among the COPD cohort as a whole, 26.0% experienced a decline in functioning measured by SPPB and 24.4% by the 6MWD. Obese subjects with COPD were significantly more likely to experience functional declines by both SPPB and 6MWD (). After adjustment for all covariates, obese subjects had a significantly increased risk of functional decline as measured by 6MWD (OR = 1.8 [95% CI 1.1, 2.9]).
Table 4. Longitudinal meaningful decline in functioning.*
Stratification by GOLD classification revealed that significant differences in SPPB decline were observed only among individuals in GOLD 3/4. After adjustment for covariates, however, this difference was no longer present. In contrast, for 6MWD, significant differences were seen between obese and not obese subjects only for GOLD 0/1 in bivariate analyses. After adjustment for covariates, however, an increased risk of decline in 6MWD performance was noted for all groups, although the risk was statistically significant only for the GOLD 3/4 group (OR = 2.3 (1.0, 5.4).
Using data from the multivariate logistic regression analyses, we calculated the population attributable fraction (PAF) for decline in 6MWD associated with obesity among the GOLD 3/4 strata where the risk was most manifest. In that group, the PAF for obesity was 27.4% for functional decline measured by 6MWD.
Discussion
Approximately half of individuals in this cohort of individuals with moderate-to-advanced COPD met the standard BMI criterion for obesity. In contrast, only 4% (who were excluded from this analysis) met the BODE criterion for low BMI (< 21 kg/m2). Regardless of the measure used to define functional status, obesity demonstrated powerful cross-sectional associations with functioning. Thus, even though higher BMI appears to be linked to better survival in severe COPD (Citation7,Citation26), obesity appears to be associated with worse functioning among individuals living with COPD. Although a relationship between obesity and poorer function is expected based on research in the general population, the role of obesity specifically in functional decline in COPD has received little attention.
In longitudinal analyses, obesity was associated with decline in 6MWD in the COPD group, but not the control group, suggesting a COPD-related phenomenon. Moreover, the risk of decline in 6MWD was statistically significant only for the most severe GOLD 3/4 group, again, consistent with a COPD-specific effect. The monotonic incremental increase in the point estimates of risk (odds of decline in 6MWD were 1.6, 1.8, and 2.3 for COPD 0/1, 2, and 3/4, respectively) is also consistent with the effect of obesity in COPD being modified by the stage of disease.
The potential limitations of our analysis should be kept in view. We used body mass index, as have most other studies of body composition. BMI, however, is only a proxy measure for fat and fat-free mass, that more accurately capture actual body composition (Citation27–30). For example, among a group of COPD patients, sarcopenia was present across all BMI categories, including 54% of those who were obese by BMI, and BMI was less predictive of functional problems than more complex measures of body composition, such as fat-free mass (FFM) or appendicular lean mass Citation(31). Therefore, even though commonly used and easy to measure, BMI is prone to misclassification of actual body composition when compared to other approaches, such as the use of whole-body dual energy absorptiometry (DXA). Nonetheless, such misclassification would likely not have explained the obesity associations that we observed in this analysis.
Obesity may be a confounder that leads to apparent protection or risk. For example, obesity-related symptoms may lead to earlier or more aggressive treatment that attenuates functional decline Citation(9), or may carry risk of co-morbidity that is the true causative factor in accelerated decline. Obese COPD patients may have greater muscle strength and mass, which may lead to better preserved exercise capacity. In addition, there are indications that overweight and obesity are risk factors for COPD misdiagnosis Citation(32). It is possible that such misdiagnosis may have masked the effects of obesity in the patients with less severe COPD.
The way in which functioning is measured may also have an impact on the observed relationships between obesity and functioning in COPD. Studies that have examined performance-based exercise capacity using cycling-based testing have found little impact of obesity, whereas testing based on walking appears to show worse functioning among obese patients Citation(8). The speculation is that, although muscular strength may be better preserved in overweight and obese patients, the stress of weight-bearing exercise negates that advantage. This may explain the divergence between our findings for 6MWD and SPPB in longitudinal studies.
The population from which we drew our study participants, Northern California Kaiser Permanente, is generally similar to the regional population, except for the extremes of income Citation(33). Even though more than one quarter of the COPD cohort was classified as stages GOLD 3 or 4, individuals with extremely severe COPD were likely to be underrepresented in the research clinic visit sample as they may have been unable to leave their homes. The prevalence of obesity in this US sample (54%) is not only significantly higher than that of our controls (42%), it is substantially higher than COPD samples from other countries (Citation34–36).
The study excluded people over age 65 at baseline, thereby truncating the age of both COPD patients and controls. However, the effects we report may present a conservative estimate of the impact of obesity on functioning in COPD given that the prevalence of obesity is increasing in older age groups, and that obesity may have an even greater impact on function and functional decline in older age group (Citation37–39). Finally, it is possible that differential participation in initial research clinic visits and selective loss-to-follow-up influenced the results we observed. Although there were no differences between controls who remained in the study and those who dropped out, members of the COPD cohort who did not remain for Wave 3 had worse function at Wave 1.
Conclusion
A high proportion of individuals in this large sample were obese. Obesity was associated not only with poor function concurrently, but also with subsequent decline in 6MWD performance, particularly among those with the worse pulmonary function. Because of the high prevalence of obesity, the population impact on function in COPD can be substantial. Among individuals classified as GOLD class 3/4 in our cohort, more than one quarter of the incidence of functional decline measured by the 6MWD was attributable to obesity.
Dyspnea and impairment caused by obesity will not respond to COPD interventions, which suggests that pulmonary rehabilitation includes a stronger focus on generalized physical activity to assist with reduction of obesity. For example, walking programs as a component of pulmonary rehabilitation appear to be effective across all BMI categories Citation(40). Smoking cessation efforts should also directly address weight gain, again perhaps including a strong component of physical activity to avoid weight gain. Combined with the high prevalence of obesity and obesity-related co-morbidities in COPD and the association of obesity with increased health care use, our findings of the impact of obesity on functional decline in COPD underscore the need for more attention to the mechanisms of the relationship and effective means of addressing obesity in clinical and rehabilitation settings.
Declaration of interest
The authors have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
Support for this project was given by the following sources: NIH/NHLBI R01HL077618 and NIH/NCRR UCSF CTSI UL1TR00004.
References
- Sturm R, Ringel J, Andreyeva T. Increasing obesity rates and disability trends. Health Aff 2004; 23(2):199–205.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal T. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160(6):1856–1861.
- Franssen F, O'Donnell D, Goossens G, Blaak E, Schols A. Obesity and the lung: 5.Obesity and COPD. Thorax 2008; 63(12):1110–1117.
- Eisner M, Blanc PD, Sidney S, Yelin EH, Lathon P, Katz PP, Tolstykh I, Ackerson L, Iribarren C. Body composition and functional limitation in COPD. Respir Res 2007; 8:7.
- Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009;179(6):509–516.
- O'Donnell D, Ciavaglia C, Neder J. When obesity and chronic obstructive pulmonary disease collide. Physiological and clinical consequences. Ann Am Thorac Soc 2014;11(4):635–644.
- Sood A. Obesity, adipokines, and lung disease. J Appl Phsyiol 2010; 108(3):744–753.
- Sava F, Laviolette L, Bernard S, Breton M, Bourbeau J, Maltais F. The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med 2010; 10:55.
- Galesanu R, Bernard S, Marquis K, Lacasse Y, Poirier P, Bourbeau J, Maltais F. Obesity and chronic obstructive pulmonary disease: Is fatter really better? Can Respir J 2014; 21(5):297–301.
- ten Hacken N. Physical inactivity and obesity: relation to asthma and chronic obstructive pulmonary disease? Proc Am Thorac Soc 2009; 6(8):663–667.
- Eisner M, Iribarren C, Blanc P, Yelin E, Ackerson L, Byl N, Omachi T, Sidney S, Katz P. Development of disability in chronic obstructive pulmonary disease: beyond lung function. Thorax 2011; 66(2):108–114.
- Celli B, Cote C, Marin J, Casanova C, de Oca M, Mendez R, Plata V, Cabral H. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10):1005–1012.
- World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: World Health Organization, 2000.
- American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1):111–117.
- Guralnik J, Simonsick E, Ferrucci L, Glynn R, Berkman L, Blazer D, Scherr P, Wallace R. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol: Med Sci 1994; 49(2):M85–M94.
- Eisner M, Blanc P, Yelin E, Sidney S, Katz P, Ackerson L, Lathon P, Tolstykh I, Omachi T, Byl N, Iribarren C. COPD as a systemic disease: impact on physical functional limitations. Am J Med 2008;121(9):789–796.
- Patel S, Mohan D, Andersson Y, Baz M, Kon S, Canavan J, Jackson S, Clark A, Hopkinson N, Natanek S, Kemp P, Bruijnzeel P, Man W, Polkey M. Phenotypic characteristics associated with reduced Short Physical Performance Battery score in COPD. Chest 2014;145(5):1016–1024.
- American Thoracic Society, European Respiratory Society. Standardization of spirometry. Euro Respir J 2005; 26(2):319–338.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159(1):179–187.
- Bestall J, Paul E, Garrod R, Garnham R, Jones P, Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54(7):581–586.
- Norman G, Sloan J, Wyrwich K. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592.
- Make B. How can we assess outcomes of clinical trials: the MCID approach. COPD 2007; 4(3):191–194.
- Perera S, Mody S, Woodman R, Studenski S. Meaningful change and responsiveness in common physical peformance measures in older adults. J Am Geriatr Soc 2006; 54(5):743–749.
- Wise R, Brown C. Minimal clinically important difference in the six-minute walk test and the incremental shuttle walking test. COPD 2005; 2(1):125–129.
- Polkey M, Spruit M, Edwards L, Watkins M, Pinto-Plata V, Vestbo J, Calverley P, Tal-Singer R, Agusti A, Bakke P, Coxson H, Lomas D, MacNee W, Rennard S, Silverman E, Miller B, Crim C, Yates J, Wouters E, Celli B. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Resp Crit Care Med 2013; 187(4):382–386.
- Schols A, Broekhuizen R, Weling-Scheepers C, Wouters E. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005; 82(1):53–59.
- Okorodudu D, Jumean M, Montori V, Romero-Corral A, Somers V-J, J, Erwin P, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes 2010; 34(10):791–799.
- Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard B, Andersen T, Sørensen T, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample. Am J Respir Crit Care Med 2006; 173(1):79–83.
- Kim S, Kang Y, Jung J, Park M, Kim Y, Kim S, Chang J, Kim E. Body mass index and fat free mass index in obstructive lung disease in Korea. Int J Tuberc Lung Dis 2014; 18(1):102–108.
- Maltais F. Body composition in COPD: looking beyond BMI. Int J Tuberc Lung Dis 2014; 18(1):3–4.
- van de Bool C, EPA R, Franssen F, EFM W, Schols A. Antagonistic implications of sarcopenia and abdominal obesity on physical performance in COPD. Eur Respir J 2015;46(2):336–345.
- Franssen F. Overweight and obesity are risk factors for COPD misdiagnosis and overtreatment. Chest 2014; 146(6):1426–1428.
- Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Publ Health 1992; 82(5):703–710.
- Steuten L, Creutzenbert E, Vrijhoef H, Wouters E. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J 2006; 15(2):84–91.
- Montes de Oca M, Tálamo C, Perez-Padilla R, Jardim J, Muino A, Lopez M, Valdiva G, Pertuzé J, Moreno D, Halbert R, Menezes A, PLATINO Team. Chronic obstructive pulmonary disease and body mass index in five Latin American cities: the PLATINO study. Respir Med 2008; 102(5):642–650.
- Vozoris N, O'Donnell D. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J 2012; 19(3):e18–e24.
- Vincent H, Vincent K, Lamb K. Obesity and mobility disability in the older adult. Obesity Rev 2010; 11(8):568–579.
- Batsis J, Zbehlik A, Scherer E, Barre L, Bartels S. Normal weight with central obesity, physical activity, and functional decline: data from the Osteoarthritis Iniative. J Am Geriatr Soc 2015; 63(8):1552–1560.
- Artaud F, Singh-Manoux A, Dugravot A, Tavernier B, Tzourio C, Elbaz A. Body mass index trajectories and functional decline in older adults: Three-City Dijon cohort study. Eur J Epidemiol 2015 Apr 9 [ Epub ahead of print].
- Greening N, Evans R, Williams J, Green R, Singh S, Steiner M. Does body mass index influence the outcomes of a walking-based pulmonary rehabilitation programme in COPD? Chron Respir Dis 2012; 9(2):99–106.