abstract
Incidence, predictors and effect of discontinuation of long-acting bronchodilators on the risk of death or hospital admission among adults with Chronic Obstructive Pulmonary Disease (COPD) were assessed in a large population-based prospective study carried out by linking Italian healthcare utilization databases. Specifically, the cohort of 17,490 beneficiaries of the National Health Service in the Italian Region of Lombardy, aged 40 years or older, who started long-acting bronchodilators therapy during 2005–2008 was followed from first dispensation until 2012. During this period, patients who experienced discontinuation of long-acting bronchodilators were identified. Hospitalizations for COPD and deaths for any cause (composite clinical outcome) were also identified during follow-up. A Cox proportional hazards model was fitted to identify predictors of discontinuation. The case-crossover design was used to assess the implications of treatment discontinuation on the clinical outcome risk. Cumulative incidences of discontinuation were, respectively, 67%, 80%, and 92% at 6 months, 1 year, and 5 years since initial treatment. Significant predictors of discontinuation were female gender, younger age, starting treatment with fixed-dose combination of inhaled bronchodilators and corticosteroids, using antibiotics, inhaled long-acting bronchodilators and corticosteroids and not using short-acting bronchodilators, other respiratory drugs and systemic corticosteroids during follow-up. Odds ratios (95% confidence intervals) for the clinical outcome associated with not discontinuing long-acting bronchodilators was 0.64 (0.50 to 0.82). In conclusion, in the real-life setting, discontinuation of inhaled long-acting bronchodilators in adults with COPD is high even after just 6 months, even though persistence to these drugs reduces the risk of severe outcomes.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a major public health issue worldwide due to its high associated morbidity, mortality, and economic burden (Citation1–4). COPD is characterized by an airflow limitation that is not fully reversible. This limitation is usually progressive and associated with an abnormal pulmonary inflammatory response to noxious particles or gases Citation(5).
Although currently available pharmacotherapies have not been shown to modify the long-term decline of lung function in COPD patients, several effective medications are available to prevent and control symptoms, improve health status, and avoid COPD exacerbations Citation(4). As established by current guidelines, a central part of COPD management is use of inhaled bronchodilators with protracted activity (about 12 or more hours). These include long-acting beta-agonists (LABAs), alone or in combination with inhaled corticosteroids (ICS), and long-acting muscarinic-antagonists (LAMAs) Citation(5).
Guidelines for COPD management are based on clinical trials Citation(5). These, however, recruit restricted groups of patients (e.g., according to acute bronchodilator reversibility and/or prior history of exacerbations) so limiting their generalizability. Furthermore, while adherence levels in clinical trials may be around 80% or over (Citation6–8), far lower adherence levels are typically observed in the real-life clinical practice (e.g., 10–40%) (Citation9–11). This is worrying, as research suggests that poor compliance to medications is a major contributor for COPD treatment failure, resulting in an increased risk of exacerbations, hospitalizations and mortality (Citation12–14).
We carried out a large population-based study, based on an unselected cohort of inhaled long-acting bronchodilators new-users, to investigate incidence, predictors, and clinical implications of COPD drug therapy discontinuation.
Methods
Setting
The data used for this study were retrieved from the healthcare utilization databases of Lombardy, a region of Italy accounting for about 16% (almost 10 million) of the national population. In Italy, the whole population is covered by the National Health Service, which in Lombardy has been associated since 1997 with an automated system of databases to collect a variety of information. Details on the healthcare utilization databases of the Lombardy Region are available elsewhere Citation(15).
Cohort selection, follow-up, and drug exposure assessment
The target population included all Lombardy residents, aged 40 years or older, who were beneficiaries of the Regional Health Service. Of these, those who received at least one a prescription of LAMAs or LABAs, either as monotherapy or fixed combination with inhaled corticosteroids (LABA/ICS), between January 1, 2005 until June 30, 2008 were considered eligible for cohort entry. The date of the first dispensation was defined as the index date.
Patients who became beneficiaries of the Regional Health Service less than 5 years prior to the index date, were excluded from the cohort.
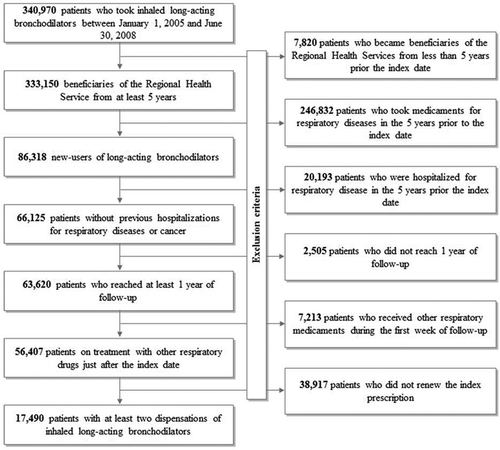
Exclusion was also extended to patients who, in the 5 years before the index date, (i) received at least one medicament for treating chronic respiratory diseases (except short-acting beta-agonists and muscarinic antagonists, respectively SABAs and SAMAs), to include only newly treated individuals, or (ii) had been hospitalized for any respiratory conditions (to identify only incident hospital admissions for respiratory diseases during follow-up) or cancer. Patients were also excluded if they (i) left the cohort (i.e. were censored, see below) within one year of the index date (to ensure at least one year of potential drug availability) or (ii) received more than one type of long-acting bronchodilators (among LABA, LABA/ICS, or LAMA) or received at least one respiratory drug other than LABAs, LAMAs, SABAs, or SAMAs at the index date or during the following seven days (to reduce the range of COPD severity at cohort entry).
Finally, patients who, in the first year after the index date, did not renew the initial prescription of inhaled long-acting bronchodilator were also excluded, based on the assumption that for these patients continuous drug treatment might not have been indicated. The remaining patients comprised the final cohort. All prescriptions of drugs used to treat chronic respiratory diseases, systemic corticosteroids and antibiotics dispensed to cohort members in the 5 years before the index date and during follow-up were identified. Cohort members accumulated person-years of follow-up from the index date until the earliest among the dates of outcome onset (dependent on the specific performed analysis; see later), death, emigration, or end of study period (i.e., December 31, 2012).
Measuring incidence and predictors of discontinuing inhaled long-acting bronchodilators
The first episode of discontinuation of long-acting bronchodilators therapy occurred after cohort entry was considered the outcome for this analysis. In this analysis, discontinuation of long-acting bronchodilators was defined as follows. First, the duration of each inhaled long-acting bronchodilators dispensed during follow-up was calculated by dividing the total amount of the prescribed drug by the defined daily dose. Then, starting from the index date, consecutively refilled prescriptions were considered uninterrupted if the time-span between the end of one prescription and the beginning of the following one (or of censoring) was 90 days or shorter. Treatment discontinuation was otherwise assumed and the date covered by the last prescription before discontinuation was taken as the outcome onset date. The curve representing the cumulative incidence of discontinuing patients was estimated according to the Kaplan–Meier approach.
A Cox regression model was fitted to estimate the hazard ratios (HRs), with corresponding 95% confidence intervals (CIs), measuring the association between selected covariates and the time of first episode of drug discontinuation. Covariates measured at baseline (i.e., gender, age at cohort entry, type of inhaled long-acting bronchodilator received at therapy start, and Charlson's co-morbidity index Citation(16) calculated from inpatient charts for the 5 years before cohort entry), as well as those measured during follow-up (i.e., use of ICS, SABAs, SAMAs, other respiratory drugs, oral corticosteroids, and antibiotics) were included in the model. As covariates measured during follow-up may change in time, they were included as time-dependent in the model Citation(17).
Measuring clinical implications of discontinuing long-acting bronchodilators
Death or first hospital admission for COPD, whichever occurred first after cohort entry, was considered the outcome for this analysis. In studying the association between bronchodilators use and outcome onset, we recognized that we could not assess the effect of several potential confounders. In fact, clinical features such as COPD severity at cohort entry, co-morbidities, and lifestyle factors were not available in our database, making it impossible to control their influence on the association of interest through conventional study designs and analyses. For this reason, we instead considered the case-crossover approach, which is suitable for controlling both measured and unmeasured confounders that do not change over time Citation(18). This approach was appropriate for our setting since we aimed to evaluate the association between transient exposures (i.e., episodes of drug discontinuation) and acute events (i.e., death or hospital admission) Citation(18).
Following the case-crossover approach, only patients who experienced the outcome (cases) were included in this analysis. We verified whether each case received a long-acting bronchodilator during two 90-day periods, one just before outcome onset (current period) and the other preceding the former by 2 months (referent period). The effect on the outcome of receiving (vs. not receiving) a long-acting bronchodilators during the current period was then estimated by contrasting cases' exposure status in the current vs. referent periods. In practice, this was done by computing a 1:1 matched odds ratio (OR) where each case served as its own control Citation(18). The exact binomial distribution was used to calculate the corresponding 95% CIs.
Two sensitivity analyses were performed to verify the robustness of the case-crossover estimates. First, we verified whether estimates were affected by the adopted width of the current and referent periods. To do so, we re-analysed data by changing the width of the current and referent periods first to 60 days and then to 120 days. Second, since case-crossover estimates could be themselves subject to bias due to confounders that vary with time (e.g., seasonality in drug prescriptions or changes in disease severity), a case-case-time-control design was used Citation(19). With this aim, for each “index case” included into the case-crossover design, a “future case” was randomly selected among those patients who developed the outcome after the index case, matching for gender, age (± 3 years) and index date (± 60 days). The crossover analysis estimating the exposure OR in future cases provides an estimate of the expected exposure trend in the time leading up to an exacerbation. The ratio of case-crossover and case-case-crossover odds ratios then provided an unbiased estimate of the association between use of long-acting bronchodilators and the considered outcome.
The SAS software (v9.3; SAS Institute, Cary, North Carolina, USA) was used for analyses. All tested hypotheses were two-tailed and p-values < 0.05 were considered statistically significant.
Results
Patients
shows that among the 340,970 prevalent users of inhaled long-acting bronchodilators, 323,480 did not met the inclusion criteria. At baseline, the remaining 17,490 cohort members had mean age of about 64 years (SD 10 years), 53% of them were men and 9% had at least a chronic co-morbidity. Most cohort members received fixed LABA/ICS combination at cohort entry (about 69%), while much less started with LAMA (21%) or LABA in monotherapy (10%). During follow-up most patients had concomitant use of antibiotics (87%), ICS (86%), oral corticosteroids (35%), and short-acting bronchodilators (mainly SABA, 25%).
Incidence and predictors of discontinuing inhaled long-acting bronchodilators
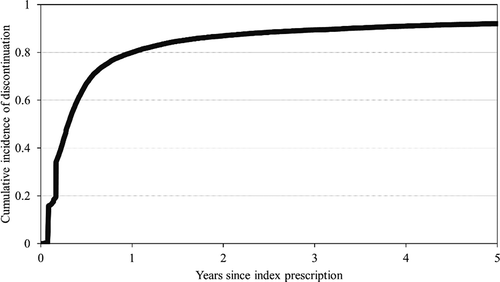
Cohort members accumulated 164,310 person-months of follow-up at risk of a first discontinuation of long-acting bronchodilators, with a median follow-up of 4 months (interquartile range, 2–9 months). Six months after starting therapy with inhaled long-acting bronchodilators, more than 67% of patients experienced a first episode of drug discontinuation (). The cumulative proportions of discontinuation at 1, 2 and 5 years were 80%, 87% and 92%.
Figure 2. Cumulative incidence of discontinuation of any inhaled long-acting bronchodilator medicament from starting therapy.
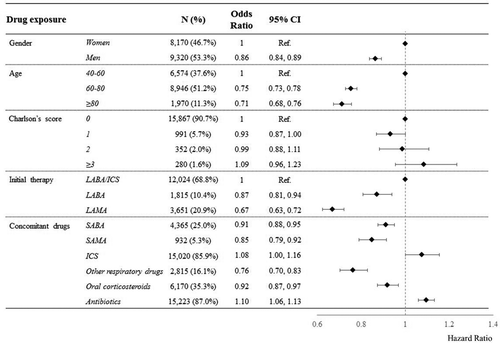
Men, patients aged 60 years or older, patients starting on monotherapy (LABA and even more LAMA), and patients who used short-acting bronchodilators, oral corticosteroids and other respiratory drugs during follow-up were at a lower risk of discontinuation. Conversely, patients who used ICS and antibiotics during follow-up had modest, but statistically significant excess discontinuation risk ().
Figure 3. Effect of selected characteristics of the included patients at cohort entry and during follow-up on the risk of discontinuation from any long-acting bronchodilator medicament. Note. ICS: inhaled corticosteroids; SABA: short-acting beta-agonists; SAMA: short-acting muscarinic-antagonists; Hazard ratios, and 95% confidence interval, estimated according to Cox proportional hazard model. Estimates are mutually adjusted for the included covariates.
Clinical implications of discontinuing long-acting bronchodilators
The 17,490 cohort members accumulated 811,878 person-months of follow-up at risk of outcome onset, with a median follow-up of 46 months (interquartile range, 36–58 months). A total of 2,074 patients experienced the outcome during follow-up of which 151 were excluded because they occurred within 1 year from cohort entry. The remaining 1,923 cases (1,454 deaths and 469 hospitalizations for COPD) entered the subsequent case-crossover analyses.
Case-crossover and case-case-time-control estimates for the association between current dispensation of respiratory drugs and other medicaments and the risk of death or hospital admission for COPD are reported in . Case-crossover estimates show evidence that current exposure to long-acting bronchodilators was associated with a 36% reduced outcome risk, yet current use of both oral corticosteroids and antibiotics was associated with risk excesses of 266% and 73%, respectively. Case-case-time-control estimates tendentially confirmed such results although weaker and less precise effects were observed.
Figure 4. Case-crossover (CC) and case-case-time-control (CCTC) odds ratios for the discontinuation-clinical outcome association. Note. ICS: inhaled corticosteroids; SABA: short-acting beta-agonists; SAMA: short-acting muscarinic-antagonists; Odds ratios, and 95% confidence interval, estimated according to conditional logistic regression contrasting within-patient medicament dispensation during current and referent time-windows (see text for further explanations).
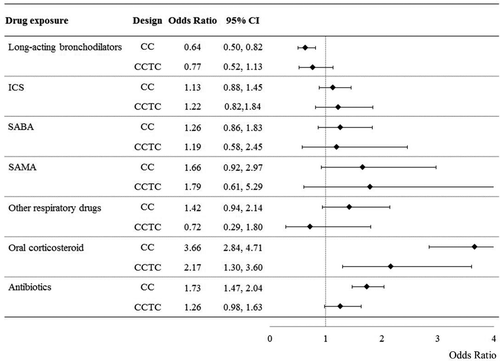
Case-crossover estimates did not generally change by shortening (60 days) or lengthening (120 days) the width of the current and referent periods, the corresponding odds ratios (95% CIs) being 0.65 (0.50 to 0.83) and 0.63 (0.50 to 0.80), respectively.
Discussion
The results of the present study confirm previous observations (Citation9–11) that, in the real-world clinical practice, initial inhaled long-acting bronchodilator drug treatment is frequently abandoned. The pattern of high treatment discontinuation during the first few months and subsequent continually declining persistence shown in this study is consistent with that observed by others Citation(20). This indicates a lack of willingness to take the medication for more than a few months. The new important finding, however, is that patients who did not discontinue treatment had a 36% reduction of the risk of COPD hospital admission or death compared to patients who discontinued. These results provide the largest available evidence on the importance of persistence with long-acting bronchodilator for the protection towards severe respiratory outcomes, adding data from the real-life setting to evidence from a randomized controlled trial (Citation6, 21).
Other results of our study deserve to be mentioned. First, consistently with others (Citation6, 14), our study suggests that women are more likely to discontinue respiratory therapy, although the reasons behind this remain unclear. Second, there is evidence that COPD patients display more adherent behaviors as they age Citation(22), and similarly our study found that patients older than 80 years had a 29% reduced risk of discontinuation than those aged 40–60 years.
Third, patients starting therapy on LAMAs had a 33% reduced risk of discontinuation with respect to those starting on LABA/ICS. This is consistent with the results of a recent observational study reporting that, among the available long-acting bronchodilators, LAMAs ensure the best profile of compliance, likely because of the higher convenience of once daily dosing Citation(14). Fourth, patients who used short-acting bronchodilators during follow-up had a reduced risk of discontinuing long-acting bronchodilators, a relationship that has been described previously (Citation14, 23).
It may be that symptomatic patients, as defined by increased use of symptom-relieving drugs, may be inclined to develop better persistence to their maintenance pharmacotherapy Citation(24). Fifth, most COPD patients in our cohort received antibiotics during follow-up, and antibiotic prescription was significantly more pronounced just before outcome onset (i.e., during the current period). The possibility that COPD exacerbations triggering the onset of a severe outcome may sometimes possibly be treated with antibiotics cannot be excluded Citation(25).
Several limitations of this study should be considered. One, because no diagnostic information was available, COPD patients were defined according to their drug use and age. Hence, we cannot exclude that patients with conditions other than COPD, such as asthma/COPD overlap syndrome Citation(5), may have been included. Nevertheless, in order to only include patients that used long-term bronchodilators for chronic obstructions of the airways, we excluded patients who did not refill long-term bronchodilators at least once within 1 year from therapy start. This implies that our findings should be relevant for individuals requiring continuous long-term bronchodilator therapy.
Two, because in our study therapy discontinuation was derived from refills of drug prescriptions, the validity of our findings is based on the assumption that prescription corresponds to use. There is, however, no guarantee that this is always the case, and indeed it is likely that in a number of patients the prescribed drugs are not consumed. This implies, however, that in the real world, discontinuation of treatment may be even worse than that reported by our study Citation(15).
Three, only exacerbations leading to a COPD hospitalization could be assessed from our data source. This implies that our results only reflect the clinical implications of discontinuing long-acting bronchodilator with respect to respiratory outcomes of high severity. Still, persistence to long-acting bronchodilator therapy may also play an important role in preventing less severe forms of COPD outcomes, such as worsening of dyspnoea symptoms (Citation5, 6).
Finally, as in all observational studies, confounding by indication is of concern for interpreting our findings. COPD severity is clearly a key factor in this setting since: (i) patients with severe airflow limitation are likely to develop better persistence to their maintenance pharmacotherapy Citation(24), and (ii) disease severity is a predictor of exacerbation risk thus of the onset of severe outcomes such as hospitalization and death Citation(5). Because healthcare utilization databases do not include lung functionality information, conventional designs are in fact vulnerable to substantial biases and hence essentially invalid when the goal is to infer about the effect of discontinuation.
In the current application, the case-crossover design was used to avoid confounding by subject-specific attributes that are constant over time Citation(26). Time-varying confounders cannot be excluded however. For example, current and past drug use may differ for the effect of seasonality in the use of respiratory medicaments. Still, deterioration of respiratory functions may be expected as time elapses, so that current use of maintenance therapy may be higher than in the past simply because of symptoms worsening. The case-case-time-control approach Citation(27) was used to account for such sources of bias. The consistence of case-crossover and case-case-time-control estimates suggests that, not only COPD severity, but also other unmeasured factors (e.g., co-morbidities, exposure to smoke and air pollution, among others) affected the reported estimates only marginally.
Conclusions
In conclusion, our data on drug utilization patterns in the real world setting offer evidence that discontinuation of therapy with inhaled long-acting bronchodilators in COPD patients is high. This is worrying, as discontinuing therapy with inhaled long-acting bronchodilators increases the risk of severe outcomes such as hospitalization and death. It is thus important to increase awareness among health professionals of the need to improve adherence and persistence with therapy. More studies are required to increase our understanding of the predictors of poor persistence and identify effective strategies for improving compliance to long-acting bronchodilators in COPD. Our results suggest that physicians may help patients persist with therapy by favoring once-daily long-acting muscarinic-antagonists when clinically appropriate.
Declaration of interest statement
This work was supported by funding from GlaxoSmithKline. GC had a collaborative relationship with the advisory boards of Novartis and Roche. For all other authors, there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated. The authors are responsible for the content and the writing of this article.
Acknowledgments
The authors wish to thank all members of the “CRD Real-World Evidence” scientific board (listed in alphabetical order) for their participation in the project. The scientific board includes: Ovidio Brignoli, Italian College of General Practitioners, Florence, Italy; Isa Cerveri, Institute of Respiratory Diseases, University of Sassari, Sassari, Italy; Giovanni Corrao, Laboratory of Healthcare Research and Pharmacoepidemiology, Unit of Biostatistics, Epidemiology, and Public Health, Department of Statistics and Quantitative Methods, University of Milano-Bicocca, Milan, Italy; Roberto de Marco, Unit of Epidemiology and Medical Statistics, University of Verona, Verona, Italy; Eugenio Guffanti, Unit of Pulmonary Rehabilitation, Research Hospital of Casatenovo, Italian National Research Centre on Aging (INRCA), Casatenovo; Adriano Vaghi, Division of Pneumology, “Guido Salvini” Hospital, Garbagnate Milanese, Italy; Marco Villa, Local Health Authority ASL Cremona, Via San Sebastiano, Cremona, Italy.
Funding
This work was supported by funding from GlaxoSmithKline. GC had a collaborative relationship with the advisory boards of Novartis and Roche. For all other authors, there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
References
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000; 117(2 Suppl):5S–9S.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765–73.
- Breekveldt-Postma NS, Koerselman J, Erkens JA, Lammers JWJ, Herings RMC. Enhanced persistence with tiotropium compared with other respiratory drugs in COPD. Respir Med 2007; 101(7):1398–405.
- Toy EL, Beaulieu NU, McHale JM, Welland TR, Plauschinat CA, Swensen A, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med 2011; 105(3):435–41.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. Available from: http://www.goldcopd.org/ (Accessed August 25, 2015).
- Vestbo J, Anderson JA, Calverley PM, Celli B, Ferguson GT, Jenkins C, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 2009; 64(11):939–43.
- Kesten S, Flanders J, Serby CW, Witek TJ. Compliance with tiotropium, a once daily dry powder inhaled bronchodilator, in one year COPD trials. Chest 2000; 118(4):191S–2S.
- Rand CS, Nides M, Cowles MK, Wise RA, Connett J. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. Am J Respir Critical Care Med 1995; 152(2):580–8.
- Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol 2006; 118(4):899–904.
- Breekveldt-Postma NS, Gerrits CM, Lammers JW, Raaijmakers JA, Herings RM. Persistence with inhaled corticosteroid therapy in daily practice. Respir Med 2004; 98(8):752–9.
- Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf 2007; 16(4):441–8.
- Antoniu SA. Adherence to inhaled therapy in COPD: effects on survival and exacerbations. Expert Rev Pharmacoecon Outcomes Res 2010; 10(2):115–7.
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med 2013; 107(10):1481–90.
- Covvey JR, Mullen AB, Ryan M, Steinke DT, Johnston BF, Wood FT, et al. A Comparison of medication adherence/persistence for asthma and chronic obstructive pulmonary disease in the United Kingdom. Int J Clin Pract 2014; 68(10):1200–8.
- Corrao G, Mancia G. Generating evidence from computerized healthcare utilization databases. Hypertension 2015; 65(3):490–8.
- Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 2008; 61(12):1234–40.
- Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. New York: John Wiley & Sons; 1995.
- Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991; 133(2):144–53.
- Wang S, Linkletter C, Maclure M, Dore D, Mor V, Buka S, et al. Future cases as present controls to adjust for exposure trend bias in case-only studies. Epidemiology 2011; 22(4):568–74.
- Cramer JA, Bradley-Kennedy C, Scalera A. Treatment persistence and compliance with medications for chronic obstructive pulmonary disease. Can Respir J 2007; 14(1):25–9.
- Balkrishnan R, Christensen DB. Inhaled corticosteroid use and associated outcomes in elderly patients with moderate to severe pulmonary disease. Clin Ther 2000; 22(4):452–69.
- Rand CS, Nides M, Cowles MK, Wise RA, Connett J. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. Am J Respir Crit Care Med 1995; 152(2):580–8.
- Elkout H, Helms PJ, Simpson CR, McLay JS. Adequate levels of adherence with controller medication is associated with increased use of rescue medication in asthmatic children. PLoS ONE 2012; 7:e39130.
- World Health Organization (WHO). Adherence to long-term therapies: evidence for action, 2003. Available from: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf (Accessed July 18, 2015).
- Roede BM, Bindels PJE, Brouwer HJ, Bresser P, de Borgie CAJM, Prins JM. Antibiotics and steroids for exacerbations of COPD in primary care: compliance with Dutch guidelines. Br J Gen Pract 2006; 56(530):662–5.
- Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health 2000; 21:193–221.
- Wang SV, Gagne JJ, Glynn RJ, Schneeweiss S. Case-crossover studies of therapeutics: design approaches to addressing time-varying prognosis in elderly populations. Epidemiology 2013; 24(3):375–8.