ABSTRACT
We investigated the effect of the long-acting muscarinic antagonist aclidinium bromide on chronic obstructive pulmonary disease (COPD) exacerbations by pooling data from five randomized, placebo-controlled, parallel-group Phase III studies of 3–6 months’ duration. Data were pooled from the aclidinium 400 μg twice-daily (BID) and placebo arms (N = 2,521) and stratified by Global initiative for chronic Obstructive Lung Disease (GOLD) group (A, B, C and D). Results showed that fewer patients experienced ≥1 exacerbation with aclidinium (any severity: 12.5%; moderate to severe: 10.9%) compared with placebo (any severity: 15.7%; moderate to severe: 13.3%) and the odds of experiencing ≥1 exacerbation of any severity were reduced in patients receiving aclidinium (odds ratio = 0.78, p = 0.039). Furthermore, aclidinium reduced the rate of exacerbations compared with placebo (any severity: rate ratio = 0.79, p = 0.026; moderate to severe: 0.80, p = 0.044). The time to first exacerbation of any severity was delayed with aclidinium compared with placebo (hazard ratio = 0.79, p = 0.026) and there was a numerical delay in time to first moderate-to-severe exacerbation. Finally, the effects of aclidinium on exacerbations versus placebo were greater in patients in GOLD Groups B and D; however, it is of note that only 10.7% of patients were classified in Group A or C. In summary, the results indicate that aclidinium 400 μg BID reduces the frequency of COPD exacerbations compared with placebo and that these effects are greater in symptomatic patients.
Introduction
Exacerbations in patients with chronic obstructive pulmonary disease (COPD) are associated with an accelerated decline of lung function (Citation1–4), impaired health status (Citation5–7) and increased mortality Citation(8). Exacerbations of COPD, particularly those requiring hospitalizations, are a major contributor to the economic burden of COPD (Citation9,10). The prevention of COPD exacerbations is an important longer-term goal of COPD therapy Citation(11).
Recent studies have provided evidence that long-acting muscarinic antagonists (LAMAs) can reduce the frequency of exacerbations versus placebo (Citation12,13) or—in a population enriched for exacerbations—versus long-acting β2-agonists (LABAs) (Citation14,15). Indeed, the 2014 Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines recommended LAMAs as maintenance therapy for patients with a high risk of exacerbation (≥2 exacerbations or ≥1 exacerbation leading to hospitalization in the previous year, or severe airflow limitation [GOLD Stage III and IV]) and/or a high level of symptoms (GOLD Groups B, C and D) Citation(11).
Aclidinium bromide 400 μg is a twice-daily (BID) LAMA indicated as a maintenance therapy for patients with COPD Citation(16). Although exacerbations have been assessed in Phase III clinical studies of aclidinium and its combination with formoterol (Citation5,17–22), the individual studies were not designed to assess exacerbations as a primary endpoint. Here, we investigated the effect of aclidinium monotherapy on exacerbations using pooled data from five Phase III studies of 3–6 months’ duration. Additionally, we performed a novel exploratory analysis to assess the differential effect of aclidinium on exacerbations according to the GOLD Groups (A, B, C and D).
Methods
Study design
Data were pooled from the aclidinium 400 μg BID monotherapy and placebo arms of five studies in patients with moderate-to-severe COPD, of which three studies were designed to assess the efficacy and safety of aclidinium monotherapy (ACCORD I [NCT00891462], ACCORD II [NCT01045161] and ATTAIN [NCT01001494]), and two were designed to assess the efficacy and safety of aclidinium/formoterol combined (ACLIFORM [NCT01462942] and AUGMENT [NCT01437397]; ). All five studies were multinational, multicenter, randomized, double-blind and parallel-group in design. Detailed methods of each study have been published elsewhere (Citation5,17–20,22). Aclidinium 400 μg and placebo were administered in the morning and evening via a breath-actuated multidose dry powder inhaler (Genuair™/Pressair®Footnote1).
Table 1. Study designs.
Patients
Male and female patients aged ≥40 years who were current or former smokers (≥10 pack-years) with moderate-to-severe COPD (post-bronchodilator forced expiratory volume in 1 second [FEV1] ≥30% and <80% predicted and FEV1/FVC ratio <70%) were eligible for inclusion in the studies. A history of ≥1 exacerbation was not an inclusion criterion. Exclusion criteria included presence or history of clinically significant respiratory disease (other than COPD) or cardiovascular conditions, respiratory infection or COPD exacerbation ≤6 weeks pre-screening and hospitalization for COPD exacerbation ≤3 months pre-screening.
Inhaled salbutamol (100 μg/puff) was permitted as relief medication, provided its use was discontinued 6 hours prior to study visits. Inhaled corticosteroids (ICS), oral or parenteral corticosteroids (equivalent to ≤10 mg/day of prednisone or 20 mg every other day), oral sustained-release methylxanthines and oxygen therapy (<15 hours/day) were allowed, provided treatment was stable ≥4 weeks pre-screening. Other long-acting bronchodilators were prohibited.
Study protocols were approved by all necessary ethics committees. All patients provided written informed consent.
Assessment of exacerbations
COPD exacerbations were assessed by the investigator using the Healthcare Resource Utilization definition (an increase of COPD symptoms during ≥2 consecutive days that required a change in COPD treatment). Exacerbations were categorized as mild (self-managed by the patient at home by increasing usual COPD medication [short-acting bronchodilator and/or ICS use]), moderate (did not lead to hospitalization, but were treated with antibiotics and/or systemic corticosteroids or an increase in dose of systemic corticosteroids) or severe (led to hospitalization [overnight stay or emergency room visit]). A COPD exacerbation was considered a new exacerbation episode if the patient had not taken oral steroids and antibiotics for ≥14 days since the previous exacerbation.
Statistical analysis
Individual data were pooled from the aclidinium monotherapy and placebo arms of the intent-to-treat (ITT) population (all patients who received at least one dose of the study medication and had a baseline and at least one post-baseline FEV1 assessment) in each study. Exacerbation rates were annualized by dividing the number of exacerbations by the number of days the patient participated and multiplying by 365.25.
For analyses of exacerbation rates and time to first exacerbation, data were stratified according to the GOLD COPD assessment (Groups A, B, C and D) based on level of symptoms (St. George's Respiratory Questionnaire [SGRQ] total score <25 [Groups A and C] or ≥25 [Groups B and D] Citation(23)), airflow limitation severity (GOLD Stage I or II vs. III or IV) and previous exacerbation history (≤1 vs. ≥2 within the previous year) Citation(24). For further analysis, patients were also pooled by level of symptoms (low [Group A+C] vs. high [Group B+D]). Data were also stratified by baseline ICS use for analyses of exacerbation rates.
Whether or not patients experienced ≥1 COPD exacerbation was analyzed based on a logistic regression model with treatment group, study, smoking status and baseline airflow limitation severity as factors. The number of COPD exacerbations was analyzed using a negative binomial regression model with the total number of COPD exacerbations as response. Exposure time was included as an offset. Time to first exacerbation was analyzed using a Cox proportional hazard model, with time from first study dose to failure (an exacerbation) right censored by study termination (withdrawal or study end – whichever occurred first). Both models included age, treatment group, study, sex, baseline ICS use, baseline airflow limitation severity and smoking status as covariates.
Results
Patients
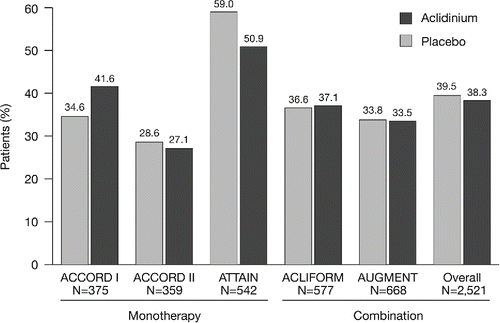
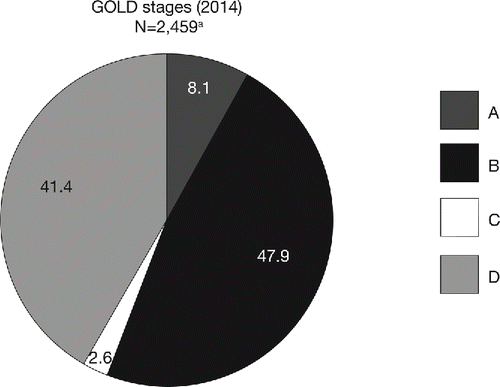
In total, 2,521 patients were included in the pooled analysis (ITT population). Patient demographics and baseline characteristics were similar across the five studies, although ATTAIN and ACLIFORM had a higher proportion of male patients and AUGMENT had fewer patients with ≥1 exacerbation in the 12 months prior to study entry (). Overall, 28.3% of patients had experienced ≥1 exacerbation in the previous year (). The majority of patients were classified in GOLD Groups B (41.4%) and D (47.9%; ). Most patients in GOLD Group D were classified as such due to a FEV1 lower than 50% of the reference. ICS use was similar between treatment groups in each study. Overall, 39.5% of patients in the placebo group and 38.3% of patients in the aclidinium group were using concomitant ICS ().
Table 2. Patient demographics and baseline characteristics (ITT population).
Figure 1. Pooled analysis population, stratified by GOLD group (ITT population). aPatients had FEV1 30–80% predicted. There were 62 patients with insufficient data for GOLD classification. ITT, intent-to-treat; GOLD, Global initiative for chronic Obstructive Lung Disease; N, number of patients in aclidinium 400 μg and placebo groups.
Number of exacerbations
In the pooled analysis, 12.5% of patients in the aclidinium group and 15.7% of patients in the placebo group had ≥1 exacerbation of any severity; the odds of a patient experiencing ≥1 exacerbation of any severity were reduced with aclidinium 400 μg versus placebo (odds ratio [OR] = 0.78, p = 0.039; ). In addition, in four of the five individual studies, the proportion of patients who experienced ≥1 COPD exacerbation of any severity was greater in the placebo group than in the aclidinium group (). Similarly, the proportion of patients who experienced ≥1 moderate-to-severe exacerbation was numerically lower with aclidinium compared with placebo in each study; however, these results did not reach statistical significance at the study level or overall ().
Table 3. Patients with ≥1 exacerbation.
Rate of exacerbations
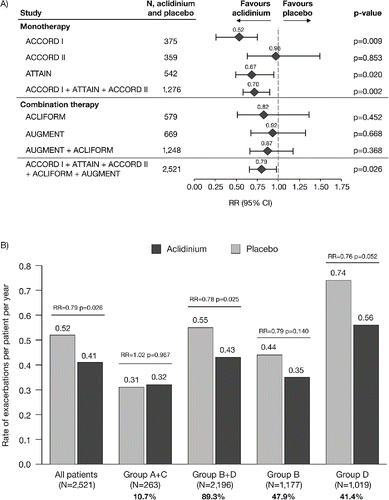
In the pooled data, aclidinium reduced the rate of exacerbations of any severity compared with placebo (rate ratio [RR] = 0.79, p = 0.026; ). By GOLD group, the reduction in the rate of exacerbations with aclidinium compared with placebo was greatest in GOLD Groups B and D () and a rate reduction was also observed with aclidinium in GOLD Group D and GOLD Group B+D (Group D: RR = 0.76, p = 0.052; Group B+D: RR = 0.78, p = 0.025; ). Results for Groups A and C are not shown individually due to the low number of patients and non-convergence of the statistical model. Additionally, the rate of exacerbations of any severity was very low in Groups A and C (placebo: 0.20 and 0.05 exacerbations per patient per year, respectively) compared with Groups B and D (placebo: 0.44 and 0.74 exacerbations per patient per year, respectively).
Figure 3. Rate of COPD exacerbations of any severity (A) overall (individual studies and pooled analyses)a and (B) stratified by GOLD group (pooled analysis). aExacerbations analyses from the individual ACLIFORM and AUGMENT studies are reported for the ITT-exacerbations populations of the aclidinium monotherapy and placebo arms only (579 and 669 patients, respectively; all patients who received at least one dose of the study medication), as specified in the study protocols and published previously (Citation20,32); pooled analyses include data from the ITT populations (577 and 668 patients, respectively; all patients who received at least one dose of the study medication and had a baseline and ≥1 post-baseline FEV1 assessment) for consistency with the ACCORD I, ACCORD II and ATTAIN study populations. Percentages are the proportion of all GOLD-classified patients (N = 2,459) in each GOLD group. CI, confidence interval; COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; ITT, intent-to-treat; RR, rate ratio; N, number of patients in aclidinium 400 μg and placebo groups.
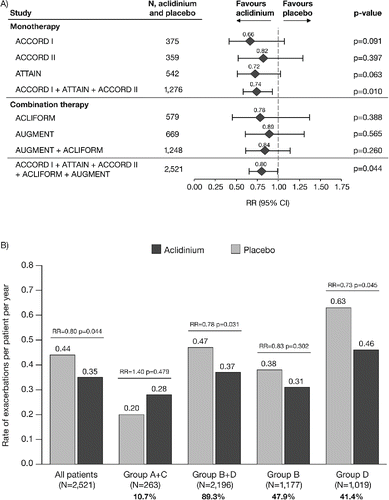
The data for moderate-to-severe exacerbations followed a similar pattern to the data for exacerbations of any severity, with a reduction in exacerbation rate with aclidinium compared with placebo (RR = 0.80, p = 0.044; ). presents data for the rate of moderate-to-severe exacerbations stratified by GOLD group. The reduction in rate of moderate-to-severe exacerbations with aclidinium versus placebo was greatest in GOLD Group D (RR = 0.73, p = 0.045) and there was a reduction in GOLD Group B+D (RR = 0.78, p = 0.031). Again, the data for Groups A and C are not presented individually for the reasons stated above. The rate of moderate-to-severe exacerbations in Groups B and D was higher than in Groups A and C (placebo rates: 0.38 and 0.63 exacerbations per patient per year vs. 0.10 and 0.01 exacerbations per patient per year, respectively).
Figure 4. Rate of moderate-to-severe COPD exacerbations (A) overall (individual studies and pooled analyses)a and (B) stratified by GOLD group (pooled analysis). aExacerbations analyses from the individual ACLIFORM and AUGMENT studies are reported for the ITT-exacerbations populations of the aclidinium monotherapy and placebo arms only (579 and 669 patients, respectively; all patients who received at least one dose of the study medication), as specified in the study protocols and published previously (Citation20,32); pooled analyses include data from the ITT populations (577 and 668 patients, respectively; all patients who received at least one dose of the study medication and had a baseline and ≥1 post-baseline FEV1 assessment) for consistency with the ACCORD I, ACCORD II and ATTAIN study populations. Percentages are the proportion of all GOLD-classified patients (N = 2,459) in each GOLD group. CI, confidence interval; GOLD, Global initiative for chronic Obstructive Lung Disease; ITT, intent-to-treat; RR, rate ratio; N, number of patients in aclidinium 400 μg and placebo groups.
When stratified by concomitant ICS use, exacerbation rates were numerically higher in patients who were using ICS compared with those who were not using ICS (). In addition, there was a reduction in exacerbation rate with aclidinium 400 μg versus placebo for exacerbations of any severity (p < 0.026) and moderate-to-severe exacerbations in the overall patient group (p < 0.044), but not those with concomitant ICS use or those without concomitant ICS use when considered separately ().
Table 4. Exacerbation rates stratified by concomitant ICS use (ITT population).
Time to first exacerbation
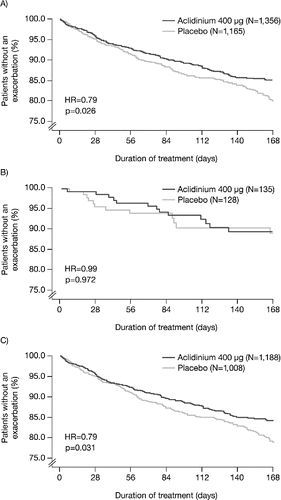
Aclidinium increased the time to first exacerbation of any severity compared with placebo overall (hazard ratio [HR] = 0.79, p = 0.026; ) and for patients in GOLD Group B+D (HR = 0.79, p = 0.031; ) but not GOLD Group A+C (HR = 0.99, p = 0.972; ). The changes in time to first moderate-to-severe exacerbation with aclidinium versus placebo followed a similar pattern (all patients: HR = 0.82, p = 0.081; Group B+D: HR = 0.81, p = 0.073; Group A+C: HR = 1.37, p = 0.511).
Figure 5. Time to first COPD exacerbation of any severity in (A) all patients; (B) patients in GOLD Groups A and C; and (C) patients in GOLD Groups B and D (ITT population). COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; HR, hazard ratio; ITT, intent-to-treat; N, number of patients.
Discussion
This pooled analysis shows that aclidinium 400 μg BID reduces the frequency of exacerbations in patients with COPD with moderate-to-severe airflow limitation. Specifically, the number of exacerbations of any severity was reduced with aclidinium compared with placebo (p = 0.039), as was the annual rate of both moderate-to-severe exacerbations (p = 0.044) and exacerbations of any severity (p = 0.026). Additionally, aclidinium 400 μg delayed the time to first exacerbation of any severity in all patients (p = 0.026); however, the delay in the time to first moderate-to-severe exacerbation did not reach statistical significance. Finally, the reduction in exacerbation frequency was most apparent in GOLD Groups B and D (i.e. patients with more symptoms), although it should be noted that patient numbers in Groups A and C were low (10.7% of the total population).
In this pooled analysis, aclidinium 400 μg reduced the rate of exacerbations of any severity and moderate-to-severe exacerbations by approximately 20% compared with placebo. These results are consistent with recent studies of other LAMAs. For example, the 26-week GLOW 1 study and the 52-week GLOW 2 study demonstrated reduced rates of exacerbations of 28–34% with glycopyrrolate 50 μg once daily (QD) versus placebo Citation(13). The 4-year UPLIFT trial showed a 14% reduction in rate of exacerbations with tiotropium 18 μg QD versus placebo (although it should be noted that LABAs and ICS were allowed as concomitant medication) Citation(12), in contrast to most short-term studies. Similar to the GLOW and UPLIFT studies, our study populations were not enriched for exacerbations, and although reductions in the rate of exacerbations were observed with aclidinium versus placebo, treatment effects may be greater in a population with a history of exacerbations. Indeed, the highest rates of exacerbation were observed in symptomatic patients (GOLD Groups B and D) and it was these patients who displayed the largest reductions in exacerbation rates with aclidinium versus placebo. The 1-year MISTRAL study included a patient population enriched for exacerbations and found a 35% reduction in rate of exacerbations with tiotropium 18 μg QD versus placebo Citation(25). Greater reductions in exacerbation rates have also been demonstrated with LAMAs compared with LABAs in patient populations enriched for exacerbations (Citation14,15). Similarly, in the present analysis, aclidinium significantly reduced exacerbations of any severity in patients with concomitant ICS use but not patients without concomitant ICS use, and this may be due to the higher exacerbation rate in patients who are prescribed ICS.
In addition to the effects on exacerbation rates, we observed a delay in time to first exacerbation of any severity with aclidinium versus placebo and a delay in time to first moderate-to-severe exacerbation. Again, these results are consistent with previous studies with other LAMAs (Citation12,13); however, it should be noted that comparing the treatment effects in our pooled analysis with those observed in other studies can be problematic due to major differences in study design (e.g. differences in patient population, treatment duration, allowed concomitant medications and exacerbation endpoints).
Investigating the effects of treatment on exacerbations according to the new GOLD classification may be useful for identifying differences in response in different GOLD groups and thus predicting which patients will respond better to a particular therapy. However, our sub-group analysis is limited by the low patient numbers and rate of exacerbations in GOLD Groups A and C. The distribution of patients across GOLD groups will depend on the methods used for GOLD classification (i.e. modified Medical Research Council vs. COPD Assessment Test vs. SGRQ and airflow limitation vs. exacerbation history vs. both) (Citation26–29). The COPDgene study used the same classification parameters (SGRQ, airflow limitation and exacerbation history) as our pooled analysis and reported a higher proportion of patients in Group A (29.4% vs. 8.1%), a lower proportion in Group B (24.7% vs. 47.9%) and a similar proportion in Groups C (4.9% vs. 2.6%) and D (41.0% vs. 41.4%) Citation(23). These results suggest that a greater proportion of patients had an SGRQ score ≥25 in our pooled analysis compared with the COPDgene study, possibly a consequence of different inclusion criteria (patients with moderate-to-severe vs. any severity of airflow limitation) or geographical variations in SGRQ due to real or perceived differences in health status (our pooled analysis included study sites in North America, Europe, Africa and the Asia-Pacific region; the COPDgene study was conducted in North America) Citation(23).
The mechanisms by which LAMAs may help prevent COPD exacerbations are not well understood Citation(30). Various mechanisms have been proposed, including improved lung mechanics and bronchodilation, reduced lung hyperinflation, reduced airway resistance, improved inspiratory capacity, reduced sputum production, inhibition of viral activity in the lung, and direct and indirect effects on lung inflammation Citation(30).
Pooling of data from studies with similar designs can confirm trends that are observed/reveal trends that may not be evident in the individual studies, particularly when the event rate is low. In this analysis, pooling of data gave a sufficient number of patients in each GOLD group for exploratory sub-group analyses.
One potential limitation of this pooled analysis is the relatively short duration of the five studies. However, a recent systematic review of tiotropium versus placebo showed similar reductions in exacerbation rates in studies of <1 year (13 studies of 3–6 months and one study of 9 months) and studies of ≥1 year (six studies of 1 year, one study of 2 years and one study of 4 years) Citation(31), suggesting that shorter studies can still provide reliable estimates of treatment effect. Another potential limitation is the use of SGRQ for segregating GOLD classification groups as GOLD only uses the modified Medical Research Council dyspnoea scale, COPD assessment test or Clinical COPD Questionnaire for this purpose Citation(11).
Conclusions
The results of this pooled analysis add to the growing body of evidence that the use of a LAMA as maintenance therapy can help prevent exacerbations of COPD by demonstrating an effect within a relatively short period of time (3–6 months). In a population of patients with COPD that is not enriched for exacerbations, aclidinium 400 μg BID may reduce the frequency of exacerbations by approximately 20% compared with placebo. The treatment effect may have been greater in symptomatic patients because these patients had higher rates of exacerbation compared with non-symptomatic patients and reductions in rate are more likely to be observed in patients with more frequent exacerbations.
Declaration of interest
This pooled analysis was funded by Almirall S.A., Barcelona, Spain. Almirall S.A. designed and conducted the pooled analysis and reviewed the data. Almirall S.A. and AstraZeneca PLC were involved in review of the manuscript. Suzanne McAllister, PhD, and Richard Knight, PhD, of Complete Medical Communications (Macclesfield, UK), provided medical writing support funded by AstraZeneca PLC, Barcelona, Spain.
JAW has received research grants from GSK, Takeda, Almirall, Novartis, Johnson & Johnson and Vifor Pharma; honoraria for advisory boards and/or lectures from Almirall, Novartis, GSK, Boehringer Ingelheim, Takeda, Johnson & Johnson, Vifor Pharma, Napp, Pfizer and AstraZeneca. AA has received research grants from Almirall, AstraZeneca, GSK, Menarini and MSD as well as honoraria for lectures or advisory boards from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, Novartis, Teva, Kyorin, Glenmark and Takeda. GD has received honoraria for advisory boards for Almirall and lecture fees from Micom SPr. FC is an employee of AstraZeneca PLC, Barcelona, Spain and former employee of Almirall S.A., Barcelona, Spain. RL is an employee of AstraZeneca PLC, Barcelona, Spain, and former employee of Almirall S.A., Barcelona, Spain. EGG is an employee of AstraZeneca PLC, Barcelona, Spain and former employee of Almirall S.A., Barcelona, Spain.
Acknowledgements
The authors would like to thank the study investigators at each of the participating centers for their contribution to the studies.
Notes
1. Registered trademark of AstraZeneca group of companies; for use within the USA as Pressair® and as Genuair™ within all other licensed territories.
References
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008;178:332–338.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–852.
- Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001;164:358–364.
- Vestbo J, Agusti A, Wouters EF, Bakke P, Calverley PM, Celli B, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med 2014;189:1022–1030.
- Jones PW, Lamarca R, Chuecos F, Singh D, Agusti A, Bateman ED, et al. Characterisation and impact of reported and unreported exacerbations: results from ATTAIN. Eur Respir J 2014;44:1156–1165.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–1422.
- Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Wang C, et al. Negative impacts of unreported COPD exacerbations on health-related quality of life at 1 year. Eur Respir J 2010;35:1022–1030.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman SP, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925–931.
- Wouters EF. Economic analysis of the confronting COPD survey: an overview of results. Respir Med 2003;97 Suppl C:S3–S14.
- Miravitlles M, Garcia-Polo C, Domenech A, Villegas G, Conget F, de la Roza C. Clinical outcomes and cost analysis of exacerbations in chronic obstructive pulmonary disease. Lung 2013;191:523–530.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]. [updated 2015; cited 2015 Mar 30]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Feb18.pdf.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–1554.
- Buhl R, Banerji D. Profile of glycopyrronium for once-daily treatment of moderate-to-severe COPD. Int J Chron Obstruct Pulmon Dis 2012;7:729–741.
- Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 2013;1:524–533.
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MPMH, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093–1103.
- AstraZeneca PLC. Summary of product characteristics Eklira Genuair 322 μg inhalation powder [Internet]. [updated 2015 Apr 28; cited 2015 May 7]. Available from: http://www.medicines.org.uk/emc/medicine/27001/.
- Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, de Miquel G, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J 2012;40:830–836.
- Kerwin EM, D'Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF, et al. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012;9:90–101.
- Rennard SI, Scanlon PD, Ferguson GT, Rekeda L, Maurer BT, Garcia Gil E, et al. ACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clin Drug Investig 2013;33:893–904.
- Singh D, Jones PW, Bateman ED, Korn S, Serra C, Molins E, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med 2014;14:178.
- Bateman E, Rennard S, Jones P, Molins E, Jin M, Leselbaum A. Effect of aclidinium bromide/formoterol fumarate fixed-dose combination (FDC) on exacerbations in moderate-to-severe COPD: Pooled analysis of two studies. Eur Respir J 2014;44:285.
- D'Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum AR, Caracta CF. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res 2014;15:123.
- Han MK, Muellerova H, Curran-Everett D, Dransfield MT, Washko GR, Regan EA, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med 2013;1:43–50.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease [Internet]. [updated 2014 Jan; cited 2014 Nov 12]. Available from: http://www.goldcopd.com/guidelines-global-strategy-for-diagnosis-management.html.
- Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J 2006;27:547–555.
- Zogg S, Durr S, Miedinger D, Steveling EH, Maier S, Leuppi JD. Differences in classification of COPD patients into risk groups A-D: a cross-sectional study. BMC Res Notes 2014;7:562.
- Kim S, Oh J, Kim YI, Ban HJ, Kwon YS, Oh IJ, et al. Differences in classification of COPD group using COPD assessment test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: a cross-sectional analyses. BMC Pulm Med 2013;13:35.
- Holt S, Sheahan D, Helm C, Tofield C, Corin A, Kocks JW. Little agreement in GOLD category using CAT and mMRC in 450 primary care COPD patients in New Zealand. NPJ Prim Care Respir Med 2014;24:14025.
- Agusti A, Hurd S, Jones P, Fabbri LM, Martinez F, Vogelmeier C, et al. FAQs about the GOLD 2011 assessment proposal of COPD: a comparative analysis of four different cohorts. Eur Respir J 2013;42:1391–1401.
- Wedzicha JA, Decramer M, Seemungal TA. The role of bronchodilator treatment in the prevention of exacerbations of COPD. Eur Respir J 2012;40:1545–1554.
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;7:CD009285.
- Bateman ED, Chapman KR, Singh D, D'Urzo AD, Molins E, Leselbaum, A, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res 2015;16:92.