ABSTRACT
Severity of resting functional impairment only partially predicts the increased risk of death in chronic obstructive pulmonary disease (COPD). Increased ventilation during exercise is associated with markers of disease progression and poor prognosis, including emphysema extension and pulmonary vascular impairment. Whether excess exercise ventilation would add to resting lung function in predicting mortality in COPD, however, is currently unknown. After an incremental cardiopulmonary exercise test, 288 patients (forced expiratory volume in one second ranging from 18% to 148% predicted) were followed for a median (interquartile range) of 57 (47) months. Increases in the lowest (nadir) ventilation to CO2 output (VCO2) ratio determined excess exercise ventilation. Seventy-seven patients (26.7%) died during follow-up: 30/77 (38.9%) deaths were due to respiratory causes. Deceased patients were older, leaner, had a greater co-morbidity burden (Charlson Index) and reported more daily life dyspnea. Moreover, they had poorer lung function and exercise tolerance (p < 0.05). A logistic regression analysis revealed that ventilation/VCO2 nadir was the only exercise variable that added to age, body mass index, Charlson Index and resting inspiratory capacity (IC)/total lung capacity (TLC) ratio to predict all-cause and respiratory mortality (p < 0.001). Kaplan–Meier analyses showed that survival time was particularly reduced when ventilation/VCO2 nadir > 34 was associated with IC/TLC ≤ 0.34 or IC/TLC ≤ 0.31 for all-cause and respiratory mortality, respectively (p < 0.001). Excess exercise ventilation is an independent prognostic marker across the spectrum of COPD severity. Physiological abnormalities beyond traditional airway dysfunction and lung mechanics are relevant in determining the course of the disease.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality worldwide Citation(1). The severity of airflow limitation [as estimated by forced expiratory volume in one second (FEV1)] only partially explains increased risk of death in these patients Citation(2, 3). More recently, lung hyperinflation Citation(4) and transfer factor Citation(2) have been found to be useful prognostic indicators. Unfortunately, a large fraction of deaths remains unpredicted by resting functional evaluation Citation(5) and a recent document co-sponsored by the American Thoracic Society and European Respiratory Society called for additional research on predictors of mortality in COPD Citation(3).
Exercise tolerance decreases in tandem with progressing COPD severity Citation(6). In fact, even simple estimates of functional capacity, such as the 6-minute walking distance, provide useful prognostic information, particularly when considered in association with severity of airway obstruction and exertional dyspnea on daily life [as reviewed in ref. Citation(5)]. Measurement of cardiocirculatory and ventilatory variables by means of cardiopulmonary exercise testing (CPET) might provide important complementary information to “field” tests as those measurements are more directly linked to the pathophysiology of COPD and may elucidate the mechanisms underlying exercise intolerance in individual patients Citation(7). Determination of CPET variables, which are more closely related to mortality are therefore key to expand our knowledge on the mechanistic bases of the reported inter-relationships between poor functional capacity, greater activity-related dyspnea and poor prognosis in COPD.Citation(5, 8)
In this context, there is a growing recognition that CPET-based markers of ventilatory inefficiency [increased ventilation (VE) relative to CO2 production (VCO2)] are related to clinically relevant outcomes in COPD (Citation9–11). These abnormalities are thought to reflect an enlarged dead space (VD) as a fraction of a lower tidal volume (VT) and/or increased neural ventilatory drive due to a low PaCO2 “set-point” and heightened afferent ventilatory stimulation Citation(12, 13). Of note, several pathophysiological derangements underlying increased VD/VT, including emphysema progression Citation(14), pulmonary microvascular abnormalities Citation(15) and mechanical VT constraints Citation(16) are ultimately relevant to prognosis in COPD Citation(5, 17). High neural ventilatory drive may be a consequence of increased pulmonary arterial pressures Citation(18), sympathetic over-excitation and increased stimulation of peripheral ergoreceptors Citation(19), which are all well-known negative prognostic markers in chronic cardiopulmonary diseases Citation(20, 21). Thus, similar to heart failure Citation(22) and pulmonary hypertension Citation(21), exercise ventilatory inefficiency may constitute a relevant physiological biomarker with negative prognostic implications in COPD.
This study aimed to determine whether incremental CPET would add to standard pulmonary function tests in estimating the risk of death in patients with mild to very severe COPD. We specifically hypothesized that ventilatory inefficiency [i.e., increased VE/VCO2 ratio at its lowest point (“nadir”)] Citation(23) would compound with established resting functional indices in predicting all-cause and respiratory mortality in these patients.
Methods
Subjects
The study population comprised 288 outpatients with established COPD presenting with a wide range of airflow obstruction who underwent resting functional and CPET evaluations from October 1999 to June 2012 at the Respiratory Investigation Unit, Kingston General Hospital. Those evaluations were performed as screening tests for potential participation in subsequent physiologic studies. Thus, patients’ diagnosis was carefully confirmed and treatment (inhaled β2-agonists, anticholinergics, and inhaled steroids) optimized according to the guidelines at the time of testing. Data from this cohort have been used in a communication dealing with the effects of COPD progression on exercise ventilation Citation(11). Causes of death were obtained from the patients’ centralized electronic medical records from Kingston General Hospital and coded as “respiratory” or “non-respiratory” by two independent reviewers (AA and JAN). Discrepancies in coding (N = 4) were resolved by consensus. Patients had no evidence of asthma or another lung disease or a known contraindication for a CPET Citation(7). The institutional review board approved the use of these retrospective, anonymous data sets and waived the need for patient informed consent (DMED-1659-13).
Measurements
Clinical variables
Age, gender, and body mass index (BMI, kg/m2) were recorded. Dyspnea was evaluated by the Baseline Dyspnea Index (BDI) scale Citation(24). Co-morbidity burden was determined by the combined 19-disease Charlson Index Citation(25).
Lung function tests
Spirometry [including inspiratory capacity (IC) measurements], body plethysmography [residual volume (RV) and total lung capacity (TLC)], and measurements of transfer factor for carbon monoxide (TLCO) were performed using automated testing equipment (2130 spirometer with 6200 Autobox DL or V6200 Autobox; SensorMedics; Yorba Linda, CA, USA).
Cardiopulmonary exercise test
Symptom-limited incremental CPET was performed on an electronically braked cycle ergometer using the Vmax229d System (SensorMedics). Tests were performed without supplemental oxygen. The rate of work rate increment was individually selected according to reported exercise tolerance and resting functional impairment (typically 5–10 W/min in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages 3 and 4 and 10–15 W/min in stages 1 and 2). VE (L/min), VCO2 (L/min), oxygen uptake (VO2, L/min), end-tidal partial pressure for carbon dioxide (PETCO2, kPa), and VT (L) were averaged at 30-second intervals. Peak VE was also expressed relative to the estimated maximal voluntary ventilation [MVV (L/min) = FEV1 × 35]. VE/VCO2 (ventilatory equivalent for CO2) was the lowest test data point Citation(23). IC maneuvers tracked operating lung volumes and, assuming a constant TLC, were used to calculate inspiratory reserve volume (IRV, L) Citation(16). Arterial oxygen saturation was measured noninvasively by pulse oximetry (SpO2, %). Breathlessness was rated according to the modified 10-point Borg category-ratio scale.
Statistical analysis
Values are reported as means ± standard deviation (SD) unless otherwise specified (IBMۚ SPPSۚ Statistics version 22.0.0.0). The time frame from CPET to the last attendance or death was recorded. Survivors and nonsurvivors were contrasted by nonpaired t or Mann–Whitney's test or a χ2 test for differences in proportions. The survival time was calculated with the life table method. Receiver operating characteristics (ROC) curve analyses selected the optimal threshold values (highest Youden's J index = Sensitivity + Specificity − 1) for the lung function and CPET variables. Areas under the curves were compared according to DeLong et al. Citation(26) (MedCalc 14.12.0, Ostend, Belgium). Univariate and multivariate binary logistic regression analyses evaluated the predictive value of the different variables, using all-cause and respiratory mortality as the outcome. Clinical variables were used as continuous variables, except the categorical variables of gender and smoking status. Results were expressed in terms of the estimated relative risks with corresponding 95% confidence intervals. Multicollinearity was checked by calculating variable's level of tolerance and the respective variance inflation factor. Kaplan–Meier analysis assessed the survival characteristics of lung function and CPET variables retained in the final logistic regression models with statistical significance being determined by the log-rank test.
Results
General characteristics
Main clinical, functional, and CPET variables describing the study population are shown in . Patients presented with a large range of resting functional impairment (e.g., FEV1 ranged from 18% to 148% pred); however, 178/288 (61.8%) patients had mild-to-moderate airflow obstruction. Exercise impairment, as indicated by peak VO2 below the lower limit of normality (83% predicted) Citation(7), was found in 209/288 (72.6%) patients. As expected, patients showed increased peak VE/MVV ratios, lower peak HR, poor ventilatory efficiency, and varying reduction in IC and SpO2. Moreover, dyspnea was the main limiting symptom in the majority of patients ().
Table 1. Clinical, resting functional, and CPET responses for the whole sample and for patients separated by mortality status.
Mortality
The mean ± SD follow-up time for the total population was 65 ± 39 months, while the median (interquartile range) was 57 (47) months. Seventy-seven patients (26.7%) died during the follow-up period. Seventy-two patients died after hospital admission and five patients arrived dead in the Hospital Emergency Department. Autopsies were allowed to be performed in 42 patients (54.5%) and the main cause of death was based on the conclusions in these patients. Thirty patients (38.9%) died from respiratory causes: COPD was listed as the main contributing factor in all but two patients. Most common nonrespiratory causes included cardiovascular disease (N = 18), cerebrovascular disease (N = 8), and cancer (N = 7). Median (95% CI) survival for the total population was 123 (105–158) months. No patient underwent lung transplant or lung volume reduction surgery during the follow-up time.
Clinical, functional, and CPET variables in survivors and nonsurvivors
shows the differences in patient characteristics separated by mortality status. Nonsurvivors were more frequently males, older, leaner, more dyspneic on daily life, and had a higher co-morbidity burden than the survivors (p < 0.05). From the most prevalent co-morbidities included in the Charlson Index Citation(25), only chronic heart failure was more frequent in nonsurvivors than survivors (). Nonsurvivors also had more severe lung function impairment as indicated by lower FEV1, worse lung hyperinflation and air trapping, and lower DLCO. Metabolic and cardiovascular variables from the CPET indicated than nonsurvivors had lower peak VO2, larger chronotropic reserves, and lower O2 pulse. They also showed greater ventilatory response at rest and during exercise, more O2 desaturation, and greater mechanical constraints. Moreover, dyspnea was more frequently cited as the limiting symptom in this group compared to survivors (p < 0.05; ).
Predictors of mortality
ROC curve analyses contrasting the resting functional variables revealed that IC/TLC has the highest area under the curve for all-cause () and respiratory () mortality prediction followed by RV and FEV1. These variables, however, were closely inter-related (r values ranging from 0.70 to 0.89; p < 0.01). A similar analysis involving the CPET responses showed better prognostic performance for VO2 peak and VE/VCO2 nadir () with a lower between-variable correlation (r = −0.57; p < 0.05). and show the optimal thresholds for all-cause and respiratory mortality prediction according to key resting and exercise variables. This analysis revealed that VE/VCO2 nadir > 34 was the best cut-off for all-cause and respiratory mortality prediction. In contrast, the IC/TLC cut-offs differed: ≤0.34 for all-cause and ≤0.31 for respiratory mortality ( and ). Univariate logistic regression analysis showed that age, BMI, and Charlson Index were also significantly associated with all-cause () and respiratory () mortality (p < 0.05).
Figure 1. Receiver operating characteristics curve analyses contrasting lung function and exercise variables performance in predicting all-cause and respiratory mortality in patients with COPD. Best cut-offs for individual variables are marked by circles and shown in and , respectively. p < 0.05: * vs. FEV1, TLC, and TLCO/VA (lung function) and vs. O2 pulse, HR, and SpO2 (exercise). Abbreviations: COPD = chronic obstructive pulmonary disease; AUC = area under the curve; CI = confidence interval; IC = inspiratory capacity; TLC = total lung capacity; RV = residual volume; FEV1 = forced expiratory volume in one second; TLCO = transfer factor; VO2 = oxygen uptake; VE = minute ventilation; VCO2 = carbon dioxide output; HR = heart rate; SpO2 = oxy-hemoglobin saturation by pulse oximetry.
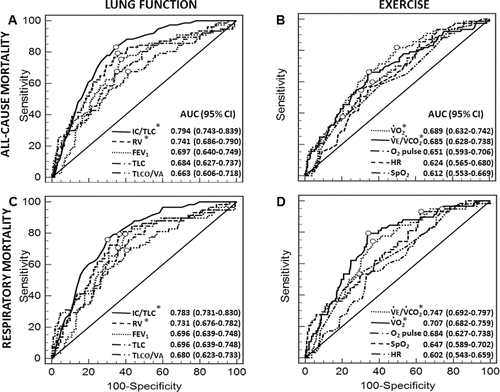
Table 2. All-cause mortality prediction: performance of optimal thresholds for individual lung function and exercise variables.
Table 3. Respiratory mortality prediction: performance of optimal thresholds for individual lung function and exercise variables.
Table 4. All-cause mortality prediction: univariate and multivariate logistic regression modeling.
Table 5. Respiratory mortality prediction: univariate and multivariate logistic regression modeling.
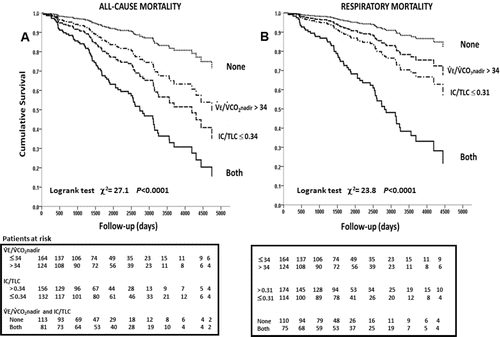
Final results for adjusting the logistic regression analysis over all potential predictors of death are shown in for all-cause and in for respiratory mortality. FEV1 and, as expected, RV and TLC showed high multicollinearity with IC/TLC (p < 0.01): these variables, therefore, did not remain in the final models in addition to IC/TLC. Age, BMI, Charlson Index, IC/TLC, and VE/VCO2 nadir were therefore the independent predictors of mortality. presents the Kaplan–Meier survival curves: compared to patients presenting with VE/VCO2 nadir ≤ 34 plus IC/TLC > 0.34, survival to all-cause () and respiratory () mortality progressively decreased in those with higher VE/VCO2 nadir or lower IC/TLC. Patients presenting VE/VCO2 nadir > 34 plus IC/TLC ≤ 0.34 had the poorest survival. For instance, when VE/VCO2 nadir was added to IC/TLC 10-year survival decreased from 54.7% to 36.8% and 68.7% to 39.0% for all-cause and respiratory mortality, respectively (p < 0.0001; ).
Figure 2. Kaplan–Meyer survival curves for resting IC/TLC and VE/VCO2 nadir optimal thresholds as defined by receiver operating characteristics curve analyses. Abbreviations: IC = inspiratory capacity; TLC = total lung capacity; VE = exercise ventilation; VCO2 = CO2 output.
We also investigated the determinants of increased VE/VCO2 nadir (>34). Compared to their counterparts with lower nadirs, patients presenting with VE/VCO2 nadir > 34 had significantly greater resting VE/VCO2 (36.8 ± 6.3 vs. 41.9 ± 9.7; p < 0.01), higher VE–VCO2 intercepts (3.6 ± 3.1 vs. 7.1 ± 4.1; p < 0.05), and a trend to lower VE–VCO2 slopes (32.1 ± 5.4 vs. 30.5 ± 7.3; p = 0.15). Moreover, the nadir was reached at a significantly lower VO2 in this group (78.5 ± 18.7% pred vs. 59.8 ± 17.1% pred; p < 0.001).
Discussion
This study investigated whether cardiocirculatory, ventilatory, and pulmonary gas exchange variables measured during incremental CPET would add to standard lung function variables in estimating the risk of mortality in patients with COPD. We found that a widely used marker of ventilatory inefficiency (increased VE/VCO2 nadir) Citation(22, 23, 27) was the only CPET variable to remain a predictor of mortality when well-established clinical (age, BMI and co-morbidity burden) Citation(5) and resting functional (IC/TLC) Citation(4) variables were accounted for in a multivariate logistic regression analysis. Of note, there was a marked increase in the risk of death when a high VE/VCO2 nadir (>34) compounded with a low IC/TLC (≤0.34 for all-cause mortality and for ≤0.31 respiratory mortality). These results indicate that ventilatory inefficiency is a key physiological abnormality in COPD (Citation10, 11, 28), which is relevant to mortality prediction across the spectrum of disease severity.
There is a large body of evidence supporting CPET for the prognostic assessment of patients with chronic cardiopulmonary diseases Citation(7, 20, 21). However, CPET remains largely underutilized for risk stratification in COPD Citation(5). In fact, the few previous studies that examined this issue in COPD confined their analysis to traditional end-exercise variables such as peak VO2 Citation(29). Moreover, those studies typically evaluated patients with severe or very severe COPD in whom variables reflecting advanced lung function impairment (e.g., low TLCO or hypoxemia) Citation(2, 17) likely overshadowed the prognostic role of CPET.
A key finding of the present study was the independent role of a submaximal, effort-independent CPET variable (VE/VCO2 nadir) Citation(22, 23, 27) in predicting all-cause and respiratory mortality in a sample with a sizeable number of patients with mild-to-moderate COPD. Of note, VE/VCO2 nadir overruled more traditional CPET markers of poor prognosis, such as peak VO2, heart rate, and SpO2 Citation(7, 29). VE/VCO2 nadir was particularly relevant in enhancing the prognostic performance of resting lung hyperinflation (IC/TLC) Citation(4). In this context, it has long been established that exercise ventilation is inversely related to PaCO2 set-point and proportional to the fraction of VT “wasted” in the VD.Citation(12) Although mechanical constraints as COPD progresses may reduce tidal volume (VT) and impair the rate of increase in VE (leading to a low VE–VCO2slope) Citation(9, 11, 14), the linear VE–VCO2 relationship shifts upward (i.e., high VE–VCO2intercept) in tandem with increasing VD/VT Citation(9, 11, 30, 31). Consequently, discrete VE values at a given VCO2 (VE/VCO2 ratio or ventilatory equivalent for CO2) Citation(23) should increase in proportion to VD/VT in order to maintain PaCO2 close to the chosen set-point Citation(12). We focus our analysis on VE/VCO2 nadir as it is not influenced by between-subject differences in lactacidotic drive Citation(12), it is easily obtained from CPET reports, and, as outlined above, conflates the physiological information provided by both VE–VCO2 slope and intercept Citation(11, 13).
In this context, we previously demonstrated that increased VD/VT was the most consistent pulmonary gas exchange abnormality associated with greater ventilatory inefficiency in patients with largely preserved FEV1 Citation(28). VD/VT ratio may increase secondary to emphysema as alveolar destruction increases VD and, by promoting lung hyperinflation (reduced IC/TLC), decreases exercise VT Citation(16). Impaired pulmonary microvascular blood flow leading to a dead space effect (high ventilation/perfusion ratios) might be a consequence of vessel loss preceding anatomical emphysema Citation(32) and/or compression by regional hyperinflation Citation(33). Hueper et al. recently found reduced pulmonary microvascular blood flow in nonemphysematous lung regions in mild COPD, suggesting pulmonary vascular destruction/dysfunction before the establishment of macroscopic emphysema Citation(32). Moreover, Holverda et al. Citation(18) and Vonbank et al. Citation(34) showed that COPD patients presenting with pulmonary hypertension at rest had greater exercise ventilation than their counterparts with normal pulmonary vascular pressures. At the other end of the disease spectrum, Armstrong et al. Citation(35) and Kim et al. Citation(36) showed that excision of regional lung units destroyed by emphysema via lung volume reduction surgery significantly lowered VE/VCO2 nadir. Taken together, these findings suggest that higher VE/VCO2 nadir in nonsurvivors resulted primarily from higher VD/VT and, accordingly, greater pulmonary microvascular abnormalities and/or larger emphysema extent Citation(5, 6, 15, 17).
It is noteworthy that approximately two-thirds of our patients had a nonrespiratory cause of death. In fact, cardiovascular and cerebrovascular causes of death jointly accounted for a similar number of deaths compared to respiratory causes. In this context, worse lung hyperinflation (low IC/TLC) may have compounded with underlying pulmonary vascular disease to further increase pulmonary vascular resistance and decrease left ventricular filling in nonsurvivors Citation(37). There is recent evidence that pulmonary endothelial dysfunction leading to impaired microvascular blood flow Citation(38) (and high VE/VCO2 nadir) might be etiologically related to low-grade systemic inflammation and oxidative stress, two well-known negative prognostic markers in cardiocirculatory diseases Citation(37). Moreover, ventilatory inefficiency has been associated with increased neural ventilatory drive secondary to sympathetic over-excitation and increased risk of cardiac death Citation(20, 22). Not unexpectedly, therefore, left ventricular dysfunction (either systolic or diastolic) has been related to higher exercise VE/VCO2 in COPD.Citation(30) It is thus conceivable that ventilatory inefficiency was associated with greater central hemodynamic impairment and/or higher sympathetic activation during exercise with ominous prognostic implications.
We confirmed the seminal results of Casanova et al. Citation(4) showing that IC/TLC is a key resting functional predictor of respiratory mortality in COPD. Patients with low IC/TLC are likely to be particularly ill-prepared to face greater expiratory flow limitation and acute-on-chronic lung hyperinflation during COPD exacerbations Citation(39). Increased ventilatory requirements secondary to greater wasted ventilation during exacerbations is likely to further increase the burden upon the already overloaded respiratory muscles in patients with increased VE/VCO2 Citation(39). This might help explain why patients showing the combination of low IC/TLC and high VE/VCO2 nadir were at a greater risk of exacerbation-related respiratory failure and death. Moreover, assuming that a high VE/VCO2 primarily reflects poorer ventilation–perfusion matching in COPD Citation(15, 28), it is possible that those with greater ventilatory inefficiency would present with more pronounced changes in arterial blood gases during exacerbations thereby increasing the risk of death Citation(39).
The current investigation has, naturally, some limitations. Our sample was relatively small compared to large epidemiological studies dealing with prognosis in COPD Citation(3), i.e., the number of patients at risk in each prognostic category was not large and further decreased after ∼ 10 years of follow-up (). In fact, treatment paradigms for COPD changed over the follow-up period of time and this might have potentially influenced patient's outcome depending on the date of CPET. Notwithstanding these limitations, it constitutes one of the largest series to date to investigate the prognostic relevance of CPET in COPD and the tests were carefully performed in a specialized referral center. The optimal threshold for VE/VCO2 nadir in COPD (i.e., >34) coincided with that proposed for heart failure Citation(22). The relevance of high VE/VCO2 nadir to predict all-cause mortality, however, cannot be fully ascribed to increased mortality in co-existent COPD–heart failure as only a quarter of deceased patients had this co-morbidity (). Although we did not assess test–retest VE/VCO2 nadir reliability, VE/VCO2 nadir has been found to be highly reproducible Citation(23, 40). Finally, only a minority of our patients had very severe COPD and it could be argued that pronounced decreases in mechanical–ventilatory reserves in GOLD stage 4 patients would preclude an adequate ventilatory response to metabolic demand. Thus, it remains to be demonstrated whether ventilatory inefficiency would remain a useful prognostic marker in hypercapnic patients with end-stage COPD.
In conclusion, this study is the first to demonstrate that ventilatory inefficiency during incremental CPET (i.e., VE/VCO2 nadir > 34) adds to established clinical (age, BMI, and co-morbidities) and resting lung function (IC/TLC) variables in estimating the risk of all-cause and respiratory mortality in patients with COPD. Thus, CPET can add important prognostic information to traditional measures of resting lung hyperinflation across the spectrum of COPD severity. These results were obtained in a sample showing predominantly mild-to-moderate airflow obstruction, a large sub-population in which the identification of negative prognostic markers may have a larger impact on disease management compared to more severe COPD. Ventilatory inefficiency constitutes a physiological biomarker linked to key clinical outcomes in COPD: exercise tolerance Citation(9, 11), dyspnea Citation(10, 28), and, as newly established in this study, risk of death. Ventilatory inefficiency in COPD likely conflates the overlapping influences of disease-related functional abnormalities and pathophysiological derangements associated with multiple co-morbidities. Our work also provides novel evidence that physiological abnormalities leading to poorly efficient pulmonary gas exchange and increased wasted ventilation during exercise are relevant in predicting the course of the disease. Therapeutic approaches aiming at improving those abnormalities and decreasing exercise ventilatory inefficiency might have beneficial effects on survival in COPD.
Additional information
Funding
References
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- Boutou AK, Shrikrishna D, Tanner RJ, Smith C, Kelly JL, Ward SP, et al. Lung function indices for predicting mortality in COPD. Eur Respir J 2013; 42(3):616–625.
- Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agustí A, Criner GJ, et al. An official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191(7):e4–27.
- Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171(6):591–597.
- Celli BR. Predictors of mortality in COPD. Respir Med 2010; 104(6):773–779.
- O'Donnell DE, Laveneziana P, Webb K, Neder JA. Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med 2014; 35(1):51–69.
- ERS Task Force, Palange P, Ward SA, Carlsen K-H, Casaburi R, Gallagher CG, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J 2007; 29(1):185–209.
- de Torres JP, Casanova C, Marín JM, Pinto-Plata V, Divo M, Zulueta JJ, et al. Prognostic evaluation of COPD patients: GOLD 2011 versus BODE and the COPD comorbidity index COTE. Thorax 2014; 69(9):799–804.
- Teopompi E, Tzani P, Aiello M, Gioia MR, Marangio E, Chetta A. Excess ventilation and ventilatory constraints during exercise in patients with chronic obstructive pulmonary disease. Respir Physiol Neurobiol 2014; 197:9–14.
- Guenette JA, Chin RC, Cheng S, Dominelli PB, Raghavan N, Webb KA, et al. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J 2014; 44(5):1177–87.
- Neder JA, Arbex FF, Alencar MCN, O'Donnell CDJ, Cory J, Webb KA, et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J 2015; 45(2):377–87.
- Whipp BJ, Ward SA, Wasserman K. Ventilatory responses to exercise and their control in man. Am Rev Respir Dis 1984; 129(2 Pt 2):S17–S20.
- Ward SA. Ventilatory control in humans: constraints and limitations. Exp Physiol 2007; 92(2):357–366.
- Paoletti P, De Filippis F, Fraioli F, Cinquanta A, Valli G, Laveneziana P, et al. Cardiopulmonary exercise testing (CPET) in pulmonary emphysema. Respir Physiol Neurobiol 2011; 179(2–3):167–173.
- Rodríguez-Roisin R, Drakulovic M, Rodríguez DA, Roca J, Barberà JA, Wagner PD. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol Bethesda 2009; 106(6):1902–1908.
- O'Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest 2012; 141(3):753–762.
- Boutou AK, Nair A, Douraghi-Zadeh D, Sandhu R, Hansell DM, Wells AU, et al. A combined pulmonary function and emphysema score prognostic index for staging in chronic obstructive pulmonary disease. PloS One 2014; 9(10):e111109.
- Holverda S, Bogaard HJ, Groepenhoff H, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Cardiopulmonary exercise test characteristics in patients with chronic obstructive pulmonary disease and associated pulmonary hypertension. Respir Int Rev Thorac Dis 2008; 76(2):160–167.
- Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, et al. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186(7):606–615.
- Ponikowski P, Banasiak W. Chemosensitivity in chronic heart failure. Heart Fail Monit 2001; 1(4):126–131.
- Ferreira EVM, Ota-Arakaki JS, Ramos RP, Barbosa PB, Almeida M, Treptow EC, et al. Optimizing the evaluation of excess exercise ventilation for prognosis assessment in pulmonary arterial hypertension. Eur J Prev Cardiol 2014; 21(11):1409–1419.
- Myers J, Arena R, Oliveira RB, Bensimhon D, Hsu L, Chase P, et al. The lowest VE/VCO2 ratio during exercise as a predictor of outcomes in patients with heart failure. J Card Fail 2009; 15(9):756–762.
- Sun X-G, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med 2002; 166(11):1443–1448.
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984; 85(6):751–758.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5):373–383.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44(3):837–845.
- Ingle L, Sloan R, Carroll S, Goode K, Cleland JG, Clark AL. Prognostic significance of different measures of the ventilation-carbon dioxide relation in patients with suspected heart failure. Eur J Heart Fail 2011; 13(5):537–542.
- Elbehairy AF, Ciavaglia CE, Webb KA, Guenette JA, Jensen D, Mourad SM, et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med 2015; 191(12):1384–1394.
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003; 167(4):544–549.
- Apostolo A, Laveneziana P, Palange P, Agalbato C, Molle R, Popovic D, et al. Impact of chronic obstructive pulmonary disease on exercise ventilatory efficiency in heart failure. Int J Cardiol 2015; 189:134–140.
- Gargiulo P, Apostolo A, Perrone-Filardi P, Sciomer S, Palange P, Agostoni P. A non invasive estimate of dead space ventilation from exercise measurements. PloS One 2014; 9(1):e87395.
- Hueper K, Vogel-Claussen J, Parikh MA, Austin JHM, Bluemke DA, Carr J, et al. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD Study. Am J Respir Crit Care Med 2015; 192(5):570–580.
- Smith BM, Hoffman EA, Basner RC, Kawut SM, Kalhan R, Barr RG. Not all measures of hyperinflation are created equal: lung structure and clinical correlates of gas trapping and hyperexpansion in COPD: the multi-ethnic study of atherosclerosis (MESA) COPD study. Chest 2014; 145(6):1305–1315.
- Vonbank K, Funk GC, Marzluf B, Burian B, Ziesche R, Stiebellehner L, et al. Abnormal pulmonary arterial pressure limits exercise capacity in patients with COPD. Wien Klin Wochenschr 2008; 120(23–24):749–755.
- Armstrong HF, Dussault NE, Thirapatarapong W, Lemieux RS, Thomashow BM, Bartels MN. Ventilatory efficiency before and after lung volume reduction surgery. Respir Care 2015; 60(1):63–71.
- Kim V, Kretschman DM, Sternberg AL, DeCamp MM, Criner GJ, National Emphysema Treatment Trial Research Group. Weight gain after lung reduction surgery is related to improved lung function and ventilatory efficiency. Am J Respir Crit Care Med 2012; 186(11):1109–1116.
- Barr RG. The epidemiology of vascular dysfunction relating to chronic obstructive pulmonary disease and emphysema. Proc Am Thorac Soc 2011; 8(6):522–527.
- Thomashow MA, Shimbo D, Parikh MA, Hoffman EA, Vogel-Claussen J, Hueper K, et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The multi-ethnic study of atherosclerosis chronic obstructive pulmonary disease study. Am J Respir Crit Care Med 2013; 188(1):60–68.
- O'Donnell DE, Parker CM. COPD exacerbations. 3: Pathophysiology. Thorax 2006; 61(4):354–361.
- Barron A, Dhutia N, Mayet J, Hughes AD, Francis DP, Wensel R. Test-retest repeatability of cardiopulmonary exercise test variables in patients with cardiac or respiratory disease. Eur J Prev Cardiol 2014; 21(4):445–453.
