Abstract
Despite the fact that metanalyses and clinical guidelines do not recommend the routine use of domiciliary non-invasive ventilation (NIV) for patients diagnosed with severe stable Chronic Obstructive Pulmonary Disease (COPD) and with chronic respiratory failure, it is common practice in some countries.
We conducted an international web-survey of physicians involved in provision of long-term NIV to examine patterns of domiciliary NIV use in patients diagnosed with COPD.
The response rate was 41.6%. A reduction of hospital admissions, improvements in quality of life and dyspnea relief were considered as the main expected benefits for patients. Nocturnal oxygen saturation assessment was the principal procedure performed before NIV prescription. Recurrent exacerbations (>3) requiring NIV and failed weaning from in hospital NIV were the most important reasons for starting domiciliary NIV. Pressure support ventilation (PSV) was the most common mode, with “low” intensity settings (PSV-low) the most popular (44.4 ± 30.1%) compared with “high” intensity (PSV-high) strategies (26.9 ± 25.9%), with different geographical preferences.
COPD is confirmed to be a common indication for domiciliary NIV. Recurrent exacerbations and failed weaning from in-hospital NIV were the main reasons for its prescription.
Introduction
Acute non-invasive ventilation (NIV) has been shown to be effective in patients with acute exacerbations of Chronic Obstructive Pulmonary Disease (COPD) with mild-to-moderate respiratory acidosis, reducing mortality, need for endotracheal intubation and length of hospital stay (Citation1, 2). Some studies have also reported positive effects with use of NIV in patients diagnosed with severe stable hypercapnic COPD (Citation3), such as: mild reduction in hypercapnia (Citation4), improvements in quality of life (QoL) (Citation5–8), reduced hospital admissions and costs (Citation9), and improvement in survival in two studies (Citation8, Citation10), only one published at the time of the survey (Citation10), while others did not (Citation11). Therefore, to date meta-analysis (Citation12) and clinical guidelines (Citation13), do not recommend the use of chronic NIV, except in very selected patients (Citation14). Despite this, COPD is a common indication for domiciliary NIV, especially in certain geographical areas (Citation15). To better understand this apparent discrepancy, we conducted a web-survey to assess pattern of domiciliary NIV use for treatment of chronic respiratory failure in stable COPD among physicians, and to determine practices for its prescription.
Methods
We conducted a web-survey between May and August 2013. Purposive sampling was used to interview physicians who were most likely to be involved in the implementation and prescription of domiciliary NIV for patients diagnosed with COPD and chronic respiratory failure. We identified 15 physicians of different European Countries, pioneer researchers in the field of NIV and invited them to participate. Each of them was asked to provide contact details of national colleagues known to be experts in the field; we combined them with contacts of members belonging to the NIV group of European Respiratory Society and built an address book.
The survey instrument (Citation16) was developed by the Steering Committee and validated by a panel of 4 inter-professional pulmonary experts who assessed it for clinical sensibility. Pre-testing and pilot testing were performed to test questionnaire's content validity, reliability, relevance, ability to discriminate among respondents and to improve wording style; the instrument was then condensed into 20 questions with multiple-choice responses, ranking and Likert scales as appropriate and formatted for the web (Citation17).
Physicians were provided with a unique username and password to access a secure internet-based questionnaire at www.nivcopd.eu. The final survey was e-mailed to 645 physicians; reminders were sent after 4 and 8 weeks.
Statistical analysis
Descriptive statistics (means, medians and proportions) was used when appropriate. We grouped respondents based on: 1) unit type (pulmonary/others); 2) number of NIV prescriptions/year (“high prescribers”: >Citation15 NIV prescriptions/year; “low prescribers”: <Citation15 NIV prescriptions/year); and 3) expertise with a specific Pressure Support Ventilation (PSV) setting (“high intensity” using PS>Citation20 cmH20 or “low intensity” using PS<20 cmH20); moreover, we defined as “experts”, physicians using one of the two PSV protocols in ≥50% of their patients.
Agreement among physicians in ranking the importance of expected benefits of domiciliary NIV, investigations performed before NIV prescription and factors indicating the need for long-term NIV, was assessed through the Kendall coefficient of concordance (Kendall's W) (Citation19, 20). Principal Component Analysis (PCA) was used to assess the ranking order expressed by physicians. The number of components extracted was based on visual inspection of the scree plot. The data set was transposed in order to have physicians’ rank vote on columns and items on rows; no centering and scaling were employed. Wilcoxon-Mann–Whitney and Kruskal–Wallis tests were used to compare continuous and categorical values, respectively. Statistical analysis was performed using SPSS version 15 (SPSS Inc. Chicago, IL, USA) and R(Citation21) version 3.2.1. A probability value of p < 0.05 was considered statistically significant.
Results
Survey's questionnaire showed overall a good internal consistency and adequate content validity (Cronbach's α ≥ 0.75, Content Validity Index ≥ 0.78) (Citation18).
Response rate and respondents’ characteristics
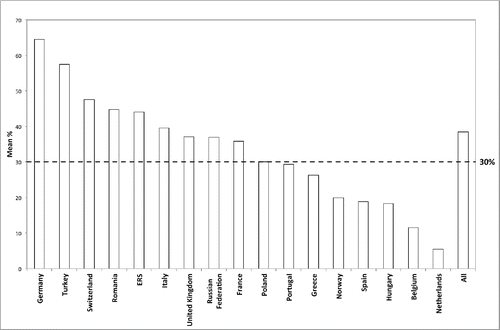
Respondents’ characteristics are shown in . Among different pathologies, the prescription rate for chronic NIV in patients diagnosed with COPD was 38.5% of the total prescriptions; domiciliary NIV prescriptions for COPD varies among countries (), with a prescription rate above 30% in 8 of the countries and in the European Respiratory Society group of respondents.
Table 1. Response rate and respondents characteristics.
Attitude towards NIV initiation
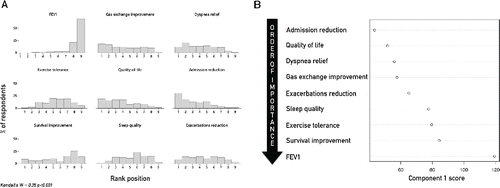
shows respondents’ ranking of main benefits expected from NIV that prompt its domiciliary prescription, with a quite low level of agreement among physicians, (Kendall's W = 0.35, p < 0.001). Importance of expected benefits from domiciliary NIV prescription was explored with PCA; the first two components extracted explained 86% and 3.4% of total variance, respectively. The analysis of component 1 score revealed the following expected advantages from long-term NIV initiation, in decreasing order of importance: reduction in hospital admission, improvement of QoL, dyspnea and blood gases, reduction of COPD exacerbation, amelioration of sleep quality and exercise tolerance and finally, improvement of survival and FEV1 ().
Figure 2. (A) Frequency distribution graph of rank values rated by physicians for each expected benefit from domiciliary NIV. Rank value range is from 1 (Important) to 9 (Irrelevant). Mean rank value for each variable is expressed at the bottom, in each panel. (B) Ranking order of expected benefits as explored by multivariate analysis. Only the first component of principal component analysis was plotted. Abbreviations: FEV1 = FEV1 improvement; Exercise tolerance = Exercise tolerance improvement; Quality of life = Quality of life improvement; Admission reduction = Reduction of hospital admission; Sleep quality = Sleep efficacy amelioration; Exacerbations Reduction = Reduction of exacerbations frequency; Kendall's W = Kendall's coefficient of Concordance.
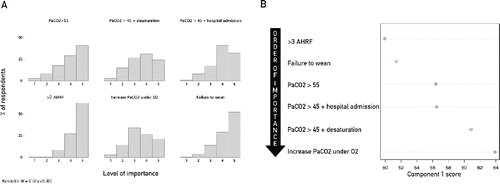
Parameters driving patients’ selection for chronic NIV varied greatly among physicians (), with a significantly low rate of concordance (Kendall's W = 0.16, p < 0.001). PCA explored factors considered important by respondents in their decision to start domiciliary NIV; the first two components extracted explained 96% and 1.2% of total variance, respectively. Several recurrent episodes of acute-on-chronic hypercapnic respiratory failure requiring NIV and inability to be weaned from NIV subsequent to an episode of acute hypercapnic respiratory failure, were recognized as the most relevant ().
Figure 3. (A) Frequency distribution graph of rank values rated by physicians (physicians’ rating) for each decision to start NIV. Rank value range is from 1 (Irrelevant) to 5 (Very Important). Mean value for each variable is expressed at the bottom, in each panel. (B) Importance rating of decisions to start NIV as explored by multivariate analysis. Only the first component of principal component analysis was plotted. Abbreviations: PaCO2 > 55 = PaCO2 > 55 mmHg; PaCO2 > 45 + Desaturation = PaCO2 > 45 mmHg + nocturnal desaturation; PaCO2 > 45 + hospital admission = PaCO2 > 45 mmHg + frequent hospitalization; >3 AHRF = Recurrent (>3) Acute Hypercapnic Respiratory Failure (AHRF) episodes requiring NIV; Increase PaCO2 under O2 = Increase in PaCO2 under oxygen; Failure to wean = Failure to wean from NIV: Kendall's W = Kendall's Coefficient of Concordance.
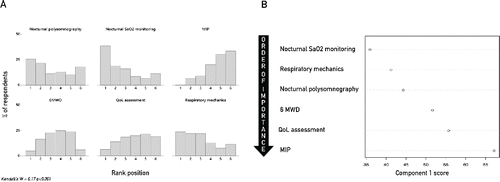
Finally, physicians expressed a low agreement about procedures need to be performed before domiciliary NIV prescription, (Kendall's W = 0.13, p < 0.001), (). PCA (with the first two components extracted explaining 84% and 4.7% of total variance, respectively) pointed out that nocturnal oxygen saturation monitoring was the main procedure performed before starting NIV, followed by measurements of respiratory mechanics and polysomnography ().
Figure 4. (A) Frequency distribution graph of rank values rated by physicians for each procedure performed before starting NIV. Rank value range is from 1 (Very important) to 6 (Irrelevant). Mean rank value for each variable is expressed at the bottom, in each panel. (B) Ranking order of procedure performed before starting NIV as explored by multivariate analysis. Only the first component of principal component analysis was plotted. Abbreviations: MIP = Maximum inspiratory pressure; 6MWD = 6 minutes’ walking distance; QoL assessment = Quality of life assessment; Kendall's W = Kendall's Coefficient of Concordance.
NIV application: Modes, settings, interfaces and humidification
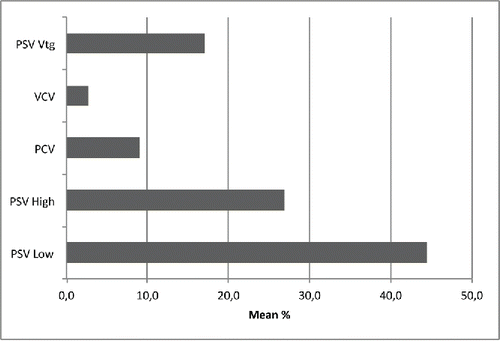
PSV mode was by far the most commonly used method of ventilation () and although “low intensity” settings (PSV-low) were the most popular (44.4%), the “high intensity” (PSV-high) ones were also prescribed frequently (26.9%). This choice was statistically influenced by “volume” of prescriptions/year, with “high prescribers” applying preferably PSV-high compare to “low prescribers” (29.2 ± 26.9% vs. 21.4 ± 22.7%, p = 0.04), but not affected by physician's specialty (30.2 ± 23.9% vs. 25.6 ± 26.6%, p = n.s.).
Figure 5. Ventilation mode utilization for domiciliary NIV in severe stable COPD patients. Abbreviations: PSV Low = PSV with low inspiratory support (Pressure support < 20 cm H2O); PSV High = PSV with high inspiratory support (Pressure support > 20 cm H2O); PCV = Pressure control ventilation; VCV = Volume control ventilation; PSV-Vtg = Pressure support with volume guarantee.
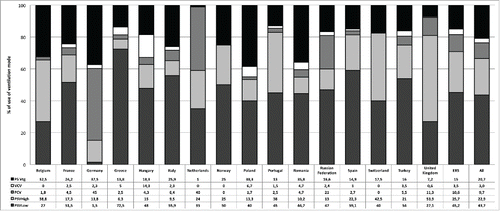
We found a statistically significant heterogeneity among countries () for the use of PSV-high (p < 0.01), PSV-low (p = 0.01) and “volume target” PSV (PSV-Vtg), (p < 0.01), respectively. Conversely, a more homogeneous utilization of Volume Control Ventilation (VCV) and PCV was observed (n.s.), with relatively lower rates, except for Germany and Netherlands where PCV was used by >40% of respondents.
Figure 6. Ventilation mode distribution by country. Abbreviations: PSV Low = PSV with low inspiratory support (Pressure Support < 20 cm H2O); PSV High = PSV with high inspiratory support (Pressure Support > 20 cm H2O); PCV = Pressure control ventilation; VCV = Volume control ventilation; PSV-Vtg = Pressure support with volume guarantee; ERS = members of European Respiratory Society (ERS) assembly on NIV; ALL = all respondents.
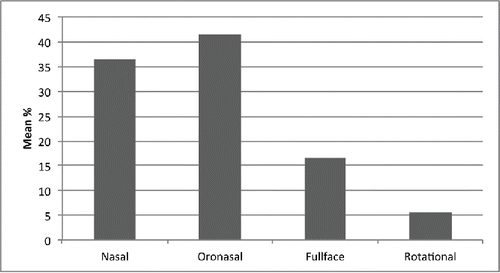
Oro-nasal and nasal interfaces were almost equally distributed and total face masks constituted almost 20% of prescriptions (). Mask choice was mainly determined by doctors’ expertise with a specific PSV protocol: although “high intensity” NIV experts use all three interfaces, they employ the total face mask much more compared to “low intensity” experts and other users (33.1 ± 35.3% vs. 11 ± 22.7% vs. 14.3 ± 20.6%, p < 0.01). In contrast, “low intensity” experts prefer the oro-nasal interface (32.9 ± 32% vs. 48.3 ± 31% vs. 36.8 ± 25.1%, p < 0.01). “High prescribers” prefer the rotational strategy almost twice as many times compared to “low prescribers” (6.4 ± 9.7% vs. 3.3 ± 6.5%, p < 0.01).
Figure 7. Interface utilization for domiciliary NIV in COPD patients. Nasal = nasal mask; Oronasal = oronasal mask; Fullface = fullface mask; Rotational = rotational strategy.
Almost 40% of respondents reported not prescribing any kind of humidification system; when humidification was recommended, active humidification, was used more frequently (39%) than heat and moisture exchangers, (22.4%); this choice was predominantly influenced by the type of ward from which patients were discharged (respiratory ward 29.6 ± 30% vs. others 42.1 ± 34.4% p = 0.02) and not by physician's expertise or number of prescriptions/year (n.s.). In-hospital acclimatization period to NIV did not vary significantly, depending on ventilation modes; generally, a time frame between 1 and 5 days was necessary. Physicians advise NIV preferably for the whole night, irrespective of ventilation mode (PSV-low 43.2%, PSV-high 35.9%, PSV-Vtg 27.7%).
Patients’ discharge and follow-up
Estimated compliance with home NIV, was considered good by more than 30% of physicians when using PSV-high (37.9%) and PSV-low (36.9%) as well as PSV-Vtg (35%), but very few respondents reported it as excellent; moreover, compliance with home NIV was considered very good only by 18.9% physicians when PSV-high was applied compared to PSV-low (30.6%) and PSV-Vtg (20.4%).
shows some other items related to home care program. The education program involved both patient and caregivers in most of the cases (76%), but formal home care programs were not available in almost 45% of the cases; telemedicine was an option for only 5% of respondents; a follow-up visit was scheduled every three months (46%).
Figure 8. Logistic management of COPD patients on domiciliary NIV. Each panel represents the distribution (percentages) of physicians’ responses for: a) Back-up ventilator prescription; b) Educational program = Type of educational program before NIV prescription; c) Visit schedule = Frequency of scheduled visits; d) Home care program = Type of home care program; e) Ventilator maintenance = Responsibility of ventilator management and technical assistance; f) Type of reimbursement. Abbreviations: Pt = patient, M.D. = physician; RT = respiratory therapist; Hosp. = hospital.
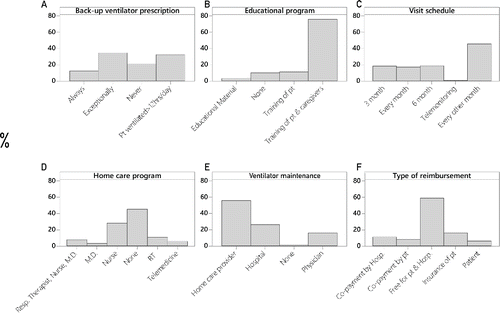
Ventilator maintenance was responsibility of home care providers in half of the cases (56%), but in a relatively high proportion also of prescribing hospitals (26%) or prescribing physicians (16%). Based on countries’ health care policy, there was no direct reimbursement for the majority of patients (59%), while co-payment (8%) or private insurance (16%) were sometimes required.
Discussion
To our knowledge, this is the first survey to assess practices for initiation and prescription of domiciliary NIV among physicians for treatment of chronic respiratory failure in stable COPD.
Although the response rate of our web-survey (42%) is not particularly high, it is perfectly in line with the available average response rate described in the literature for physicians’ web questionnaires (Citation22–24).
Despite a paucity of scientific evidence on the use of domiciliary NIV for severe stable COPD (12), NIV prescription in these patients is quite high, reaching an overall average of 38.5% with a prescription rate above 30% for 8 of the countries and European Respiratory Society group of respondents. Reduction of hospital admissions and improved QoL are perceived as the most important expected benefits. Settings and modes of ventilation varied mainly depending on geographical location and physician's expertise.
The Eurovent survey (15) published 10 years ago, demonstrated an almost identical percentage of prescriptions for patients diagnosed with COPD, and similar heterogeneity of geographical distribution, compare to the present study. There are clearly many factors contributing to this country variation. First, the estimated prevalence of COPD differs among countries; second, it is likely that different national attitudes to the potential value of long-term ventilation in COPD exist; third, the reimbursement system is more strict in certain areas (i.e., Belgium) and more liberal in others (i.e., Italy and Germany); fourth, it might be related to historical reasons (15).
Considering the lack of clear scientific evidence supporting the use of home NIV for patients diagnosed with COPD, expected benefits from NIV were mainly related to the clinical benefits demonstrated in non-RCTs, such as reduction of hospital admission and improved QoL, rather than the expectancy of an improved survival. Interestingly, even though QoL is perceived as a major benefit, QoL questionnaires are rarely performed before NIV initiation (). Indeed, “solely” improving arterial blood gases was not considered the main reason for starting NIV; this is mainly because hypercapnia has never been clearly shown to be a determinant of mortality (Citation25), though a very recent Swedish study showed a possible prognostic role for PaCO2 in patients diagnosed with COPD on long-term oxygen treatment (Citation26).
Patients diagnosed with COPD are likely to develop nocturnal hypoventilation, especially during REM sleep when upper airway tone and accessory muscle activity is impaired. Nearly half (42%) of patients diagnosed with COPD in one study had a >10 mmHg increase in PaCO2 at night, resulting in progressive resetting of respiratory control to higher PaCO2 values (Citation27, 28). Therefore, it is not surprising that most clinicians ranked respiration assessment during sleep as the most important clinical evaluation to perform before prescribing domiciliary NIV; interestingly, in contrast to the Eurovent study (Citation15), VCV has almost completely disappeared; this has happened despite no study having shown a clear advantage in terms of gas exchange and sleep quality of one mode over the other.
The so-called “low intensity” approach was the preferred setting in almost half of the prescribers, but nearly half of the respondents used techniques aimed at maximally reducing PaCO2 (PCV and PSV-high). We cannot exclude, however, that some of respondents may have confused PCV with “high intensity” ventilation, since the latter is sometimes incorrectly called “pressure controlled” mode. If this was the case, “low” and “high” approaches were equally distributed, but very differently according to the geographical location. In this regard, the original description of “high intensity” NIV refers to assist/control NIV using high back-up rates (Citation29–32).
For example, in Germany and some Northern nations, high level of inspiratory support (i.e., > Citation25/Citation30 cmH2O) are recommended by national Guidelines (Citation33). Indeed, data from a recent multicenter trial (8, 34) showed that this strategy, which is targeted to greatly reduce hypercapnia, is associated with a significantly better long-term survival and QoL compared with long-term oxygen treatment, but no comparisons with lower pressure settings are available. A dual-controlled mode like “volume target” PSV was also applied by a small percentage of respondents, and this sound reasonable since data on clinical effectiveness of this hybrid mode are still controversial (Citation35).
We observed a heterogeneous distribution of interface use; in particular, the oro-nasal and even the total face mask were commonly prescribed. In both acute and chronic settings all these interfaces have been shown to improve similarly blood gases vs. spontaneous breathing (Citation36), but it is usually thought that nasal mask is more largely used because of better tolerance. New developments in mask design and materials, however, have probably minimized the difference in discomfort. There is no clear evidence of what is the best humidification system for long-term NIV; (Citation37) therefore, it is not surprising that active humidification and heat and moisture exchangers were equally distributed. However, it is noteworthy that about half of respondents reported not using any type of humidification.
This may be due to the absence of clear recommendations and/or to the system of reimbursement. Without humidification, nasal symptoms and resistances may increase, especially in presence of leaks therefore, contributing to reduce compliance with continuous positive airway pressure (Citation38–40).
Concerning attitudes towards patients’ discharge, respondents indicated that compliance with NIV was not optimal at hospital discharge, despite whether patients may have spent a considerable time to get acclimatized to NIV, probably because physicians think that tolerance will improve over a longer period of time. It was very reassuring that almost all respondents had an in-hospital program dedicated to patients and caregivers’ education, as this has been suggested to be probably the best tool to improve compliance with NIV.
The minority of respondents reported to use a formal home care program, and this is once more dependent on the reimbursement system and it may also be related to legal issues, since very rarely institutional insurance covers clinicians’ actions outside the hospital “walls.” Individuals or institutions taking technical responsibility for the ventilator varied largely, primarily related to geographical location and system of payment.
Our study has some limitations:
As in every survey, we dealt with physicians’ perceptions rather than data collection on real patients. Therefore, our results may underestimate actual practice variation.
We chose a non-probability sampling design, surveying physicians with specific skills on domiciliary NIV, because we wanted to explore practices of “domiciliary NIV users/prescribers” in patients diagnosed with COPD. This method has an inherent intentional bias that represents at the same time its strength, allowing us to assess competent informant. Therefore, our findings may not be totally generalized.
Conclusions
COPD is confirmed to be a common indication for domiciliary NIV. This is the first survey about NIV practice in stable COPD patients; we have demonstrated that there is a quite large variability in percentage of prescription rate and ventilatory settings, among different Countries. Recurrent exacerbations and failed weaning from in-hospital NIV were the main reasons for prescription. Lacking clear data from RCTs, the practice of “chronic NIV” still relies on empirical and subjective decisions, and this may eventually lead to under- or over-prescription of chronic ventilator support.
Declaration of interest statement
No conflicts exist for any of the following authors: Crimi, Noto, Princi, Masa, Simonds, Nava.. Cuvelier, Elliott, Wijkstra, and Windisch report personal fees from companies dealing with mechanical ventilation, outside the submitted work. Wijkstra and Windisch also report a research grant from companies dealing with mechanical ventilation. No grant support was used for this research. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors would like to thank the 15 national delegates, representative for each country: Dr. João Valença (Centro Hospitalar Lisboa Norte, Hospital de Santa Maria, Lisboa, Portugal), Wilfried de Backer (Department of Pulmonary Medicine, Antwerp University Hospital, Antwerp, Belgium), Sergey Avdeev (Pulmonology Research Institute, Moscow, Russia), Jean-Paul Janssens (Department of Pulmonary Diseases, Hôpitaux Universitaires de Genève, Switzerland), Florin Mihaltan (Institute of Pneumologie “Marius Nasta,” Bucharest, Romania), Jacek Nasilowski (Department of Internal Medicine, Pneumology and Allergology, Medical University of Warsaw, Poland), Giorgia Pitsiou (Respiratory Unit, G.H. Papanikolaou, Thessaloniki, Greece), Zuhal Karakurt (Pulmonology Department-Medical Intensive Care Unit, Marmara Medical Faculty, Istanbul, Turkey), Marian Johnson (Physiotherapy Department, A.M.N.C.H, Tallaght, Dublin, Ireland), József Lukácsovits (Dept of Pulmonology, Semmelweis University, Budapest, Hungary), Kasper Ankjærgaard (University Hospital, Gentofte, Copenhagen, Denmark), Heidi Øksnes Markussen (Department of Thoracic Medicine, Haukeland University Hospital, Bergen, Norway; Norwegian National Centre of Excellence in Home Mechanical Ventilation, Haukeland University Hospital, Bergen, Norway), Bengt Midgren (Avd för lungmedicin Department of Lung Medicine, University Hospital Lund, Sweden), Miodrag Vukcevic (School of Medicine, University of Belgrade, Belgrade, Serbia), Georg-Christian Funk (Department of Respiratory and Critical Care Medicine, Otto Wagner Hospital, Vienna, Austria). In addition, the authors would like to acknowledge the support provided by Dr. Raffaele Scala (U.O. Pneumologia, Ospedale S. Donato, ASL 8, Arezzo, Italy) in providing the ERS address and to thank all the physicians who took part in the survey.
References
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326(7382):185.
- Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 2000; 355(9219):1931–1935.
- Budweiser S, Hitzl AP, Jorres RA, Heinemann F, Arzt M, Schroll S, et al. Impact of noninvasive home ventilation on long-term survival in chronic hypercapnic COPD: a prospective observational study. Int J Clin Pract 2007; 61(9):1516–1522.
- Casanova C, Celli BR, Tost L, Soriano E, Abreu J, Velasco V, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118(6):1582–1590.
- Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20(3):529–538.
- Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152(2):538–544.
- Windisch W. Impact of home mechanical ventilation on health-related quality of life. Eur Respir J 2008;32(5):1328–1336.
- Kohnlein T, Windisch W, Kohler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014; 2(9):698–705.
- Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003; 58(10):867–871.
- McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64(7):561–566.
- Struik FM, Sprooten RT, Kerstjens HA, Bladder G, Zijnen M, Asin J, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax 2014; 69(9):826–834.
- Struik FM, Lacasse Y, Goldstein R, Kerstjens HM, Wijkstra PJ. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 6:CD002878.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- National Institute for Health and Care Excellence (NICE). Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care [Internet]. 2010. Available from: http://www.nice.org.uk/guidance/cg101
- Lloyd-Owen SJ, Donaldson GC, Ambrosino N, Escarabill J, Farre R, Fauroux B, et al. Patterns of home mechanical ventilation use in Europe: results from the Eurovent survey. Eur Respir J 2005; 25(6):1025–1031.
- Burns KE, Duffett M, Kho ME, Meade MO, Adhikari NK, Sinuff T, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 2008; 179(3):245–252.
- Crimi C, Noto A, Princi P, Esquinas A, Nava S. A European survey of noninvasive ventilation practices. Eur Respir J 2010; 36(2):362–369.
- Cronbach L. Coefficient alpha and the internal structure of tests. Psychometrika 1951; 6:297–334.
- Kendall MG, Babington Smith B. The problem of m rankings. Ann Math Stat 1939; 10(3):275–287.
- Siegel S, Castellan NJJ. Nonparametric Statistics for the Behavioral Sciences. 2nd ed. New York, NY: McGraw-Hill, 1998.
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2008 Available from: http://www.R-project.org (accessed 9 January 2015).
- Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol 1997; 50(10):1129–1136.
- Tse-Hua Shi XF. Comparing response rates in e-mail and paper surveys: A meta-analysis. Educ Res Rev 2009; 1(4):26–40.
- Cook C, Heath F, Thompson R. A meta-analysis of response rates in web- or internet-based survey. Educ Psychol Measure 2000; 60(6):821–836.
- Aida A, Miyamoto K, Nishimura M, Aiba M, Kira S, Kawakami Y. Prognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapy. Am J Respir Crit Care Med 1998; 158(1):188–193.
- Ahmadi Z, Bornefalk-Hermansson A, Franklin KA, Midgren B, Ekstrom MP. Hypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective study. Respir Res 2014; 15(1):30.
- Becker HF, Piper AJ, Flynn WE, McNamara SG, Grunstein RR, Peter JH, et al. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med 1999; 159(1):112–118.
- O’Donoghue FJ, Catcheside PG, Ellis EE, Grunstein RR, Pierce RJ, Rowland LS, et al. Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J 2003; 21(6):977–984.
- Windisch W, Haenel M, Storre JH, Dreher M. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int J Med Sci 2009; 6(2):72–76.
- Dreher M, Ekkernkamp E, Walterspacher S, Walker D, Schmoor C, Storre JH, et al. Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest 2011; 140(4):939–945.
- Windisch W. Noninvasive positive pressure ventilation in COPD. Breathe 2011; 8(2):114--123. doi:10.1183/20734735.011511doi:
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65(4):303–308.
- Windisch W, Walterspacher S, Siemon K, Geiseler J, Sitter H. Guidelines for non-invasive and invasive mechanical ventilation for treatment of chronic respiratory failure. Published by the German Society for Pneumology (DGP). Pneumologie 2010; 64(10):640–652.
- Elliott M. Domiciliary NIV for COPD: where are we now? Lancet Respir Med 2014; 2(9):672–673.
- Carlucci A, Schreiber A, Mattei A, Malovini A, Bellinati J, Ceriana P, et al. The configuration of bi-level ventilator circuits may affect compensation for non-intentional leaks during volume-targeted ventilation. Intensive Care Med 2013; 39(1):59–65.
- Pisani L, Carlucci A, Nava S. Interfaces for noninvasive mechanical ventilation: technical aspects and efficiency. Minerva Anestesiol 2012; 78(10):1154–1161.
- Nava S, Cirio S, Fanfulla F, Carlucci A, Navarra A, Negri A, et al. Comparison of two humidification systems for long-term noninvasive mechanical ventilation. Eur Respir J 2008; 32(2):460–464.
- Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest 1999; 116(2):403–408.
- Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care 2009; 54(1):71–84.
- Tuggey JM, Delmastro M, Elliott MW. The effect of mouth leak and humidification during nasal non-invasive ventilation. Respir Med 2007; 101(9):1874–1879.