Abstract
The safety and tolerability of nebulized amoxicillin clavulanic acid were determined in patients with stable COPD and during severe exacerbations of COPD. Nine stable COPD patients received doses ranging from 50:10 mg up to 300:60 mg amoxicillin clavulanic acid and eight patients hospitalised for a COPD exacerbation received fixed doses 200/40 mg twice daily. Safety was evaluated by spirometry before and after inhalation. Tolerability was evaluated by questionnaire. Plasma and expectorated sputum samples were assayed for amoxicillin content.
Seventeen patients underwent in total 100 nebulizations with amoxicillin clavulanic acid. In this safety and tolerability study no clinically relevant deteriorations in FEV1 were observed. Nebulized amoxicillin clavulanic acid produces sputum concentrations well above the Minimal Inhibiting Concentration of 90% for potential pathogenic micro-organisms, with low concentrations in the central compartment (low systemic exposure).
Based on spirometry and reported side effects, inhalation of nebulized amoxicillin clavulanic acid seems to be safe and well tolerated, both in stable patients with COPD as in those experiencing a severe exacerbation. Levels of amoxicillin were adequate.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a common preventable and treatable disease. COPD is a leading cause of mortality and morbidity worldwide. Exacerbations in COPD and its co-morbidities contribute to the overall severity in individual patients. The pulmonary disease is characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases (Citation1). Furthermore COPD is characterized by periodic exacerbations that typically occur one to three times a year (Citation2).
Exacerbations are events with a heterogeneous presentation that are thought to be caused by complex interactions between the host, viruses, bacteria and environmental pollution, leading to an increase in the inflammatory burden (Citation3). In literature, it is suggested that 50–70% of exacerbations are due to respiratory infections (including bacteria, atypical organisms and respiratory viruses), 10% are due to environmental pollution (depending on season and geographical placement), and up to 30% are of unknown etiology (Citation3). Effectiveness of antibiotics in acute exacerbations of COPD (AECOPD) is still under discussion, and depends on the correct prescription of antibiotics and probably also on the concentration of antibiotics reached in the target tissue. Theoretically, to be effective, the antibiotic concentration in target tissues should reach the Minimal Inhibiting Concentration of 90% (MIC90) for potential pathogenic micro-organisms (PPM) in COPD such as Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis (Citation4,5).
Some clinical studies have suggested that with regard to clinical efficacy of amoxicillin, sputum concentration is a better predictor of efficacy than serum concentration (Citation6,7). Previous research has shown that an amoxicillin concentration in sputum higher than the Minimal Inhibiting Concentration of 90% (MIC90) reduced the mean length of hospitalization for a COPD exacerbation from 11 to 7 days (Citation8). However, only one-third of the COPD patients receiving treatment either orally or intravenously reaches an amoxicillin concentration in sputum higher than the MIC90 (Citation8,9). Inhalation of amoxicillin clavulanic acid may be a method of administration resulting in better pharmacological treatment in COPD exacerbations by achieving higher and more effective antibiotic levels locally.
Exploring the safety and effectiveness of local treatment with amoxicillin clavulanic acid in the airways by inhalation of nebulized amoxicillin clavulanic acid is of great interest. With regard to bronchiectasis nebulization of amoxicillin has been described in literature and was well tolerated (Citation10,11). Inhalation of amoxicillin clavulanic acid, however, has not been described before in literature. Therefore, the objective of this study was to explore the safety and tolerability of inhalation of nebulized amoxicillin clavulanic acid in patients with stable COPD first, and second in hospitalized patients suffering from an exacerbation of COPD.
Methods
Design
We performed two subsequent studies: STONAC 1 and STONAC 2. Both studies were phase 1 single-arm prospective intervention studies that assessed safety and tolerability of nebulization of amoxicillin clavulanic acid either in stable COPD (STONAC 1) or in hospitalized patients suffering from an exacerbation of COPD (STONAC 2). STONAC 1 and STONAC 2 each designed for eight subjects.
Study population
Both studies included subjects with a clinical diagnosis of COPD (as defined by GOLD criteria (Citation1), able to produce sputum, age 40 years or over, and current or former smoker. Exclusion criteria included allergy for penicillin, amoxicillin or clavulanic acid, current pneumonia, and FEV1 post bronchodilator < 1.2 l (STONAC 1 only). In the STONAC 2 systemic use of amoxicillin was an exclusion criterion as well. An exacerbation was defined as an event in the natural course of the disease characterized by a change in the patient's baseline dyspnoea, cough, and/or sputum that is beyond normal day-to-day variations, is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD (Citation1).
Both studies were performed according to the Helsinki declaration and were approved by the medical ethical review board of Twente and the hospital board. A data safety monitoring board (DSMB) was established. Study subjects were informed by the investigators and a written informed consent was obtained.
Study drug/dose
Prior to each nebulization with amoxicillin clavulanic acid, subjects used a bronchodilator (200 μg salbutamol). The amoxicillin clavulanic acid used for nebulization in this study was a commercially available product of the brand Sandoz: amoxicillin/clavulanic acid: 1000 mg/200 mg powder for solution for injection, registered for intravenous administration, Registry nr (RVG): 28025. After reconstitution conform Summary of Product Characteristics file (SMpC: registration file for marketing authorization), a solution with a concentration of 50:10 mg/ml amoxicillin/clavulanic acid was obtained. After reconstitution the desired dose was manually poured into the nebulizer. The particle size of the nebulized solution was determined by laser diffraction technique (HELOS particle size analysis). The particle size of both used nebulizers (Ventstream® and Sidestream plus®) complied to the desirable size range (1–5 um). The solution of amoxicillin clavulanic acid prepared according to SmPC is slightly hyper osmotic but complies well to the physical requirements for aerosolized delivery as does the pH of the solution (Citation12).
The optimal dose of amoxicillin clavulanic acid through inhalation is unknown. In former inhalation studies a dosage of 500 mg amoxicillin twice daily was used (Citation10,11). The nebulizers in those days had an efficiency of approximately 10% (Citation13). The nebulizers used in this study (Ventstream® and Sidestream plus®) have an efficiency up to approximately 30% (Citation14).
In STONAC 1 the starting dose of amoxicillin clavulanic acid was 50:10 mg ( = 1 ml) followed by ascending doses of 100:20 mg, 200:40 mg and 300:60 mg (at weekly intervals). The starting dosage was rather low and therefore on the safe side. In STONAC 2 study, a fixed dose of 200:40 mg was used, according to the results of STONAC 1.
Nebulizer
The drug was nebulized using a Portaneb® compressor and a Ventstream® (STONAC 1) or Sidestream plus® (STONAC 2) nebulizer, equipped with expiration filters. Those nebulizers are breath-enhanced jet nebulizers designed to improve drug delivery through the delivery of an aerosol with a high respirable fraction in a short nebulization time (Citation15). These nebulizers are both jet nebulizers in which denaturation of the drug is unlikely. Time of nebulization of a solution of amoxicillin clavulanic acid (50/10 mg/ml) was expected to be 2–3 min/ml (Citation14). Nebulization continued until the nebulizer started sputtering. To prevent nasal aerosols, a nose clamp as used in spirometry was used.
Pulmonary function tests
A pulmonary function test was performed directly before inhalation and 15 minutes after inhalation of amoxicillin clavulanic acid. Forced expiratory volume in 1 second (FEV1) was measured. A reduction in FEV1 of 20% or more was considered clinically relevant.
Questionnaire
After every nebulization the subject completed a questionnaire on adverse events (). The questionnaire is similar to a questionnaire used in a pilot study with colistin nebulizations (Citation16).
Table 1. A questionnaire was filled in by the subject after nebulization.
Sputum and blood sampling and analysis
Analysis of sputum and plasma samples was performed using a validated analytical method using HPLC-MS-MS. The MIC90 (2 mg/l) used in this study is derived from local susceptibility tests from the Regional Laboratory of Public Health and is comparable with data published by the European Committee On Susceptibility Testing (Eucast) (Citation17).
STONAC 1
During STONAC 1 eight outpatients with stable COPD were recruited between January 2012 and October 2012. Subjects received ascending doses of amoxicillin clavulanic acid by inhalation. Each patient received four doses with an interval of at least 7 days between each dose.
After each nebulization the data safety monitoring board (DSMB) received a report with results of the nebulization (FEV1 and questionnaire).The patient only received a higher dosage when the last dosage given was tolerated well and was approved by the DSMB.
After inhalation of amoxicillin clavulanic acid subjects were kept under observation for 3 hours. During the observation period the subjects completed the questionnaire, expectorated sputum was sampled, and at the end a venous blood sample was collected.
STONAC 2
During STONAC 2 eight patients, hospitalized for AECOPD were recruited between October 2013 and July 2014. Subjects received doses of amoxicillin clavulanic acid 200:40 mg by inhalation, twice daily during hospitalization (with a maximum of 7 days). The nebulizations were added to regular care, i.e., oral steroids. Patients were nebulized on each day of their hospitalization, with a maximum of 7 days according to standard care. In case patients were discharged from the hospital before day 7, the nebulizations were stopped immediately.
Before and after the first nebulization a pulmonary function test was performed. After every nebulization subjects completed the questionnaire. Volunteers were asked to expectorate sputum before the second nebulization on day 1, at three moments between the nebulizations on day 3 (top, mid and trough), and before the last nebulization during inclusion in the study. Sputum expectorated at other moments than described above was also collected and analyzed on concentration of amoxicillin. Three hours after the first nebulization at day three, a venous blood sample was collected.
Following hospital discharge, the data of the treated patient, including a report with results of the nebulizations (FEV1 and questionnaires) were sent for assessment to the DSMB.
Results
In STONAC 1 one subject discontinued participation for non-medical reasons. Therefore, a ninth subject was included conform study protocol. Patient characteristics of both patients included in the STONAC 1 and 2 study are listed in . In STONAC 1, 34 nebulizations were performed, in STONAC 2, 66 nebulizations were performed.
Table 2. Baseline characteristics of enrolled subjects.
Safety
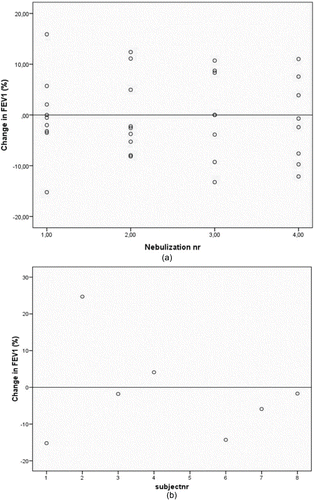
Neither in STONAC 1, nor in STONAC 2, any subject showed a clinically relevant deterioration of their FEV1 (>20%). None of the subjects experiencing a reduction in FEV1 sensed discomfort due to this reduction. The change in FEV1 is shown in .
Figure 1. (A) the change in FEV1 before-after nebulization in stable COPD patients. (STONAC 1). Change in FEV1 was measured at each nebulization. No clinical relevant reduction of FEV1 (> 20%) occurs after nebulizations of amoxicillin clavulanic acid. (B) the change in FEV1 before-after nebulization in COPD exacerbation patients. (STONAC 2). Change in FEV1 was measured once for each subject. No clinical relevant reduction of FEV1 (> 20%) occurs after nebulizations of amoxicillin clavulanic acid.
Tolerability
STONAC 1
Three adverse events (AE) were reported. There was one AE noted at a first inhalation and two AE's at second inhalations. All AE's were experienced as minor. The AE's consisted of cough (twice) and shortness of breath (once). Three subjects mentioned a slight bitter taste but did not classify this as an AE. No severe adverse events occurred during the study.
One subject experienced an exacerbation prior to the third nebulization. Three days after the second nebulization the patient suffered from coughing and shortness of breath. A consulted physician diagnosed an AECOPD and started treatment. Five weeks later the patient continued the study without further problems or adverse events.
STONAC 2
In 47% of the nebulizations no AE's were reported, and in 32% minor AE's were reported, while in 8% and 12% moderate and tolerable AE's were reported, respectively. The reported AE's were cough, shortness of breath, and an unpleasant bitter taste.
Following one nebulization (1.5%) the subject classified an AE as serious. The subject experienced cough and shortness of breath during the fourth nebulization and within 0-10 minutes post nebulization. During this hospitalization the subject had an increase in symptoms. This subject stopped participation because of the impaired clinical condition, which lasted up to at least 36 hours’ post nebulization. The DSMB assessed the causality and decided the worsening symptoms were not caused by the nebulizations but due to an exacerbation in COPD.
Several subjects could not continuously perform the nebulization, but needed short stops. Those subjects perceived nebulizations as tiring, partly because it lasted for about 10 minutes. In addition they experienced wearing a nose clip as unpleasant.
Sputum and blood sampling and analysis
STONAC 1
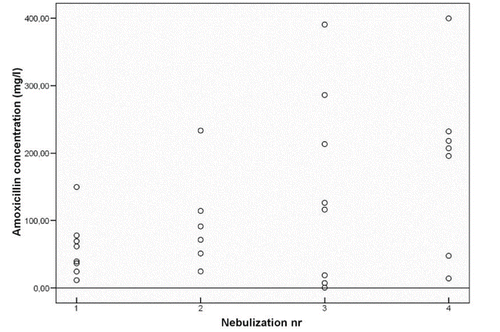
Out of 34 nebulizations, 30 sputum samples were obtained. One sample was too small to quantify amoxicillin levels. The remaining 29 sputum samples were analyzed for amoxicillin quantification. Only one sample had a concentration below MIC90. All other samples had a concentration > 6.9 mg/l (). In all collected plasma samples amoxicillin concentration was <1.0 mg/l.
STONAC 2
Of 66 nebulizations 16 sputum samples were obtained of which five samples were obtained before the second nebulization (trough samples) (see ). One sample had a concentration below MIC90. All obtained top (n = 4) and mid (n = 3) samples at day 3 and the samples collected at other moments (n = 4) showed an amoxicillin concentration >200 mg/l. (No trough samples at day 3 were collected.)
Table 3. STONAC 2: Amoxicillin concentration in sputum samples collected before the second nebulization (trough samples)
Seven plasma samples were collected. One patient was discharged from the hospital before day three, so no plasma sample was obtained from that patient. In all collected plasma samples amoxicillin concentration was < 1.0 mg/l.
Discussion
In STONAC 1 and STONAC 2 a total of 17 patients underwent in total 100 nebulizations with amoxicillin clavulanic acid. The objective of both studies was to evaluate the safety and tolerability of inhalation of nebulized amoxicillin clavulanic acid in patients with COPD. To our knowledge inhalation of amoxicillin clavulanic acid has not been studied before.
Safety
In this safety and tolerability study no clinically relevant deteriorations in FEV1 were observed. STONAC 1 showed that stable COPD patients receiving a second, third or fourth nebulization did not necessarily show larger reductions in FEV1, while some also showed increases in FEV1 compared to the first nebulization. The rise in FEV1 could be due to a late effect of the bronchodilator given before nebulization. Since extra lung function tests at a later moment post nebulization were not part of the study protocol, no data is available on time to return to baseline FEV1. In STONAC 2 FEV1 was measured only before and after the first nebulization because at the first nebulization the subject was expected to be in the worst clinical condition. The FEV1 measurements showed similar results as in STONAC 1.
Tolerability (questionnaires)
In STONAC 1 and 2 similar adverse events were reported by the subjects: cough (minor), shortness of breath (major), and a bitter taste (minor). Shortness of breath was assessed as major, because some of the subjects could not continuously maintain nebulization. In all cases with shortness of breath the nebulizations could be resumed after a few minutes of rest without any further problems. In STONAC 2 subjects explained they found it difficult to distinguish between adverse events and symptoms of their exacerbation of COPD. In general AE's like cough are common when inhaling antibiotics. To prevent or reduce AE's like cough and chest tightness or bronchospasm pretreatment with a bronchodilator is advised (Citation12).
Possibly, development of a dry powder inhalator can lead to a more patient friendly way of inhaling amoxicillin clavulanic acid. Dry powder inhalation has several advantages, such as shortened inhalation time, less contamination of the surrounding area, and no (electronic) nebulization equipment is needed.
Amoxicillin levels
In STONAC 1 and STONAC 2 all plasma levels showed amoxicillin concentrations <1.0 mg/L, indicating that systemic exposure is low. Systemic exposure can be explained in three ways: by swallowing amoxicillin deposited in the mouth and throat area; by swallowing expectorated sputum; or, by transport from the lung tissue to the blood. No information on the exposure route can be drawn from this study.
Nebulization of amoxicillin clavulanic acid results in sputum amoxicillin levels well above MIC90. In STONAC 1 even at the lowest doses given by inhalation the amoxicillin levels in sputum, obtained within 3 hours after nebulization, showed to be clearly above the MIC90. One sample taken after the third dose showed an unexpected low sputum amoxicillin concentration below MIC90. We have no explanation for this, especially in light of the fact that the sputum concentration at the lowest (first) dose was well above MIC90 in this subject.
In STONAC 2 in 15 out of 16 sputum samples an amoxicillin concentration above the MIC 90 was measured. Those sputum samples included 6 trough samples. This indicates that with dosing twice daily continuous concentrations above the MIC 90 can be reached. Not all patients were able to able to produce sputum at the moment of collection. In previous studies two thirds of the patients did not reach a concentration >MIC90 in sputum after systemic administration of amoxicillin clavulanic acid (Citation8,9).
STONAC 1 and STONAC 2 were performed in patients with stable COPD and during severe exacerbations of COPD, respectively, and can therefore not be extrapolated to other pulmonary diseases or pulmonary infections. Whether sputum is the target for treatment is unclear. Previous research showed effective amoxicillin clavulanic acid levels in bronchial mucosa following oral administration. Poor clinical response may be due to a reservoir of organisms in the bronchial secretions where effective antibiotic levels are not easily obtained (Citation18). This strengthens the idea of nebulizing amoxicillin clavulanic acid in order to reach effective levels in sputum.
Study limitations
Although 100 nebulizations have been performed, the total number of patients is still low. In STONAC-1 and STONAC-2 we showed it is possible to obtain effective amoxicillin levels in sputum by nebulizing amoxicillin clavulanic acid. Due to the low number of samples it is not possible to tell whether the levels will be effective (theoretically). An additional study is needed to show whether an effective sputum level is present over the day (for effectiveness of B-lactam antibiotics: concentration time > MIC). Further research is needed to show whether nebulizing amoxicillin clavulanic acid is also more effective than oral or parenteral amoxicillin clavulanic acid.
From the present studies no information on the effect on the microbial profile is available. Also, the effect of nebulization on the microbial profile has to be investigated in future studies.
Conclusion
These clinical, phase 1 studies on nebulized amoxicillin clavulanic acid have shown that nebulizing amoxicillin clavulanic acid is safe and well tolerated in single doses up to 300:60 mg of amoxicillin-clavulanic acid in patients having stable COPD and in doses of 200:40 mg twice a day in patients hospitalized for an exacerbation of COPD. The amoxicillin levels in sputum found in STONAC 1 and STONAC 2 are promising. The use of aerosolized antibiotics is particularly attractive, as this mode of administration has the potential to deliver high concentrations at the site of infection. Further research on clinical efficacy by a randomized trial is needed. If this antibiotic inhalation therapy is clinically effective, a large COPD population can be treated more effectively for their exacerbations.
Declaration of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
Authors Nijdam and Assink contributed equally to this work. The authors wish to thank Mr. P. Robberts (Mediq Romedic Netherlands) for providing the nebulizers and the particle size measurements of the nebulized amoxicillin clavulanic acid.
References
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176(6):532–555. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17507545.
- Sethi S, Evans N, Grant BJB, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 347(7):465–471. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12181400.
- Larsen M V, Janner JH, Nielsen SD, Friis-Møller A, Ringbaek T, Lange P. Bacteriology in acute exacerbation of chronic obstructive pulmonary disease in patients admitted to hospital. Scand J Infect Dis 2009; 41(1):26–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18855228.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106(2):196–204. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3492164.
- Van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C, Hendrix R. Clinical predictors of bacterial involvement in exacerbations of chronic obstructive pulmonary disease. Clin Infect Dis 2004; 39(7):980–986. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15472849.
- Braude AC, Cohen RD, Penner JL, Preston MA, Rebuck AS. Pulmonary disposition of moxalactam. Chest 1984; 86(6):881–883. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6499551.
- Maesen FP, Davies BI, Baur C. Amoxycillin/clavulanate in acute purulent exacerbations of chronic bronchitis. J Antimicrob Chemother 1987; 19(3):373–383. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3494724
- Brusse-Keizer M, ten Bokum L, Movig K, van der Valk P, Kerstjens H, van der Palen J, et al. Relation between amoxicillin concentration in sputum of COPD patients and length of hospitalization. COPD 2011; 8(2):66–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21495834.
- Brusse-Keizer M, VanderValk P, van der Zanden R, Nijdam L, van der Palen J, Hendrix R, et al. Amoxicillin concentrations in relation to beta-lactamase activity in sputum during exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015; 10:455–461.
- Stockley RA, Hill SL, Burnett D. Nebulized amoxicillin in chronic purulent bronchiectasis. Clin Ther 1985; 7(5):593–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4053147.
- Hill SL, Morrison HM, Burnett D, Stockley RA. Short term response of patients with bronchiectasis to treatment with amoxycillin given in standard or high doses orally or by inhalation. Thorax 1986; 41(7):559–65. Available from: http://www.pubmedcentral.nih.govarticle render.fcgi?artid = 460390&tool = pmcentrez&rendertype = abstract.
- Le J, Ashley ED, Neuhauser MM, Brown J, Gentry C, Klepser ME, et al. Consensus summary of aerosolized antimicrobial agents: application of guideline criteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2010; 30(6):562–584. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20500046.
- J. Denyer et al. Does inhalation duty cycle in vitro predict inhaled dose during domiciliary nebulizer use? The aerosol society, Drug delivery to the lungs VIII. 1998; doi:10.1089/jam.1998.11.177.
- Ho SL, Kwong WT, O’Drowsky L, Coates AL. Evaluation of four breath-enhanced nebulizers for home use. J Aerosol Med 2001; 14(4):467–475. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11791687.
- Le Brun PP, de Boer AH, Gjaltema D, Hagedoorn P, Heijerman HG, Frijlink HW. Inhalation of tobramycin in cystic fibrosis. Part 1: the choice of a nebulizer. Int J Pharm 1999; 189(2):205–214. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10536249.
- Westerman EM, Le Brun PPH, Touw DJ, Frijlink HW, Heijerman HGM. Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: A pilot study. J Cyst Fibros 2004; 3(1):23–28.
- European Committee on Microbial Susceptibility Testing (Eucast). Available from: http://www.eucast.org/clinical_breakpoints/ (accessed 12 November 2013).
- Gould IM, Harvey G, Golder D, Reid TM, Watt SJ, Friend JA, et al. Penetration of amoxycillin/clavulanic acid into bronchial mucosa with different dosing regimens. Thorax 1994; 49(10):999–1001. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7974318.