ABSTRACT
This is a proof of concept study that aims to establish feasibility and safety of a new strategy that includes an action plan for early treatment of acute exacerbations of COPD (AECOPD) with doubling dose of a combination of a long-acting beta2 agonist and an inhaled corticosteroid, and to explore its potential for avoiding the requirement of prednisone and its safety. Thirty-seven COPD outpatients with previous exacerbations were enrolled and followed-up for 12 months. The written action plan included a standing prescription to be used in the event of an AECOPD: Antibiotic, for 5 days (for purulent exacerbations) and doubling a combination of Salmeterol and Fluticasone Propionate for 10 days. The primary outcome was “treatment success” defined as “no need of prednisone within 30 days of the onset.” Twenty-seven patients experienced an AECOPD and doubled their combination dose. Among the 27 patients, there were 21 patients (78%) who did not require prednisone, and none of those had cardiovascular events, pneumonia, ER and hospital admissions. We have assessed that an early treatment of AECOPD with doubling the dose of a combination of Salmeterol and Fluticasone Propionate appears to be safe, well-tolerated and adhered to, and results in no requirement of systemic corticosteroid in a large proportion of patients presenting with mild-to-moderate worsening of dyspnea. This trial has the potential to change the approach of treatment of AECOPD and reduce the use of oral corticosteroids.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the 4th leading cause of mortality worldwide; the morbidity it causes contributes significantly to the burden of disease (Citation1, 2). Acute exacerbations of COPD (AECOPD), defined by a change in symptoms in excess of day-to-day variation Citation(3) are hallmarks of the disease. They are associated with a faster decline in lung function (Citation4, 5) and have a negative impact on quality of life Citation(6). They often lead to emergency visits, to hospitalizations and mortality (Citation7–9). Sixty percent of the global costs of COPD are associated with the management of acute exacerbations (Citation10, 11).
A history of AECOPD has been found to be the strongest predictor of future exacerbations Citation(12), and interrupting this vicious cycle may have significant implications. Presently, exacerbations attracting medical attention are typically treated with antibiotics and/or systemic corticosteroids (Citation3, Citation13) and increasing the frequency of short-acting β2-agonists (SABA)(14). Time to the initiation of treatment, from the onset of symptoms, has been shown to be a key variable in determining the success of management strategies, as delays in treatment have been associated with increased emergency visits and hospitalizations Citation(15). A written action plan that includes the prompt initiation of oral corticosteroids and antibiotics at the onset of an exacerbation has been associated with a decreased risk of hospitalization, and exacerbation recovery time (Citation16, 17).
Reducing the dose and frequency of systemic corticosteroids could potentially be achieved by substituting them with inhaled corticosteroids. Inhaled corticosteroids have a high level of topical anti-inflammatory activity and a low level of systemic activity (Citation18,19), and could potentially modify the airway inflammation that is characteristic of COPD exacerbations. This is especially true of the significant eosinophilic response that is associated with viral infections Citation(20).
The objective of this proof of concept study was to establish the feasibility and acceptability of a new action-plan inclusive strategy for early treatment of AECOPD with doubling dose of a combination of Salmeterol (LABA) and Fluticasone Propionate (ICS), and to explore its potential for avoiding the requirement of prednisone and its safety. Furthermore, we assessed adherence of patients using the combination inhaler as maintenance therapy.
Material and methods
Study design and subject selection
The study was conducted at the COPD clinic of the Montreal Chest Institute, Quebec, Canada. It was an open-label single-arm proof of concept trial. All patients signed a written informed consent. The study was approved by the Institutional Review Board.
Patients with a diagnosis of stable COPD, 40 years or older, with at least 10 pack-years cigarette smoking, at least two exacerbations requiring prednisone treatment in the past 3 years, dyspnea ≥ 2 out of 5 (MRC), and who were using a written action plan for acute exacerbations were consecutively recruited over a 6-month period. Patient had to be on a combination of Salmeterol and Fluticasone Propionate BID as a maintenance therapy or able to switch to this combination if already taking another combination medication (with the consent of their treating physician).
Subjects were excluded if they had a history of asthma or allergic rhinitis; using oxygen, oral corticosteroids or antibiotics; unstable or life threatening co-morbid conditions; having pre-existing medical conditions or on concomitant medications contraindicated with salmeterol or fluticasone propionate (e.g., monoamine oxidase inhibitors and tricyclic antidepressants, beta-adrenergic receptor blocking agents, non-potassium-sparing diuretics, inhibitors of cytochrome P450 (ritonavir, ketoconazole)); treated with theophyllines; colonized with Pseudomonas aeruginosa. We also excluded women of child-bearing potential.
An AECOPD was defined as a sustained worsening of dyspnea, cough or sputum production leading to an increase in the use of maintenance medication and/or supplementation with additional medication. In addition, exacerbation was further defined as either purulent or non-purulent to determine the need for antibiotic therapy.
Study intervention
Study medications
At recruitment, all rescue medication was replaced by Salbutamol. Patients were provided with a maintenance dose of the combination of Salmeterol and Fluticasone Propionate (metered dose inhaler) BID, and Salbutamol (metered dose inhaler) for the duration of the study, plus 1 extra container for exacerbation. Lot numbers were recorded for all medication provided; patients were asked to bring back empty containers at each follow-up visit. Smartinhalers, which are electronic inhalers containing a microprocessor that records/stores medication adherence were used for monitoring the patients' use of their maintenance therapy with the combination of Salmeterol and Fluticasone Propionate.
Intervention with action plan and instructions given to the patient for AECOPD
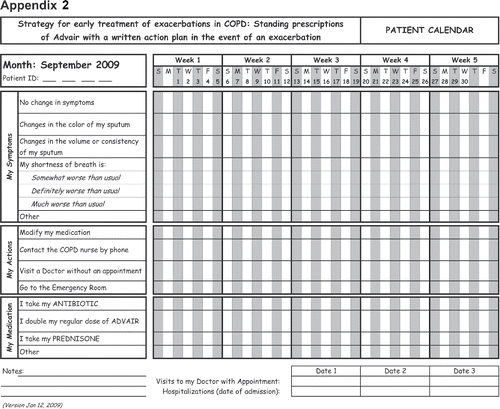
The intervention consisted of a written action plan, a group education session and nurse case-manager support. Patients first received a written action plan and one group education session based on the self-management program “Living well with COPD” Citation(21) and a standing prescription for doubling the regular dose of a combination of Salmeterol and Fluticasone Propionate, to be self-administered in the event of an exacerbation. Patients were instructed and trained to start treatment within 48 hours of experiencing respiratory symptom worsening and to call their case manager as soon as they recognize having an AECOPD and/or after starting their self-administered prescription. The case managers could communicate with the treating respirologists and the patient could be seen in clinic if needed. Case managers were available for patients' calls during business hours. Patients were followed by their case-manager by phone calls at 2 weeks after the group education session, at 1 month and every month thereafter for reinforcement.
The action plan from the second edition of “Living Well with COPD” was adapted for the study (). On a daily basis patients assessed their dyspnea symptoms, using a diary card “calendar” (), which asked the patient to choose one the following options for completing the statement “Your shortness of breath today is”: a) No change in symptoms b) Somewhat worse than usual c) Definitely worse than usual d) Much worse than usual. They were then asked to record which symptom(s) they perceived to be worse or of new onset, using a Yes/No questionnaire. “Increased sputum amount” and/ or “change in sputum colour” were considered major symptom changes, while a “cold, such as runny or blocked nose,” “increased wheezing or chest tightness,” “sore throat,” “increased cough,” or “fever” were considered minor symptom changes. Patients were reminded that they had to contact their case-manager as soon as they had a symptom change or any time they need it.
Based on severity of the worsening of the respiratory symptoms, instructions given to patients were as follows:
Moderate change in respiratory symptoms
If the patient reported dyspnea that was “somewhat or definitely worse than usual,” he/she was advised to initiate immediately the increased dose of his combination therapy (patient could already have started the treatment by himself/herself). The dose of the combination therapy was to be doubled for 10 days. If the patient was on the combination of Salmeterol and Fluticasone Propionate 250/50 BID, the dose was increased to 500/100 BID, and for those on 500/50 BID, the dose was increased to 1000/100 BID. Patients had to call the case-manager immediately to report this exaberbation; following this initial contact, the case-manager called them back after 1–3 days for follow-up. Prednisone was initiated if there was worsening of symptoms of dyspnea, despite the increased dose of the combination of Salmeterol and Fluticasone Propionate after 72 hours, or if the patient required to visit the ER department or to be hospitalized because of AECOPD.
Severe change in respiratory symptoms
If the patient reported dyspnea that was “much worse than usual,” he/she was advised to immediately initiate prednisone (40 mg once daily for 7–10 days) and further deterioration required an immediate visit to the clinic; the case-manager could decide at any time that the patient needed to be seen at the clinic. Following the patient's initial telephone contact, the case-manager called the patient after 1–3 days for follow-up.
In addition to the preceding, patients with purulent exacerbations (defined as those associated with changes in the color of secretions), were treated with an antibiotic (Avelox 400 mg daily for 5 days), unless the treating physician determined that an alternative antibiotic regimen was warranted, the reason for which was recorded (e.g., allergies).
Evaluation during the study
Patients underwent an initial evaluation with the research coordinator (person not involved in the patient care), which included assessment of vital signs and visual check for oral candidiasis, 12-lead ECG, pre- and post-bronchodilator spirometry.
For the follow-up visits, the Research Coordinator contacted the patient on a monthly basis. At 6 months, patients underwent an evaluation at the MCI, in which all the baseline tests were repeated. Completed monthly calendars and used metered-dose inhalers of Salmeterol and Fluticasone Propionate and Salbutamol were collected from the patients. Smart-inhalers data was downloaded, and devices returned to patients. Patients received medication for the next 6 months.
At the onset of an exacerbation (within 3 days of the patient reporting an exacerbation to the COPD nurse), the Research Coordinator visited the patient at home in order to measure vital signs, do a visual check for oral candidiasis and to perform a spirometry test. Two weeks following the initial home visit, the Research Coordinator went back to the patient's home and repeated these measurements. In addition to the home visits, the Research Coordinator called patients at day 7 (±2), 10 (±2), and 30 (±2). If at any time during these phone follow-ups the patient reported no improvement or worsening symptoms the Research Coordinator alerted the Case Manager who contacted the patient immediately to see if further treatment or visit to the MCI or ER was required. At each home visit or phone call, respiratory symptoms, medications added or increased, use of healthcare resources (scheduled and unscheduled) were collected.
Outcome measures and defining treatment success
The primary outcomes measured were “no requirement of prednisone within 30 days of the onset (worsening of dyspnea) of an AECOPD” following the new intervention, e.g., the doubling dose of the combination of Salmeterol and Fluticasone Propionate and “absence of cardiovascular events, pneumonia, emergency room visits and hospital admissions.” Secondary outcomes measured included adherence to the combination of Salmeterol and Fluticasone Propionate inhaler.
Statistical methods
We based our calculation on the expectation that 100% of patients experiencing an increased in dyspnea would receive prednisone. We expected that doubling the dose of the combination of Salmeterol and Fluticasone Propionate would prevent the patient to require prednisone in at least 50% of exacerbations, and considered this outcome to be clinically acceptable. We expected that at 12 months at least 51.7% of patients would have presented with at least one exacerbation Citation(22). From our experience, out of these exacerbations 17% were expected to require hospitalization. Considering an attrition of 15%, we estimated a sample size of 40 patients in order to have at least 15 episodes of AECOPD. Assuming that 50% of these AECOPD would result after doubling the dose in “no requirement of prednisone within 30 days of the onset (worsening of dyspnea) of an AECOPD,” the 95% exact confidence interval around 50% would be (25%–75%). For a pilot study, we considered this interval to be precise enough to indicate a signal of benefit using the new treatment, which could lead to a larger trial. All data were analyzed using STATA version 11.0 (StataCorp, College Station, TX, USA).
Results
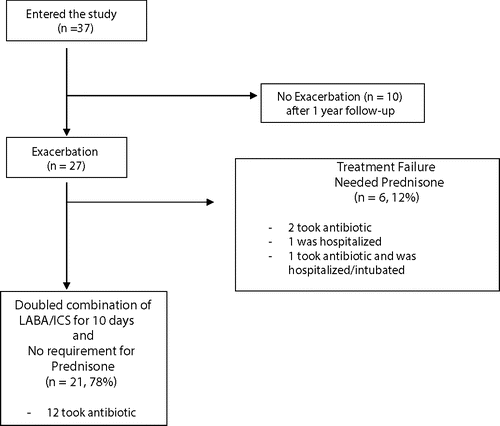
summarizes the study flow. Thirty-seven (37) patients were enrolled between January and June 2010. Of these, twenty-seven Citation(27) patients had at least one acute exacerbation during the 12-month study period.
shows baseline characteristics of the patients. Patient age varied from 48 to 85 years, and FEV1 from 14 to 73% of predicted values. When patients who experienced an AECOPD during the study period were compared with those who did not, sex differences were observed, while the spirometry results were well balanced, as were the other variables. However, a greater proportion of patients with AECOPD had higher MRC dyspnea grades (Citation4 and Citation5), and used higher doses of the combination of Salmeterol and Fluticasone Propionate (500 mcg).
Table 1. Characteristics of study patients.
Among the 27 patients who had an AECOPD, there were 21 patients (78%) who didn't require prednisone within 30 days of the onset of exacerbation (worsening of dyspnea) of an AECOPD. At continued follow-up, five Citation(5) patients experienced a second exacerbation and they responded with the same success rate (75%) (results not shown). The amount of increase of the maintenance dose of salbutamol and fluticasone combination did not seem to play a role: the same proportion of success was seen in patients with initial doses of 250 mcg increasing to 500 mcg (7 out of 9), as those with initial doses of 500 mcg doubling to 1000 mcg (14 out of 18). For comparison to the two years previous to enrolment, those same 27 patients experienced a total of 82 AECOPD, out of which 71 (86.6%) required treatment with prednisone, 19 (23.2%) required a visit to the emergency room and 10 (12.2%) a hospital admission (see ). With respect to the safety profile of the doubling dose, there was no report of cardiovascular events, pneumonia, emergency room visits and hospital admissions.
Table 2. Medication and health resources used for the AECOPD patients (n = 27).
There were 6 patients (12%) who doubled their combination of Salmeterol and Fluticasone, but ended up requiring a Prednisone treatment within 30 days onset of symptoms. Of these, 2 also took antibiotics, 1 took antibiotics and was hospitalized (and intubated), and 1 was hospitalized but did not need antibiotics.
displays the Smartinhaler results for adherence to the meter dose inhaler combination of Salmeterol and Fluticasone Propionate BID. More patients were missing the afternoon or evening dose than the morning dose, particularly in patients with no AECOPD. However, missing both doses, e.g., morning and afternoon or evening, was much higher in patients with AECOPD.
Table 3. Adherence to maintenance therapy based on Smartinhaler results for the study.
Discussion
The present proof of concept trial, using doubling dose of combination Salmeterol and Fluticasone Propionate therapy for early treatment of AECOPD showed that prednisone was not required in over 75% of the COPD patients. This new treatment approach was safe with respect to the absence of adverse events, emergency room visits and hospital admissions. These preliminary results apply to men and women with COPD, patients of all ages and those with moderate to severe airflow obstruction (GOLD 2 to 3), and they seemed to be independent of the level of the maintenance dose of combination used. In patients with recurrent AECOPD, a second acute event responded as well than the first exacerbation did with this new therapeutic approach.
The typical use of systemic corticosteroids for treating patients with exacerbations that come to medical attention has shortcomings. Other approaches have been recently reported as alternative of treatment for COPD exacerbations. Aaron and colleagues Citation(23) reported a trial of anti-TNF antibody, which was not more effective than prednisone for treatment of acute exacerbations of COPD. Systemic use of corticosteroids may not be trivial as it is associated with adverse effects such as hyperglycemia, myopathy Citation(24), and osteoporosis Citation(25). It has also been reported that short-term, high-dose corticosteroid treatment is associated with suppression of the adrenal response Citation(26). These adverse events are likely to have a cumulative effect in patients with COPD, who suffer repeated exacerbations Citation(27).
The treatment of exacerbations also includes increasing the use of SABA as needed. However, during a COPD exacerbation, the duration of effect of SABA is decreased Citation(28), thereby potentially depriving patients of sustained bronchodilation, which can play an important role in the symptomatic management of these patients. Sustained bronchodilation could be provided by replacing SABA with long-acting β2-agonists (LABA), and there is evidence to suggest an equal efficacy (Citation29–31). Reducing the duration of systemic corticosteroids Citation(32) is a strategy that could overcome these problems, but until now no alternative therapy to systemic corticosteroids has been studied.
Our study is the first trial to test the concept that doubling dose of combination therapy (Salmeterol and Fluticasone Propionate) for early treatment of AECOPD can prevent the use of systemic corticosteroids. Previous studies have been done using increased doses of nebulized budesonide for the treatment of acute exacerbations (Citation33–35). In these studies, the treatment did not aim at an early treatment of acute exacerbation but instead at replacing systemic corticosteroids with inhaled corticosteroids. Furthermore, the setting of these studies was that patients were treated in the emergency department with the usual medical supervision.
Our study highlights another potential important issue, e.g., adherence to regular treatment with inhaled medication such as the combination of Salmeterol and Fluticasone Propionate BID. Missing both morning and afternoon or evening doses was particularly a problem in patients who experienced AECOPD. This problem is likely to be underestimated in real-life clinical practice. Poor adherence to maintenance treatment could have consequences on the frequency of AECOPD. For some patients who had poor adherence, this may explain in part that they didn't require systemic corticosteroid as the action plan allows reintroducing combination therapy for early treatment of AECOPD.
Our study has several strengths. The study group was heterogeneous, including men and women, of all ages and including patients with different severity of COPD. The pharmacological and non-pharmacological components of the intervention were clearly defined and documented, preventing contamination of the results from the effects of varying interventions. All the patients used the written action plan promptly. No adverse events were reported that could be related to this new therapeutic strategy, i.e., doubling dose of a combination therapy (Fluticasone Propionate 1000 mcg BID for the vast majority of the patients who participated in the trial).
However, our study has limitations. Our study was designed as a proof of concept with a pre- and post- treatment comparison. The study was not a randomized clinical trial, not blinded and the main issue was the absence of a control group. This is important because of the relatively high success rate that is found in placebo groups in studies involving the treatment of COPD exacerbations. For example, in one study published by Aaron and colleagues Citation(21) involving patients with COPD experiencing an acute exacerbation, success which was defined as no requirement for additional treatment in the following 30 days, occurred in 57% of patients with COPD belonging to the placebo group (no systemic steroids). In our study, the success rate of over 75% could be explained in part by the fact that many patients didn't need additional treatment. Although this has to be recognized as an important limitation, these results may suggest an overuse of systemic corticosteroids in the treatment of COPD exacerbations.
Other limitations included the outcomes measured in the trial that were limited to “preventing the use of prednisone.” No comparison was made as to the effectiveness and/or treatment equivalence between ICS and OCS on patient reported outcomes (PRO) and extra pulmonary effects, i.e., systemic inflammation and/or co-morbidities. Furthermore, this therapeutic approach was used in only one centre and this centre is known to be exemplary in the field of self-management with the use of a written action plan for AECOPD “Living Well with COPD.” Patients selected for this study were familiar with the prompt use of an action plan in the event of an exacerbation. The results of the study may not be generalizable with respect to the benefits and safety of this approach. However, the intention of this proof of concept study was to test if a novel approach could be promising for early treatment of exacerbations and amenable to a large randomized clinical trial.
In conclusion, we have assessed that early treatment of AECOPD with doubling the dose of a combination of Salmeterol and Fluticasone Propionate, as part of an intervention that includes a written action plan, a group education session and nurse case-manager support, appears to be safe, well-tolerated and adhered to, and results in no requirement of systemic corticosteroid in a large proportion of patients presenting with mild-to-moderate worsening of dyspnea.
Randomized controlled trials, which include patient-reported outcomes, and long-term benefits of such a strategy, are warranted. Although we are waiting such a trial, this novel approach should not be considered unless the patient has already demonstrated that he can use promptly an action plan in the event of an exacerbation. Particular attention should also be given to ensure proper maintenance treatment adherence to reduce the rate of AECOPD. Furthermore, this should be part of an integrated approach of care with the supervision of a well-trained and qualified health care professional and physician always in support.
Funding
The entire study was funded as an investigator-initiated study by GlaxoSmithKline. Trial registration number: NCT2136875 at clinicaltrials.gov.
Declaration of interest statement
Dr. Bourbeau reports grants from Almirall, AstraZeneca, Boehringer, Ingelheim, GlaxoSmithKline and Novartis outside the submitted work. These grants are all administered by the Research Institute of the McGill University Health Centre. None of the other authors have any conflict of interest in relation to this work. The authors alone are responsible for the content and writing of the article.
References
- Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006; 27(1):188–207. PubMed PMID: 16387952.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006; 28(3):523–532. PubMed PMID: 16611654.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106(2):196–204. PubMed PMID: 3492164.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57(10):847–852. PubMed PMID: 12324669. Pubmed Central PMCID: 1746193.
- Kanner RE, Anthonisen NR, Connett JE, Lung Health Study Research G. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001; 164(3):358–364. PubMed PMID: 11500333.
- Bourbeau J, Ford G, Zackon H, Pinsky N, Lee J, Ruberto G. Impact on patients' health status following early identification of a COPD exacerbation. Eur Respir J 2007; 30(5):907–913. PubMed PMID: 17715163.
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. AAch Intern Med 2003; 163(5):585–591. PubMed PMID: 12622605.
- Collet JP, Shapiro P, Ernst P, Renzi T, Ducruet T, Robinson A. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. The PARI-IS Study Steering Committee and Research Group. Prevention of Acute Respiratory Infection by an Immunostimulant. Am J Respir Crit Care Med 1997; 156(6):1719–1724. PubMed PMID: 9412546.
- Connors AF, Jr, Dawson NV, Thomas C, Harrell FE, Jr., Desbiens N, Fulkerson WJ, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996; 154(4 Pt 1):959–967. PubMed PMID: 8887592.
- Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, Masa F, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004; 59(5):387–395. PubMed PMID: 15115864. Pubmed Central PMCID: 1746989.
- Spencer S, Jones PW, Group GS. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 2003; 58(7):589–593. PubMed PMID: 12832673. Pubmed Central PMCID: 1746751.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12):1128–1138. PubMed PMID: 20843247.
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010; 303(23):2359–2367. PubMed PMID: 20551406.
- O'Donnell DE, Aaron S, Bourbeau J, Hernandez P, Marciniuk DD, Balter M, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J 2007; 14 Suppl B:5B–32B. PubMed PMID: 17885691. Pubmed Central PMCID: 2806792.
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169(12):1298–1303. PubMed PMID: 14990395.
- Bischoff EW, Hamd DH, Sedeno M, Benedetti A, Schermer TR, Bernard S, et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax 2011; 66(1):26–31. PubMed PMID: 21037270.
- Sedeno MF, Nault D, Hamd DH, Bourbeau J. A self-management education program including an action plan for acute COPD exacerbations. COPD 2009; 6(5):352–358. PubMed PMID: 19863364.
- Brogden RN, McTavish D. Budesonide. An updated review of its pharmacological properties, and therapeutic efficacy in asthma and rhinitis. Drugs 1992; 44(3):375–407. PubMed PMID: 1382936.
- Johansson SA, Andersson KE, Brattsand R, Gruvstad E, Hedner P. Topical and systemic glucocorticoid potencies of budesonide and beclomethasone dipropionate in man. Eur J Clin Pharmacol 1982; 22(6):523–529. PubMed PMID: 7128664.
- Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis 2006;1:39--47.
- Bourbeau J, Nault D, Sedeno M, et al. Content and images from the program Living Well with COPD (2nd Edition). 2005. Available from: www.livingwellwithcopd.com
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007 Jan 15;175(2):144–149. PubMed PMID: 17053207.
- Aaron SD, Vandemheen KL, Maltais F, Field SK, Sin DD, Bourbeau J, et al. TNFalpha antagonists for acute exacerbations of COPD: a randomised double-blind controlled trial. Thorax. 2013; 68(2):142–148. PubMed PMID: 23161645.
- Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med 1994; 150(1):11–16. PubMed PMID: 8025735.
- McEvoy CE, Ensrud KE, Bender E, Genant HK, Yu W, Griffith JM, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. A Am J Respir Crit Care Med 1998; 157(3 Pt 1):704–709. PubMed PMID: 9517579.
- Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet 2000; 355(9203):542–545. PubMed PMID: 10683005.
- Osman IM, Godden DJ, Friend JA, Legge JS, Douglas JG. Quality of life and hospital re-admission in patients with chronic obstructive pulmonary disease. Thorax 1997; 52(1):67–71. PubMed PMID: 9039248. Pubmed Central PMCID: 1758400.
- Bernasconi M, Brandolese R, Poggi R, Manzin E, Rossi A. Dose-response effects and time course of effects of inhaled fenoterol on respiratory mechanics and arterial oxygen tension in mechanically ventilated patients with chronic airflow obstruction. Intens Care Med 1990; 16(2):108–114. PubMed PMID: 2332537.
- Cazzola M, Califano C, Di Perna F, D'Amato M, Terzano C, Matera MG, et al. Acute effects of higher than customary doses of salmeterol and salbutamol in patients with acute exacerbation of COPD. Respir Med 2002; 96(10):790–795. PubMed PMID: 12412978.
- Cazzola M, D'Amato M, Califano C, Di Perna F, Calderaro E, Matera MG, et al. Formoterol as dry powder oral inhalation compared with salbutamol metered-dose inhaler in acute exacerbations of chronic obstructive pulmonary disease. Clin Ther 2002; 24(4):595–604. PubMed PMID: 12017404.
- Cazzola M, Matera MG. Long-acting beta(2) agonists as potential option in the treatment of acute exacerbations of COPD. Pulm Pharmacol Ther 2003;16(4):197–201. PubMed PMID: 12850121.
- Leuppi JD, Schuetz P, Bingisser R, Bodmer M, Briel M, Drescher T, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309(21):2223–2231. PubMed PMID: 23695200.
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G, Mutlu LC, In E. The role of nebulised budesonide in the treatment of exacerbations of COPD. Eur Respir J 2007; 29(4):660–667. PubMed PMID: 17251232.
- Maltais F, Ostinelli J, Bourbeau J, Tonnel AB, Jacquemet N, Haddon J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002; 165(5):698–703. PubMed PMID: 11874817.
- Mirici A, Metal M, Akgun M. Comparison of the efficacy of nebulised budesonide with parenteral corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Clin Drug Investig 2003; 23(1):55–62. PubMed PMID: 23319094.