ABSTRACT
The purpose of this study was to systematically review the efficacy and safety of long-acting β-agonist/long-acting muscarinic antagonist (LABA/LAMA) and LABA/inhaled corticosteroid (ICS) combinations in patients with advanced chronic obstructive pulmonary disease (COPD). Randomized clinical trials of at least 12 weeks of duration comparing LABA/LAMA and LABA/ICS combinations were included. We chose forced expiratory volume in 1 second (FEV1), St. George's Respiratory Questionnaire (SGRQ) score, Transitional Dyspnea Index (TDI), COPD Assessment Test (CAT) score, COPD exacerbations, mortality, and other safety parameters as outcome assessment criteria. We included six randomized controlled trials with a total of 4,319 patients. Most patients did not have a history of exacerbation. LABA/LAMA was associated with greater improvement in FEV1 than LABA/ICS (mean difference (MD) 0.09L, 95%confidence interval (CI) 0.07 to 0.11L; high certainty). Two treatments appeared clinically equivalent in improving SGRQ (MD −0.12, 95%CI −1.16 to 0.92; high certainty), TDI (MD 0.15, 95%CI −0.05 to 0.35; high certainty), and CAT scores (MD 0.28 95%CI −0.29 to 0.85; moderate certainty). LABA/LAMA was associated with an absolute reduction of approximately 8% in the incidence of pneumonia compared with LABA/ICS (risk ratio 0.41, 95%CI 0.18 to 0.94; moderate certainty). There was no significant difference in safety and exacerbation outcomes. However, equivalence of two treatments could not be concluded due to imprecision especially for mortality, cardiac serious adverse events, and severe exacerbations. Our findings support the use of dual long-acting bronchodilators for patients with advanced COPD but without frequent exacerbations given the excess risk of pneumonia with LABA/ICS.
Introduction
In chronic obstructive pulmonary disease (COPD), inhaled therapies are used to improve lung function, symptoms, and quality of life and reduce COPD exacerbations. Short-acting bronchodilators are used on an as-needed basis to provide immediate relief, and long-acting bronchodilators are used for maintenance therapy in symptomatic patients with advanced disease Citation(1).
Global initiative for chronic obstructive lung disease (GOLD) recommends combined therapy in patients with moderate to very severe disease (GOLD stage II-IV) whose symptoms are not well controlled with a single long-acting bronchodilator. In this subpopulation of COPD, the addition of an inhaled corticosteroid (ICS) is recommended for those with frequent exacerbations (i.e., ≥2 exacerbations per year or one hospitalization for an exacerbation per year) and a second class of long-acting bronchodilator is recommended for those without frequent exacerbations Citation(2).
In real-world practice, a combination of long-acting β-agonist (LABA) and ICS has been widely used even for patients with milder form of COPD or without frequent exacerbations although the evidence for such subpopulations are less compelling (Citation3,4). In addition, there has been considerable concern that the use of ICS, alone or in combination with LABA, is associated with an increased risk of pneumonia, osteoporotic fracture, early-onset diabetes, cataracts, and even tuberculosis Citation(5).
A recent meta-analysis showed that LABA/long-acting muscarinic antagonist (LAMA) combinations were associated with improved lung function, quality of life, symptom scores, and reduced COPD exacerbations as compared with monotherapies Citation(6). There are conflicting data concerning whether addition of an ICS reduces COPD exacerbations when a patient is already on dual long-acting bronchodilators Citation(7). Data on the efficacy and safety of fixed-dose LABA/LAMA combinations are accumulating. However, an important clinical question is how the efficacy and safety of LABA/LAMA combinations compare with those of LABA/ICS combinations in patients with advanced disease.
The purpose of this study was to systematically review the efficacy and safety of LABA/LAMA and LABA/ICS combinations. We hypothesized that a greater bronchodilator effect of LABA/LAMA would translate into improved clinical outcomes when compared with LABA/ICS in advanced COPD.
Methods
Identification of trials
We identified all relevant clinical trials which evaluated the clinical efficacy and safety of LABA/LAMA versus LABA/ICS combinations in patients with advanced COPD. Two authors (YO and AVC) independently searched Ovid Medline database for studies published from 1946 to November 30, 2015 using the MeSH headings and keywords: randomized controlled trial AND Pulmonary Disease, Chronic Obstructive AND aclidinium, glycopyrronium, NVA237, tiotropium, umeclidinium or QVA149 AND indacaterol, formoterol, olodaterol, salmeterol, or vilanterol AND beclomethasone, budesonide, ciclesonide, flunisolide, fluticasone, mometasone, or triamcinolone. In addition, we searched Scopus, CINAHL, Google Scholar, ClinicalTrials.gov, and online trial registries of manufacturers of fixed-dose LABA/LAMA and LABA/ICS combination products. Bibliographies of all the selected articles and review articles which included information on LABA/LAMA versus LABA/ICS combinations in COPD were also reviewed for other relevant articles.
We included any randomized clinical trial, published or unpublished, evaluating a LABA/LAMA combination versus a LABA/ICS combination. Randomized control trials had to be of at least 12 weeks' duration. Both combinations had to be fixed dose (two-in-one inhaler). We chose change from baseline (CFB) in forced expiratory volume in 1 second (FEV1) in liter, CFB in St. George's Respiratory Questionnaire (SGRQ) score, Transitional Dyspnea Index (TDI), CFB in COPD Assessment Test (CAT) score, COPD exacerbation, mortality, total serious adverse event (SAE), cardiac SAE, and dropout due to adverse event (AE), as the outcome assessment criteria.
Data abstraction and quality assessment
Two authors (YO and AVC) independently screened studies by title and abstract to evaluate whether a trial met the inclusion criteria. We extracted data on COPD exacerbations as moderate and severe. Moderate was generally defined as “worsening respiratory status which required treatment with systemic corticosteroids and/or antibiotics” and severe as “rapid deterioration which required hospitalization” or COPD SAE which was defined as COPD-related death, life-threatening deterioration, hospitalization, or other serious events requiring treatment in an emergency room Citation(8). Data were abstracted on study design, study size, population, severity of illness, and the end points of interest. Disagreements regarding values or analyses were resolved by discussion.
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system to assess the quality of evidence as it related to the studies that contributed data to the meta-analysis Citation(9). Certainty of evidence takes into consideration the study design; risk of bias (adequacy of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other bias); precision; consistency; directness of the evidence; and publication bias.
We were not able to pool more than 10 trials, so the presence of small study bias was considered when the number of patients was less than 50 per study, 1,000 per pooled analysis, or 100 per arm (Citation10,11). Heterogeneity was assessed using visual inspection of the results, a test for heterogeneity, and the I2 statistic (I2 > 30% was considered substantial heterogeneity) Citation(12). We used optimal information size calculations as an objective measure of imprecision for grading evidence, with an α of 0.05 and a β of 0.80 Citation(13).
Data analysis
Mortality, SAEs, dropouts due to adverse event, pneumonia, and COPD exacerbations were dichotomous variables. FEV1 and quality of life and symptom scores were continuous variables. The data analysis was performed using meta-analysis software (Review Manager 5.3.5, Cochrane Collaboration, London, England). The results were expressed as Mantel–Haenszel risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes, along with their 95% confidence intervals (CIs). We used random-effects models for all analyses. We conducted a priori sensitivity analyses by excluding studies at high risk of bias for variation of outcome measurements as well as using a fixed-effects model.
Results
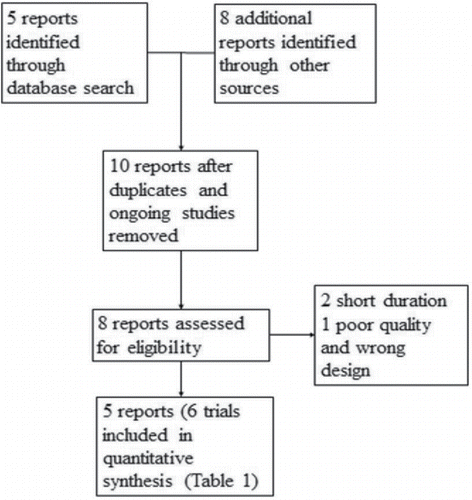
The electronic database searches identified five citations and we found eight reports from other sources. Five studies were excluded for duplicates and ongoing trials. Two studies were excluded on more detailed review. We included 6 randomized controlled trials with a total of 4,319 patients in which 2,159 were randomized to a LABA/LAMA combination and 2,160 randomized to a LABA/ICS combination () (Citation14–18).
Characteristics of included studies are summarized in . The duration of studies ranged from 12 to 26 weeks. Included studies were conducted across the world including Europe, North and South Americas, South Africa, and East Asia. Sample sizes ranged from 522 to 933. The mean age ranged from 61.6 to 65.0 years. The proportion of male patients and current smokers ranged from 65% to 91% and 26% to 59%. The mean baseline post-bronchodilator FEV1 ranged from 1.34 to 1.70 L. Post-bronchodilator FEV1 percent predicted ranged from 49% to 60%.
Table 1. Study characteristics of included trials.
Fixed-dose LABA/LAMA combinations included aclidinium/formoterol, indacaterol/glycopyrronium, and umeclidinium/vilanterol. Fluticasone/salmeterol combination was used in all studies as a LABA/ICS combination. Short-acting β-agonists were allowed for rescue treatment in all studies. All trials excluded patient with asthma, a concomitant lung disease, and a significant cardiac disease. All studies except for one excluded patients with long-term oxygen therapy Citation(15). All studies were sponsored by a pharmaceutical company. The involvement of the sponsor in the data interpretation and preparation of manuscripts was unclear in one trial Citation(14) but the sponsor was probably involved in all studies.
All trials had low risk of bias except for TDI outcome in one study Citation(14) (Appendix Table 1). The lost to follow-up rate ranged from 6% to 17% and two trials had attrition greater than 15% (Citation14,16). An intention-to-treat or a full analysis set analysis was conducted in all trials except for one study for TDI in which per-protocol population was used Citation(14). The per-protocol population contributed 23% of a pooled analysis for TDI. Reasons for non-completion of study were provided and were balanced between two groups in all trials. A sensitivity analysis was performed for TDI excluding the study which used per-protocol population and the results were unchanged. Small study bias was considered unlikely given all included trials had a sample size of at least 400 patients and each arm of all included comparisons had at least 200 patients (Citation10,11). One study did not report SGRQ scores but selective reporting was considered unlikely given all included trials were registered with ClinicalTrials.gov. GRADE evidence profile and summary of findings are presented in
Table 2. GRADE evidence profile and summary of findings: LABA/LAMA versus LABA/ICS combinations for patients with COPD.
Six trials reported CFB in FEV1 including a total of 4,077 patients. Four reported CFB in trough FEV1 (Citation15,17–18), one reported CFB in peak FEV1 Citation(14), and the other reported CFB in FEV1 area under curve 0–12 hours Citation(16) (FEV1 was measured at pre-dose and at several serial time points after the treatment. The area under curve was then calculated from the time–FEV1 curve. Appendix Table 2). LABA/LAMA combinations were associated with a greater increase of FEV1 compared with LABA/ICS arm (MD 0.09 [95% CI, 0.07–0.11L]; I2 = 0%; high certainty; ). The point estimate was less than the established minimal clinically important difference of 100 mL Citation(19) but its CI exceeded the minimal clinically important difference cut-off (Appendix Figure 1).
Figure 2. Summary effects of LABA/LAMA combination versus LABA/ICS on changes in FEV1 (L). FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist.
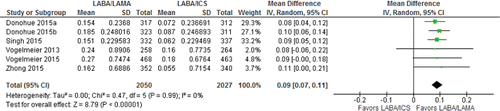
Six trials reported moderate-to-severe exacerbations including a total of 4,319 patients. One sixty five of 2,159 patients (7.6%) in the LABA/LAMA arm had a moderate-to-severe exacerbation as compared with 191 of 2,160 patients in the LABA/ICS arm (RR 0.87 [95%CI 0.68–1.11] I2 = 22%; moderate certainty; and Appendix Table 3). Eleven fewer patients per 1,000 would have a moderate or severe exacerbation on LABA/LAMA compared with LABA/ICS, but the CIs ranged from 10 more to 28 fewer. The results were similar in severe exacerbations (RR 0.80 [95%CI 0.37–1.75]; I2 = 41%; low certainty; and Appendix Table 3). LABA/LAMA may be associated with 3 fewer severe exacerbations per 1,000 compared with LABA/ICS, but the CIs ranged from 10 to 12. The effect was too imprecise to determine whether one treatment reduced exacerbations more than the other especially for severe exacerbations.
Figure 3. Summary effects of LABA/LAMA combination versus LABA/ICS on COPD exacerbations (A: moderate-to-severe; B: severe). ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist.
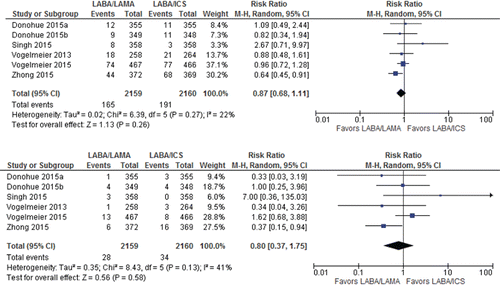
CFB in SGRQ, TDI, and CFB in CAT scores were reported in 5, 6, and 4 trials including 3,127, 4,091, and 2,686 patients, respectively (Appendix Table 2). LABA/LAMA arm was associated with slightly greater improvement in CFB in SGRQ and TDI but lesser improvement in CFB in CAT compared with LABA/ICS arm (CFB in SGRQ: MD −0.12, [95%CI −1.16 to 0.92]; I2 = 0%. TDI: MD 0.15, [95%CI −0.05 to 0.35]; I2 = 0%. CFB in CAT: MD 0.28 [95%CI −0.29 to 0.85]; I2 = 0%; ). The effect and its CIs were not in the range of established minimal clinically important difference [4 points for SGRQ Citation(19), 1 point for TDI Citation(19), and 2 points for CAT Citation(20)], and crossed the line of no effect, so the difference between the treatments was unlikely to be of clinical significance. The evidence was rated to be of high quality in these outcomes except for CFB in CAT wherein certainty of evidence was downgraded to moderate due to suboptimal sample size (Appendix Table 4).
Figure 4. Summary effects of LABA/LAMA combination versus LABA/ICS on health status (A: change from baseline in SGRQ; B: TDI; C: change from baseline in CAT). CAT, COPD assessment test; ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; SGRQ, St. George's Respiratory Questionnaire; TDI, transitional dyspnea index.
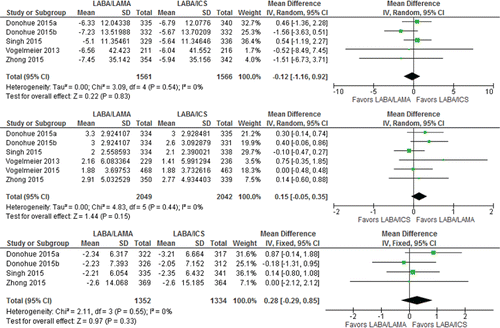
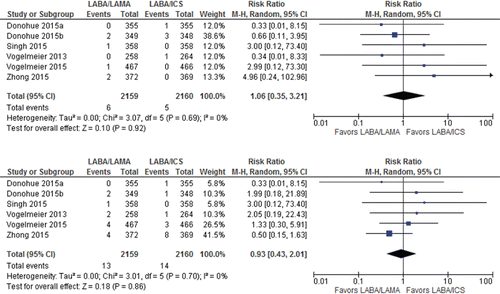
The CIs were too wide to determine whether mortality (RR 1.06 [95%CI, 0.35–3.21] 4,319 patients; 6 trials; I2 = 0%) and cardiac SAEs (RR 0.93 [95%CI 0.43–2.01] 4,319 patients; 6 trials: I2 = 0%) were more likely with LAMA/LABA or LABA/ICS () because fewer events occurred in the trials (a total of 11 and 27 patients for mortality and cardiac SAEs, respectively, Appendix Table 4). The estimate suggested an equal number of patients would die or have cardiac SAEs on LABA/LAMA or LABA/ICS, but the CIs ranged from 2 to 5 people per 1,000 in mortality or 4 to 6 per 1,000 in cardiac SAEs. Therefore, certainty of evidence was rated down two levels too low for both outcomes ().
Figure 5. Summary effects of LABA/LAMA combination versus LABA/ICS on (A) mortality and (B) cardiac SAEs. ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; SAE, serious adverse event.
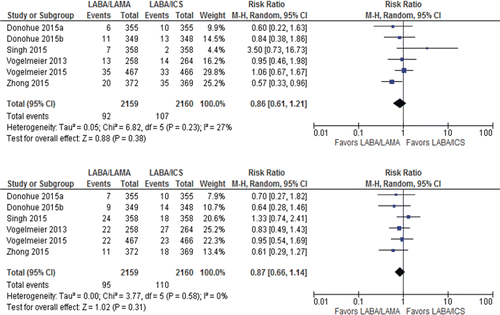
Less patients on LABA/LAMA had a serious adverse event or dropped out of study due to AE than patients on LABA/ICS, but the difference was not statistically significant (total SAEs: RR 0.86 [95%CI 0.61–1.21] 4,319 patients; 6 trials; I2 = 27%; moderate certainty. Dropouts due to AE: RR 0.87 [95%CI 0.66–1.14] 4,319 patients; 6 trials; I2 = 0%; moderate certainty, ). The CIs were relatively tight but not so much that equivalence of the two treatments could be concluded.
Figure 6. Summary effects of LABA/LAMA combination versus LABA/ICS on (A) total SAEs and (B) dropouts due to adverse event. ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; SAE, serious adverse event.
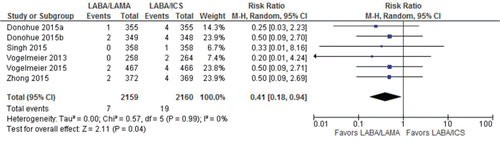
All included trials (4,319 patients) evaluated risks for pneumonia. Results showed a statistically significant reduction in the risk for pneumonia with LABA/LAMA compared with LABA/ICS (RR, 0.41 [95%CI, 0.18–0.94]; I2 = 0%— and Appendix Table 3). The estimate suggested 84 fewer patients per 1,000 [95%CI, 9 fewer to 117 fewer] would have pneumonia on LABA/LAMA compared to LABA/ICS when incidence of pneumonia was estimated to be 143 per 1,000 patient-years while on an ICS/LABA for COPD Citation(21). Certainty of evidence was rated down one level to moderate because of a small number of events (26 total, ).
Figure 7. Summary effects of LABA/LAMA combination versus LABA/ICS on pneumonia. ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist.
A subgroup analysis of severe exacerbations comparing type of LABA/LAMA formulations showed a statistically significant difference in effect favoring indacaterol/glycopyrronium over other LABA/LAMA formulations (test for subgroup differences: I2 = 65.7%, p-value = 0.05; Appendix Figure 2.3). A subgroup analysis assessing variation of FEV1 measurements and the doses (moderate vs. high dose) of ICS component in the incidence of pneumonia had no evidence of significant subgroup differences (Appendix Figures 3 and 4). Two trials allowed a history of COPD exacerbation within a year prior to enrollment and four others did not. The exacerbation history did not show a significant interaction in any of the outcomes (Appendix Figure 5). Sensitivity analyses using a fixed-effects model did not affect the results. One trial used a per-protocol population for TDI Citation(14) but this population was kept in the pooled analysis because the results were unchanged when analyzed without it (Appendix Figure 6).
Discussion
We included 6 randomized controlled trials with a total of 4,319 patients because most of them did not have a COPD exacerbation within a year prior to enrollment. Our findings showed with high certainty that LABA/LAMA was associated with greater improvement in FEV1 than LABA/ICS. It appeared highly certain that two treatments were clinically equivalent in improving SGRQ and TDI scores and moderately certain in improving CAT scores. LABA/LAMA was associated with an absolute reduction of approximately 8% in the incidence of pneumonia with a corresponding number needed to treat of 12 to prevent one case of pneumonia as compared with LABA/ICS (moderate certainty).The possibility of increased risk of pneumonia with an ICS-containing regimen was also suggested in previous studies (Citation22,23).
The subgroup analysis suggested a significant reduction in severe exacerbations with indacaterol/glycopyrronium compared with LABA/ICS (RR 0.37 [95% CI 0.16–0.87]; Appendix Figure 2.3) while other LABA/LAMA combinations did not show such a reduction. Although it is possible that indacaterol/glycopyrronium is superior to other LABA/LAMA formulations in reducing severe exacerbations, we believe that the observed effect is probably spurious given the result was based on between-study rather than within-study comparisons and infrequent event rates and there was no evidence of significant subgroup differences in other outcomes (Appendix Figure 2) Citation(22).
Our findings are in keeping with the current GOLD strategy which recommends dual long-acting bronchodilators rather than adding an ICS in patients with advanced COPD. The symptoms in such patients are not well-controlled with a single long-acting bronchodilator especially for those without frequent exacerbations given the equivalent efficacies on symptom control and quality of life scores between two treatments and the possibility of increased risk of pneumonia with an ICS-containing regimen which is also suggested in previous studies (Citation23,24).
It is noteworthy that fluticasone/salmeterol was the only LABA/ICS combination used in the included trials because an excess risk of pneumonia was not observed with a budesonide-containing regimen in a meta-analysis Citation(25). However, it remains to be seen whether an intraclass difference exists among ICS formulations with regard to the excess risk of pneumonia Citation(26). An ICS-containing regimen should probably be reserved for patients with advanced COPD with frequent exacerbations or with an asthmatic component as suggested in a recent review Citation(27). This notion is further supported by the current analysis which included both efficacy and safety outcomes with the strength of GRADE ratings Citation(9).
Our review has limitations. First, the duration of included studies was 6 months or less. A longer duration may affect the point estimates and their precisions. Two 52-week trials are currently ongoing comparing one combination each of LABA/LAMA and LABA/ICS (Citation28,29). In our recent meta-analysis comparing LABA/LAMA combinations with monotherapies, the point estimates of treatment effect did not seem to be affected by the duration of studies which would make it less likely that the inclusion of ongoing 52-week trials would significantly affect the treatment effects although they may enhance the precisions of treatment effects when analyzed with greater sample sizes and numbers of events.
Second, there were some variations in the measurements of FEV1. The subgroup analysis in CFB in FEV1 did not show significant interaction among the different FEV1 measurements (i.e., peak, trough, and area under curve 0–12 hours). We assumed that a bias, if there is any, would be minimal because the outcome measures were not absolute FEV1 values but the differences between the baseline and at the end of studies. We did not rate down for this difference because visual inspection of the forest plot showed consistent point estimates from trial to trial ranging within 30 mL from each other.
Third, we assumed a class effect for both LABA/LAMA and LABA/ICS combinations. Fluticasone/salmeterol was the only LABA/ICS combination and three different LABA/LAMA combinations were used (i.e., aclidinium/formoterol, indacaterol/glycopyrronium, and umeclidinium/vilanterol) in the included trials leaving the optimal choice of agent open to question. Subgroup analyses for type of LABA/LAMA combinations showed no evidence of interaction except for the possibility of superior effect of indacaterol/glycopyrronium in reducing severe exacerbations but the observed effect is probably spurious for the reasons described above. One way to better assess intraclass effects would be including more clinical trials using a network meta-analysis with a careful assessment of transitivity. We are currently conducting such an analysis which could not only examine the intraclass effects but also improve the inference for outcomes with a small number of events (e.g., mortality, severe exacerbations, and cardiac SAEs).
Forth, most studies excluded patients with a history of COPD exacerbation within a year prior to enrollment. Application of our findings to such a population is therefore questionable, although our subgroup analysis showed no evidence of interaction for all outcomes between trials which included or excluded patients with a history of COPD exacerbation (Appendix Figure 5). The above-mentioned 52-week trials (Citation28,29) are recruiting only those with a history of exacerbation which would shed light on patients at a higher risk of COPD exacerbation for which the GOLD recommends an ICS-containing regimen Citation(2).
Conclusions
Our findings support the current COPD strategy which recommends dual long-acting bronchodilators rather than adding an ICS for patients with advanced disease whose symptoms are not well-controlled with a single long-acting bronchodilator, but without frequent exacerbations given the excess risk of pneumonia with an ICS containing regimen. Further validations are needed to determine if a LABA/ICS combination is preferred over a LABA/LAMA combination for those with repeated exacerbations.
Data_Supplement.docx
Download MS Word (577.7 KB)References
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012; 379(9823):1341–1351. Epub 2012/02/09.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–65. Epub 2012/08/11.
- Price D, West D, Brusselle G, Gruffydd-Jones K, Jones R, Miravitlles M, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis 2014; 9:889–904. Epub 2014/09/12.
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 9:CD006829. Epub 2012/09/14.
- Ernst P, Suissa S. Systemic effects of inhaled corticosteroids. Curr Opin Pulm Med 2012; 18(1):85–89. Epub 2011/11/25.
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax 2015; 71(1): 15–25. Epub 2015/10/23.
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014; 371(14):1285–1294. Epub 2014/09/10.
- Halpin DM, Decramer M, Celli B, Kesten S, Leimer I, Tashkin DP. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-source UPLIFT(R) trial. Lung 2011; 189(4):261–268. Epub 2011/06/17.
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64(4):380–382. Epub 2010/12/28.
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013; 346:f2304. Epub 2013/04/26.
- Nuesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010; 341:c3515. Epub 2010/07/20.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414):557–560. Epub 2003/09/06.
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 2011; 64(12):1283–1293. Epub 2011/08/16.
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten A, et al. Efficacy of aclidinium/formoterol fixed-dose combination versus salmeterol/fluticasone in COPD. Eur Respir J 2015; 46(suppl 59):PA2960.
- Zhong N, Wang C, Zhou X, Zhang N, Humphries M, Wang L, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis 2015; 10:1015–1026. Epub 2015/06/18.
- Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med 2013; 1(1):51–60. Epub 2013/12/11.
- Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med 2015; 109(7):870–881. Epub 2015/05/27.
- Singh D, Worsley S, Zhu CQ, Hardaker L, Church A. Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm Med 2015; 15:91. Epub 2015/08/20.
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014; 189(3):250–255. Epub 2014/01/05.
- Kon SS, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med 2014; 2(3):195–203. Epub 2014/03/14.
- Janson C, Larsson K, Lisspers KH, Stallberg B, Stratelis G, Goike H, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS). BMJ 2013; 346:f3306. Epub 2013/05/31.
- Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010; 340:c117. Epub 2010/04/01.
- Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008; 300(20):2407–2416. Epub 2008/11/27.
- Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med 2009; 169(3):219–229. Epub 2009/02/11.
- Sin DD, Tashkin D, Zhang X, Radner F, Sjobring U, Thoren A, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet 2009; 374(9691):712–719. Epub 2009/09/01.
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 3:CD010115. Epub 2014/03/13.
- Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. Eur Respir J 2015; 45(2):525–537. Epub 2014/12/30.
- Novartis Pharmaceuticals. QVA vs. Salmeterol/Fluticasone, 52-week Exacerbation Study. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT01782326 (accessed 21 December 2015).
- GlaxoSmithKline. A Study Comparing the Efficacy, Safety and Tolerability of Fixed Dose Combination (FDC) of FF/UMEC/VI With the FDC of FF/VI and UMEC/VI; Administered Once-daily Via a Dry Powder Inhaler (DPI) in Subjects With Chronic Obstructive Pulmonary Disease (COPD). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT02164513 (accessed 21 December 2015).