Abstract
The development of adhesion bonds, either among cells or among cells and components of the extracellular matrix, is a crucial process. These interactions are mediated by some molecules collectively known as adhesion molecules (CAMs). CAMs are ubiquitously expressed proteins playing a central role in controlling cell migration, proliferation, survival, and apoptosis. Besides their key function in physiological maintenance of tissue integrity, CAMs play an eminent role in various pathological processes such as cardiovascular disorders, atherogenesis, atherosclerotic plaque progression and regulation of the inflammatory response. CAMs such as selectins, integrins, and immunoglobulin superfamily take part in interactions between leukocyte and vascular endothelium (leukocyte rolling, arrest, firm adhesion, migration). Experimental data and pathologic observations support the assumption that pathogenic microorganisms attach to vascular endothelial cells or sites of vascular injury initiating intravascular infections. In this review a paradigm focusing on cell adhesion molecules pathophysiology and infective endocarditis development is given.
INTRODUCTION
During the last decade, there is enough scientific information in the literature supporting the idea of an important role for cell adhesion molecules in normal human pathophysiological mechanisms as well as in human diseases (CitationEidelman 1991, CitationCharalabopoulos 2001, CitationGolias 2005, CitationSkubitz 2002, CitationCharalabopoulos 2004). In multicellular organisms primary tissue composition during embryogenesis, tissue growth and their physiologic function as well as their lifetime architectural preservation is thoroughly controlled by a set of reactions either between cells (cell-cell) or between cells and extracellular matter (cell-matrix). The adherence of cells to each other, their extracellular matrices and endothelial surfaces is mediated by a variety of membrane proteins collectively known as cell adhesion molecules (CAMs).
The adhesion molecules are distinguished according to this property into mediators of cell to cell reactions (cell-cell adhesion molecules, CAMs) and mediators of cell to extracellular matrix reactions (cell-subsrtatum adhesion molecules, SAMs). Moreover there are adhesion molecules that can mediate both types of reactions functioning both as SAMs and CAMs. Thus, adhesion is a vital property of cells. In general it provides a stable environment for cell growth and differentiation and allows cells to migrate. The interaction between cells and their extracellular matrices is also an important factor in the regulation of further protein deposition. Likewise, matrix proteins can influence cellular function thus creating a complex feedback mechanism. CAMs are responsible for those cellular interactions belonging to a complex mechanism which come into play at the receptors on the cell surface. In this mechanism apart from cell adhesion molecules, many other soluble cell mediators like cytokines and components of the tissue matrix like fibronectin, collagen, etc. play a crucial role (CitationMousa 2008, CitationPozzi 2003, CitationEl Hariry 1997).
Nowadays, a large number of adhesion molecules-more than one hundred- have been identified functioning as SAMs or CAMs or both. Their structure, molecular genetic profile, functional characteristics and biochemical role is fully elucidated to the bibliography. Cell adhesion molecules are substances with a protein character expressed on the cell surface of all tissues. They function as receptors that trigger intracellular pathways and participate in the control of basic vital processes such as embryogenesis, migration, cellular growth and differentiation, cell death, ensuring the interaction of cells with the environment (CitationGolias 2007, CitationRojas 1999). Specifically, adhesion molecules are membrane receptors that mediate several interactions, recognized to play a major role in a variety of normal and pathological phenomena related with traffic and interactions between cells, cell-matrix contact and determining, furthermore, the specificity of cell-cell binding (CitationRojas 1999, CitationJaitovich 2004, CitationCharalabopoulos 2002, CitationBatistatou 2006). A variety of recently identified glycoproteins have been implicated in cell-cell interactions that are critical for normal homeostasis, immune surveillance, and vascular wall integrity. These CAMs are known to mediate blood cell (leukocyte, platelet)-endothelial cell interactions that can occur in all segments of the microvasculature under certain physiological (e.g., homeostasis) and pathological (e.g., inflammation, immune responses, cancer) conditions (CitationKrieglstein 2001, CitationObene-Abuakwa 2000, CitationPafilis 2007, CitationBatistatou 2006, CitationKyzas 2006). From the immunological point of view they are involved in virtually every process of cell interactions, involving thymic selection and antigen priming, antigen recognition and cell activation, cytotoxicity and lymphocyte recirculation (CitationHorvathova 2000). On the other hand, in the development of inflammation, adhesion molecules play an essential role. In general, cytoadhesion molecules play an important role in the pathophysiology of cardiovascular, neoplastic, infectious and skin diseases. Some cardiovascular diseases are associated with pathological impairment of the structure and function of endothelial cells with appearance endothelial dysfunction (CitationBorowska 2006). Particularly, cell adhesion molecules are ubiquitously expressed proteins playing a central role in controlling cell migration, proliferation, survival, and apoptosis. Besides their key function in physiological maintenance of tissue integrity, adhesion molecules play an eminent role in various pathological processes involved in endothelial dysfunction and activation processes. In cardiovascular disorders, cell adhesion molecules are particularly involved in atherogenesis and atherosclerotic plaque progression, myocardial infarction and reperfusion damage, allograft vasculopathy, myocarditis, hypertrophic myocardiopathy and a minor role in valvular stenosis and cardiomyopathy etc (CitationJaitovich 2004, CitationHope 2003).
At present, the main classes of cytoadhesion molecules known are integrins, cadherins, selectins, members of the immunoglobulin gene superfamily (IgSF), and CD44 (CitationGeorgolios 2005, CitationGeorgolios 2006). Vascular endothelium plays a key role in regulation of the inflammatory response. Adhesion molecules such as selectins, immunoglobulin receptors, integrins, and cadherins as well as connexins expressed on the endothelial cells surface, participate in several interactions. In normal circumstances, they mediate endothelial cell-matrix interactions and regulate vascular permeability. The surface expression of adhesion molecules changes during the process of inflammation. Subsequently, those receptors participate in interactions between leukocytes and activated endothelium surface, in the process of leukocyte activation and their extravasation. Thus, levels of adhesion molecules may be a diagnostic marker of the systemic endothelial injury (CitationBelohlavkova 1999). In addition to the standard adhesion molecules, a novel approach to the understanding of cell adhesion research is the development of “cell adhesion networks” which include both intrinsic molecules and external regulators that control adhesion function (CitationZaidel-Bar 2007, CitationParis 2008, CitationCirillo 2009). In the present review we present the standard adhesion molecules, their physiology and pathophysiology focusing in inflammation and we give a paradigm model of a complicated, life threatening, difficult and sometimes late diagnosed as well as fluently misdiagnosed infectious disease in the daily clinical practice, like infective endocarditis.
INTEGRINS
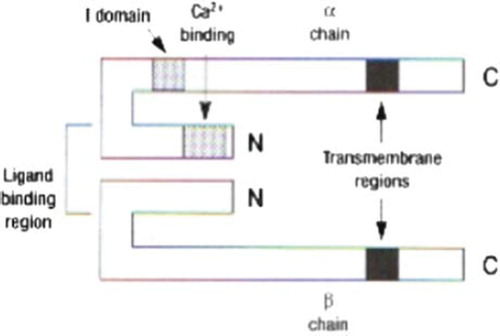
The integrins are transmembrane glycoproteins, heterodimers comprised of α and β chains that are linked together with disulphide bonds (). Integrins are secreted by the epithelial cells as well as from many other types of cells. There are at least 15 different types of α chain and 9 different types of β chains. Due to this fact a significant number of integrin molecular distribution. The primary classification of integrins was based upon their β-subunit. On the other hand due to the fact that some α-subunits are linked with more that one type of β-chains it has been introduced a more approachable classification according to the extracellular matrix molecules with which the specific integrin receptors bind. The majority of integrins are members of the β1 subgroup or VLA (Very Late Antigen) and they are expressed from a variety of cellular types. VLA antigens are located to the lymphocytes a few days after they are are triggered from mitogens (CitationReddy 2003). Moreover, β2 integin subgroup is consisted of the lymphocyte receptor LFA-1(Leukocyte Function Associated molecule-1), Mac-1 integrin and p150, 95 integrin all being expressed exclusively at the leukocytes as well as integrin αdβ2 (CitationReddy 2003, Citationvan den Vieren 1995). LFA-1(CD11a/CD18 or αLβ2) is a molecular weight of 275 kDa integrin which is expressed at the vast majority of the white blood cells, implicated in reactions between leukocytes and leukocyte with endothelium. In addition to the inflammatory process LFA-1 integrin is implicated to the adhesion of the cytotoxic T lymphocytes to the “target” cells, to the mixed type lymphocytic reactions and to the lymphocyte proliferation following antigen stimulation (CitationMc Ever 2007). LFA-1 has been implicated in cases of metastatic lymphomas and it is postulated that it is overexpressed in the small cell type lung carcinoma (CitationArnaout 2002). Some adhesion molecules such as ICAM-1, ICAM-2 and ICAM-3 of the IgSF are ligands of LFA-1 integrin. Mac-1 integrin (CD11b/CD18 or αMβ2) is a molecular weight of 265 kDa protein which is expressed at most of the white blood cell types conducting an important role at the adhesion reactions between leukocytes and leukocyte to endothelium (especially those reactions between neutrophils and endothelial cells). Specifically, Mac-1 is connected to the linkage reactions with the complement (CitationMc Ever 2007). Ligands for Mac-1 are considered ICAM-1, ICAM-3, fibrinogen, C3bi complement fragment (CitationArnaout 2002). Integrin p150,95 is a molecular weight 245 kDa protein (Leu M5 or CD11c/CD18 αXβ2) which is expressed at most of the white blood cell types at has a role at the cross reactions between leukocytes and leukocyte to endothelium (CitationHogg 1986). This integrin is implicated at the inflammation and chemotactic process, B-cell activation and CTL mediated cellular destruction. Regarding p150, 95 integrin fibrinogen and C3bi are postulated as its ligands (CitationPignatelli 1998). The β3 integrin subgroup the so called cytoadhessins, they are expressed mainly at the endothelial cells and the platelets. They consist of a glycoprotein called gpIIbIIIa and the fibronectin receptor. gpIIb/ IIIa platelet integrin is a glycoprotein of a 250 kDa molecular weight (CD41/CD61 or αΙβ3) that is expressed with resting platelets and links with fibrinogen. When platelets are activated its role as a gpIIb/IIIa receptor is activated causing a subsequent high affinity relationship to fibronectin and von Willebrand factor leading finally to platelet aggregation (Citationvan den Vieren 1995, CitationMc Ever 2007, CitationArnaout 2002, CitationPignatelli 1998, CitationMc Ever 2009, Bluchbern Citation1993). Integrin ligands comprise the bacterial and viral proteins, coagulation and fibrinolysis factors complement proteins and cellular anti-receptors, as well as the abovementioned adhesion molecules of the IgSF family ICAM-1, ICAM-2, ICAM-3, VCAM-1 and CD31 (CitationArnaout 2002, CitationHogg 1986, CitationMc Ever 2009) the classical integrin ligands. It is noticeable to mention that although integrins are expressed they do not link with their receptors unless they are activated. Both integrin and their ligand stereochemical configuration are considered significant in order to cross react. From our best of knowledge, there are three integrin activation mechanisms; cellular activation through receptors such as TCR, cytokine activation like MIP-1β and CD31 molecule mediated activation (CitationMc Kay 1993). Moreover, regulation of integrin – mediated adhesion is achieved by both robust and highly dynamic adhesions, events occurring at the same time. Therefore interactions between cell adhesion molecule components are transient and it is the dynamic nature of the adhesion site that makes the respective bond both sensitive and responsive to an external stimulus. Over the years a large number of proteins are identified as components of integrin – mediated cell – ECM (extracellular matrix) adhesions. As the adhesive network grows in complexity and connectivity it is obvious that in order robustness and dynamic plasticity to be achieved at the adhesion site most of these interactions can be switched “on” and “off”. Basic switching mechanisms such as the interaction – partner switch, the conformational switch, the tyrosine – phosphorylation switch, the serine/threonine – phosphorylation switch, the phospholipid switch, the Rho – GTPase switch and the cleavage switch, are found in integrin adhesion networks (CitationZaidel-Bar 2010).
Integrins and disease
Integrins have been implicated in processes such as inflammation, cellular growth, intercellular adhesion bonds formation and polarization process (CitationReddy 2003). Additionally, integrins are implicated to scientific fields such as hematology, neurobiology, thrombosis, cancer biology, inflammation, AIDS (CitationArnaout 2002). Leukocyte Adhesion Deficiency syndrome (LADs) is a disease characterized at the molecular level of a deficit in β subunit of integrins. The patients suffering from this syndrome present remittent bacterial infection sometimes proved life threatening. Integrin secretion is significantly high at the cellular surface of these organisms (CitationReddy 2003, CitationAnderson 1987). In the literature we find evidence that integrins mediate the adhesive reactions between cells functioning as cell adhesion molecules. Such integrins are α4β1 and lymphocyte function associated integrin-1 (LFA-1). Moreover, when integrins function as CAMs they provoke cell adhesion of heterotypic character linking preferably with some of the IgSF like ICAM-1, ICAM-2 and VCAM as well as with some members of the selectin family like endothelial leucocyte adhesion molecule-1, ELAM-1 or L-selectin (CitationElices 1990, CitationSpringer 1990, CitationBechter 1999). Cell adhesion molecules play a key role in the inflammation process and they are worldwide under investigation with much scientific work in clinical and laboratory level. In the inflammation process the complement is activated followed by macrophage activation with the Fc fragment and the complement receptors to produce cytokines such as IL-1β and TNFa, most probably α4β1 integrin mediated (CitationElices 1990, CitationSpringer 1990, CitationBechter 1999). Cytokines produce excessive secretion of ICAM-1. In addition with the intervention of αMβ2 integrin complement activating products, chemokines and the increased endothelial adhesiveness accompany neutrophil recruitment and activation. In order for neutrophils to migrate successively from the vascular space to the site of infection through the tissues molecules such as platelets have to act synergically mediated by αΙbβ3 integrin (gpIIbIIIa) platelet integrin a very interesting platelet adhesion molecule. This platelet integrin s related with increased prevalence of thrombus formation though directly implicated with thrombotic cerebral stroke (CitationMc Ever 2009, Bluchbern 1993, CitationSumpio 2002). During the last decade specific drug functioning against this platelet integrin is used successfully worldwide. In summary, integrins represent a large family of adhesion receptors that are widely expressed and mainly interact with extracellular matrix components. The affinity and the avidity of integrins can be modulated by different mechanisms triggered either from the extracellular milleu or through intracellular signals. Integrins exert an important role as signal-transducing receptors, activating different biochemical pathways. Additionally integrins modulate key intracellular phenomena, including cell activation, proliferation and apoptosis. They are involved in inflammatory, allergic and neoplastic diseases. Their role is also emphasized in organ response to trauma and in skin lesion redevelopment. Knowing integrin molecular basis and understanding their pathophysiological role contribute significantly to the creation of different strategies for the adequate modification of cellular adhesion and therefore the creation of new diagnostic and therapeutic perspectives controlling the pathogenic processes.
IMMUNOGLOBULIN GENE SUPERFAMILY (IgSF)
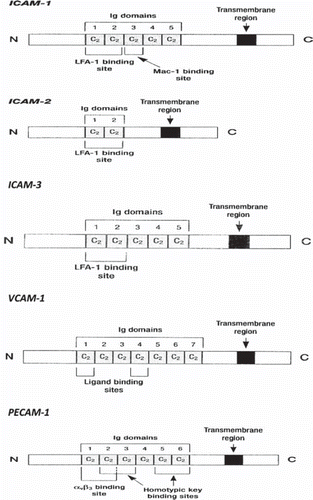
IgSF is the most abundant family of cell surface molecules, accounting for 50% of leukocyte surface glycoproteins. Their structure is characterized by repeated domains, similar to those found in immunoglobulins, built from a tightly packed barrel of β strands (). By mutation and selection, the Ig domain has evolved to serve many different functions including; receptors for growth factors, receptors for the Fc region of Ig, and as adhesion molecules, which now seems to be a function of the majority (CitationKrieglstein 2001, CitationHolness 1994). Some members of this family that are of relevance to vascular diseases include intercellular cell adhesion molecules-1 and -2 (ICAM-1, ICAM-2), vascular cell adhesion molecule-1 (VCAM-1), platelet-endothelial cell adhesion molecule (PECAM)-1, and the mucosal addressin cell adhesion molecule-1 (MAdCAM-1). Other molecules include neural cell adhesion molecule (NCAM) and carcinoembryonic antigen (CEA). Additionally, at this family nowadays some other molecules that are implicated at the cell recognition process are introduced like major histocompatibility complex antigens (MHC), T-cell receptor, platelet derived growth factor receptor (PDGF), deleted in colon cancer gene product (DCC), and colony stimulating factor-1 receptor (CSF-1). Leukocyte rolling is a prerequisite for eventual firm adherence to blood vessels. However selectin mediated adhesion of leukocytes does not lead to firm adhesion and transmigration unless members of the IgSF are involved. Additionally, they undergo increased expression in chronic immunological inflammatory processes (CitationKlein 1998, CitationZimmermann 1992). For endothelial cell-T cell interactions the most important members of this family are ICAM-1, ICAM-2 and VCAM-1, which serve as surface ligands for the LFA-1 and VLA-4 integrins (CitationPignatelli 1990).
Figure 2. Schematic presentation of the structure of some members of IgSF CAMs family (ICAM-1, ICAM-2, ICAM-3, VCAM-1, PECAM-1).
Intercellular Adhesion Molecule – 1 (ICAM-1 or CD54)
ICAM-1 (CD54) is a transmembrane glycoprotein of 90 kDa molecular weight with five extracellular immunoglobulin simulated sites. It is basally expressed on many cell types, but its expression is regulated on endothelial cells (CitationDustin 1986), where it exhibits remarkable heterogeneity between vascular beds (CitationPanes 1995, CitationHenninger 1997). The most important ligands for ICAM-1 are considered the β2 integrins LFA-1 and Mac1 (CD11B/CD18 that are expressed in leukocytes. Subsequently, ICAM-1 mediates the leukocyte-ICAM-1 presenting cells adherence. Additionally, ICAM-1 adheres with fibrinogen, hyaluronic acid, red blood cells infected with plasmodium falciparum, and CD 43 (sialoforin). Immunohistologically ICAM-1 is detected either as a transmembrane protein or as a soluble form in blood serum. It is expressed at the endothelial and epithelial cells, at the lymphocytes, monocytes, eosinophils, keratinocytes, dendritic cells, ancestral hematopoietic cells, fibrinoblasts and hepatocytes (CitationGearing 1992). There are molecules that up regulate ICAM-1 concentrations like some cytokines (TNF-α; tumor necrosis factor-α, IFN-γ; interferon-γ, IL-1; interleukin-1) and other that down regulate like glucocorticoids. ICAM-1 is found in a biologically active form in serum, probably as a result of proteolytic cleavage from the cell surface, being elevated in patients with various inflammatory syndromes such as septic shock, LAD, cancer and transplantation (CitationRao 2007). ICAM-1 is expressed to the endothelial and epithelial cells, lymphocytes, monocytes, eosinophils, keratinocytes, dendritic cells, ancestral haemopoietic cells, liver cells and fibroblasts. Deregulation of ICAM-1 expression leading to increased levels is triggered by infectious cytokines (tumor necrosis factor-alpha, TNF-α; Interferon-γ, INF-γ; Interleukin-1, IL-1), while decreased expression is observed when inflammatory factors such as glycocorticoids are induced. The immune cell circulation related function of ICAM-1 is the best studied till today. Inflammatory cytokines increase the expression of ICAM-1 in the vascular endothelial cells and on the other hand activate the leucocytic integrins LFA-1 and Mac-1 at the site of inflammation. Subsequently, this fact leads to leukocytic adherence to the regional endothelium which is considered to be a necessary step for leukocyte migration at the site of inflammation. There is the cellular type of ICAM-1 with a stable linkage to the cellular membrane and the ELISA (enzyme-linked immunospecific assay) detected soluble form of ICAM-1 (sICAM-1). Soluble forms of ICAM-1 have been reported in biological fluids such as blood serum bronchoalveolar lavage and cerebrospinal fluid. In general, increased serum levels of soluble ICAM-1 are related with different inflammatory conditions cased by bacteria, viruses, autoimmune diseases and kinds of neoplasms. Additionally, different levels of ICAM-1 have been monitored at the adult respiratory distress syndrome (ARDS). Corticosteroids that are used therapeutically at ARDS inhibit the secretion of ICAM-1 and ELAM-1 (endothelial leukocyte adhesion molecule-1) (CitationCronstein 1992). Several studies have demonstrated that in some cases of septisaemia soluble forms of ICAM-1 present flunctuations that are related to the levels of some endotoxins, tumor necrosis factor and different types of cytokines (CitationLeone 2003, CitationMundhekar 2006). Moreover in the literature we find proof for the implication of ICAM-1 and VCAM in glomerular disease as well as the importance of ICAM molecule in the migration of leukocytes to the brain being implicated in cases of encephalitis and other immunological type disorders of the central nervous system (CitationWong 2007).
Intercellular Adhesion Molecule – 2 (ICAM-2)
ICAM-2 is a 55kDa molecular weight molecule found in high concentrations at the resting endothelial cells and secreted by most of leukocytes. In contrast to ICAM-1, ICAM-2 expression is not increased on activated endothelial cells and it is not triggered by cytokine activation (CitationNortamo 1991). It is considered a truncated form of ICAM-1 that is basally expressed on endothelial cells (Citationde Fougerolles 1991). The expression of ICAM-2 is not triggered by cytokines activation. Moreover, it is found in low levels at leukocytes, epithelial cells and generally in latent phase cells while on the other hand it is stimulated by IFN-γ, TNF-a, IL-1 and LPL (lipopolisaccharide) (CitationCartwright 1995, CitationVerhamme 2006).
Intercellular Adhesion Molecule – 3 (ICAM-3 or CD50)
ICAM-3 (CD50) is a glycoprotein of 120kDa molecular weight and is considered a ligand for leukocytic integrins LFA-1 (CD11a/CD18, αLβ2). ICAM-3 is constitutively expressed at high levels by all resting leukocytes, such as monocytes, lymphocytes and neutrophils, as well as antigen presenting cells, showing a pattern of expression clearly distinct from those of ICAM-1 and ICAM-2 (Hollness 1995). During the latent status of T-cells ICAM-3 molecule is considered the ligand for LFA-1. It is possible for ICAM-3 to have a very important role in the activation cascade of the immunologic response, the cellular adhesion and signal transduction if we take into account the fact that it causes increased adhesion through the β1 and β2 integrin pathways (CitationWong 2007, CitationAcevedo 1993). Additionally, it has been postulated that ICAM-3 is related with lymphomas and myelomas considering that the vascular endothelium in such conditions secretes increased amounts of ICAM-3 (CitationCampanero 1993, CitationDoussis-Anagnostopoulou 1993). Soluble forms of ICAM-3 are found in serum as a result of proteolytic cleavage from the cellular surface. In general ICAM molecules comprise as ligands for integrins mediating heterotypic adherence reactions of cell to cell type (CAMs).
Vascular Cell Adhesion Molecule-1 (VCAM-1 or CD106)
VCAM-1 or CD106 is a 90 kDa glycoprotein which exhibits low to negligible expression on unstimulated endothelial cells, can be profoundly upregulated after cytokine challenge. This molecule is expressed on the surface of activated endothelium and a variety of other cell types including bone marrow fibroblasts, tissue macrophages, and dendritic cells. It can be upregulated by inflammatory mediators such as interleukin1β (IL-1β), IL-4, CD44, tumor necrosis factor-α (TNFα), and interferon- γ (IFN-γ) (CitationSteeber 2000). VCAM-1 is a ligand for leukocytic integrins α4β1 (VLA-4) in cells including eosinophils and for α4β7 integrins at the activated T-cells at the periphery. VCAM molecule is detected at the blood serum using ELISA most probably as a result of proteolytic cleavage.
Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1)
PECAM-1 also known as CD31 or endoCAM is a 120 kDa molecular weight glycoprotein constitutively expressed on platelets, monocytes and neutrophils and in large amounts on endothelial cells at intercellular junctions and on T-cell subsets (CitationAlbelda 1991, CitationMuller 2003, CitationVaporciyan 1993). PECAM-1 can mediate adhesion through either homophilic or heterophilic interactions (Citationde Lisser 1994). In lower doses it is produced by platelets, monocytes and neutrophils (CitationMuller 2003, CitationVaporciyan 1993). It is linked either homotypically with itself or heterotypically with integrin ανβ3. PECAM-1 is highly implicated to the emigration of leukocytes through the vascular endothelium via intercellular junctions (CitationMuller 2003). We detect the molecule in soluble form in blood serum and this PECAM isotype is responsible for transendothelial migration of leukocytes. Furtheremore, it is implicated in the cross reactions of CD8 + and T-cells with the intercellular adhesion site molecules by stimulating the via integrin adhesion process (CitationArnaout 2002).
Mucosal adhessin cell adhesion molecule (MAdCAM-1)
The mucosal adhessin MAdCAM-1 is a 58 kDa glycoprotein found on HEV (High Endothelial Venules) and mainly expressed on high endothelial venules of Peyer's patches, on venules in small intestinal lamina propria, on the marginal sinus of the spleen, and on high endothelial venules of embryonic lymph nodes (CitationStreete 1988). It is involved in tissue-specific homing of lymphocytes in lymph nodes and mucosal lymphoid tissues (CitationKrieglstein 2001, CitationBriskin 1993). The molecule is composed of two amino-terminals Ig like regions presenting a very strong bond with ICAM-1 and VCAM-1 molecules which they present a mucine like region ending in an IgA simulating region. Ligands for MadCAM-1 are α4β7 integrin and L-Selectin at the leukocyte surface (CitationBuckley 1996).
Neural cell adhesion molecule (NCAM)
Neural cell adhesion molecule (NCAM) is secreted by a large variety of cell type mostly mesenchymal and neural derived ones. NCAM is implicated mostly in cancer process being present in a variety of neural, neuroendocrine, and mesenchymal tumors. Such tumors are Wilm's tumor, pituitary adenomas, pheochromocytoma, and small cell lung carcinoma. NCAM is considered a ligand for adhesion molecule α4β1 integrin found in leukocytes (CitationAoki 1991, CitationThomson 1991, CitationMolenaar 1998).
Carcinoembryonic antigen (CEA)
Carcinoembryonic antigen, (CEA) was discovered on 1965 as a 180 kDa cancer embryonic glycoprotein present in the blood serum of patients with large intestine cancer (CitationGold 1965). It mediates the adhesion of cell to substratum matter (CitationPaxton 1987, CitationLevin 1991).
Deleted in Colorectal Cancer molecule (DCC)
DCC is a transmembrane polypeptide composed of 1447 aminoacids processing an external fragment with 4 immunoglobulin like regions and 6 fibronectin like type III regions, showing a homologous character towards neural cell adhesion molecule (NCAM) (CitationFearon 1990). DCC acts as a NCAM stimulating neuritic growth through a specific intracellular signaling process (CitationPierceall 1994, CitationFigarella-Bronger 1990).
SELECTINS
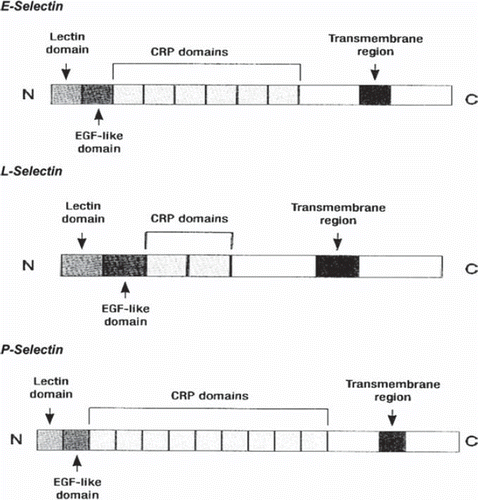
Selectins are lectin like binding transmembrane glycoproteins that mediate the initial low-affinity leukocyte-endothelial cell interaction that is manifested as leukocyte rolling. This transient binding results in further leukocyte activation and subsequent firm adhesion and transendothelial migration of leukocytes () (CitationKrieglstein 2001, Chavakis 2006, CitationPetri 2006, CitationTailor 2000). With the presence of calcium lectin region binds with carbohydrates e.g Lewis antigen, in neighbouring cells. Specifically, selectins are implicated in heterotypic interactions between blood cells and endothelial cells during leukocyte migration and firm adhesion (CitationBarthel 2007). Their role is manifested during the initial adherence of the circulating leukocytes to the vascular wall that follows their “rolling” as a response to an infective or a carcinogen mechanism. Additionally, as a response to infection mediators, leukocyte gathering is considered to be crucial for the adequate defence of the organism to any kind of injury or infection. Selectins mainly recognise ligands that possess a carbohydrate region structures that have sialyl-Lewisx (sLex) antigen. These reactions selectin – carbohydrates are considered as unstable permitting leukocytes to “roll” on vascular endothelium towards blood flow. There are three closely related members of selectin family each expressed on leukocytes (L-selectin), endothelial cells (E-selectin, P-selectin), and platelets (P-selectin) (CitationBarthel 2007). Each member contains a N-terminal C-type lectin domain (carbohydrate recognition domain), followed by an epidermal growth factor (EGF)-like motif, varying numbers of short consensus repeats similar to those found in complement – regulatory proteins (CRP), a transmembrane domain, and a short cytoplasmic tail. Studies using chimeric selectins indicate that both the lectin and the EGF domains are directly involved in cell adhesion and may determine the specificity of ligand binding (CitationTedder 1995).
Figure 3. The structure of some members of selectin CAMs family (E-Selectin, L-Selectin, P-Selectin).
Contrary to most of the other CAMs selectin role is strictly restricted to the interactions between leukocytes and the vascular endothelium. In general, selectins share an important role in human physiology. In Leukocyte Adhesion Deficiency II syndrome (LAD II), were selectin ligands are absent; there is an inability to recruit neutrophils into sites of inflammation so that they can not fulfil their role as effector cells in the immune system (Citationvon Adrian 1993). Soluble circulating forms of the selectins can be detected in plasma, where elevated levels have been reported in serum of animals and patients with inflammatory diseases (CitationGearing 1993).
P-selectin (CD 62P or GMP-140 or PADGEM)
P-selectin (CD 62P or GMP-140 or PADGEM) has a molecular weight of 140 kDa and it is stored in specific granules that are present in platelets (α-grannules) and endothelial cells (Weibel-Palade bodies) from where it can be rapidly mobilized to the cell surface in response to a variety of inflammatory agents such as thrombin, histamine complement factors, free radicals and cytokines (Tender 1995, Citationvan Gils 2009). Cell surface expression of P-selectin is generally short lived (minutes), which makes it an ideal candidate for mediating early leukocyte-endothelial interactions. Ligand for P Selectin is considered the P-selectin glycoprotein ligand-1 (PSGL-1). PSGL-1 undergoes special glycosylation in order to function as a ligand. P-Selectin mediates as well neutrophil as monocyte adherence to stimulated thrombocytes and stimulated endothelial cells. Additionaly mediates the in vitro captivation of stimulated B cells together with a subpopulation of T cells in the stimulated endothelium. E-selectin (CD62E, ELAM-1), is expressed by cytokine – activated endothelial cells (CitationFang 2009, CitationMc Ever 2004).
E-selectin
E-selectin mediates neutrophil, monocyte and some memory T-cell adhesion to vascular endothelium, and may function as a tissue –specific homing receptor for T cell subsets (CitationTedder 1995). It is broadly expressed within the vasculature at sites of inflammation. Additionally, it is found in arthritic joints, in heart and renal allograft undergoing rejection, and in cutaneous vessels of inflamed skin with psoriasis, contact dermatitis, and delayed type hypersensitivity reactions (CitationTedder 1995). E-selectin is found in a biologically active form in serum, as a result of proteolytic cleavage from the cell surface (CitationGearing 1992, CitationMadri 2000). Many ligands for E selectin have been reported and are expressed by neutrophils, monocytes and lymphocytes such as ESL-1 ligand (E-Selectin ligand-1) (85) and PSGL-1 (P-Selectin Glycoprotein ligand-1) (CitationMc Ever 2004). Although there is no preformed (storage) pool of E-selectin in endothelial cells, increased cell surface expression can occur in response to transcription-dependent protein synthesis (CitationFries 1993).
L-selectin (CD26L, LECAM-1, LAM-1, gp90MEL-14)
L-selectin (CD26L, LECAM-1, LAM-1, gp90MEL-14) is a 74 kDa molecular weight protein constitutively expressed by leukocytes. It is expressed continuously throughout myeloid differentiation, and is expressed mostly by most circulating neutrophils, monocytes and eosinophils. L-selectin mediates leukocyte binding to activated endothelium at inflammatory sites, and lymphocyte binding with integrin receptors mediation to high endothelial venules of peripheral lymph node during lymphocyte homing (CitationTedder 1995). Through this mechanism the metastatic procedure of tumors to the lymph nodes takes place. The broad expression of L-selectin allows it to play a role in the trafficking of all leukocyte lineages. Primary forms of red cell line progenitors express L-selectin while the mature red cells does not. Ligands for L selectin that are recognised till today include MadCAM-1 and other broad spectrum tissues including those of central nervous system. Soluble form of L-selectin is 3 kDa smaller than surface L-selectin and is bioefficient (CitationWalcheck 1996). Elevated levels of L selectin are reported in patients with acquired immunodeficiency syndrome, leukemias and malignant tumors. Decreased levels are reported in patients with ARDS.
Cytokines, bacterial toxins, and oxidants are known to promote the synthesis of E- and P-selectin in endothelial cells. The major ligands for all three selectins are cell surface glycans that possess a specific sialyl-LewisX-type structure (CitationHallahan 1997), L-selectin may also serve as a ligand for P- and E-selectin (CitationKrieglstein 2001, Citationvon Adrian 1993, CitationPatel 1995).
PATHOPHYSIOLOGY OF CAMs – PARADIGM MODEL IN INFECTIVE ENDOCARDITIS
Pathohysiology of CAMs
The trafficking of leukocytes within the microcirculation is critical for normal immune surveillance of tissues. In the development of inflammation, adhesion molecules play an essential role in the localization of the inflammatory response. At this level, the vascular endothelium, a governing barrier for the exchanges between blood and the tissues, plays an active part in regulation of the transcapillary permeability, control of proliferation of haematopoietic cells and the phases of the inflammatory response (Moussa 2008). The process of leukocyte recruitment is tightly regulated by the sequential expression and activation of specific adhesion molecules on the surface of leukocytes and endothelial cells (). These adhesion molecules mediate distinct steps in the recruitment of leukocytes in the microcirculation. Selectins mediate leukocyte rolling, whereas glycoproteins belonging to the integrin and immunoglobulin supergene families enable leukocytes to firmly adhere and emigrate in venules. The leukocyte-endothelial cell adhesion that is mediated by these adhesion molecules has been shown to alter the function of endothelial cells in all segments of the vasculature (i.e., in arterioles, capillaries, and venules) (CitationTailor 2000). In vivo observations of the behaviour of leukocytes in venules has led to a model of leukocyte-endothelial cell interactions that predicts three sequential and coordinated steps for leukocyte recruitment: rolling, firm adhesion (adherence), and emigration of leukocytes finally (CitationKrieglstein 2001).
Figure 4. Schematic presentation of the stages of leukocyte recruitment (leukocyte rolling, arrest, firm adhesion and migration).
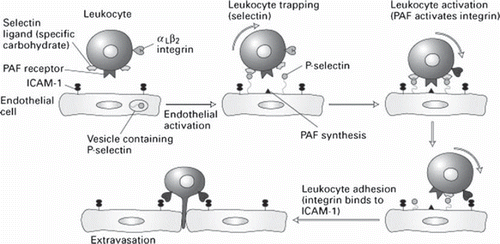
In the dormant state, leukocytes and endothelial cells do not interact. Selectin-binding sites are present on leukocytes but dormant endothelial cells do not express selectins. In case of damage or inflammatory processes of the blood vessels due to the action of released cytokines increased expression of adhesion molecules of the integrin, selectin and immunoglobulin groups occurs and subsequently increased adhesion and migration of inflammatory cells across the vascular wall is observed (CitationGolias 2007). Specifically, to establish an adhesive interaction with endothelial cells, circulating leukocytes must, at first, move from the central stream of flowing blood towards the vessel wall. It is now well accepted that endothelial cell activation results in selectin expression and subsequent interaction of selectins and with their ligands mediating thus, the weak (low-affinity) adhesive interactions that are manifested as leukocyte rolling (CitationKrieglstein 2001, CitationPetri 2006, CitationCalder 2006). Once leukocyte activation is fulfilled leukocyte integrins bind with IgSF glycoproteins such as ICAM-1 and VCAM-1, permitting firm adhesion. Although other CAM (e.g., VLA-4, VCAM-1, MadCAM-1, and members of the β7 subfamily of integrins) have also been implicated in leukocyte transiently binding (tethering) and rolling their quantitative significance remains unclear. The tethered leukocytes are then exposed to low concentrations of chemo- attractants/inflammatory mediators that result in leukocyte activation and subsequently elicit integrin-Ig-dependent leukocyte adherence, with a simultaneous downregulation (shedding) of L-selectin. Leukocyte activation is also associated with an increased avidity of the integrins, which can be elicited by chemokines, bacterial peptides, platelet activating factor (PAF), and leukotriene B4 (CitationPetri 2006, CitationCalder 2006). After they have marginated, the active cells migrate by diapedesis towards the site of inflammation by creation of chemotactic signals as the adhesion between the cells is insufficient to induce their migration (CitationMousa 2008). Leukocyte migration is an important mechanism in the pathogenesis of inflammatory diseases, the regulation of hematopoiesis and hemostasis. The transendothelial migration of leukocytes begins with locomotion of adherent leukocytes toward the endothelial cell-cell junctions. Transendothelial migration is mediated by additional IgSF members like PECAM-1 (CitationKrieglstein 2001). During this process the cell steadily establishes new adhesive contacts at the migration front while reducing adhesive interactions at the tail occur (CitationEriksson 2000). It is shown that CD11/CD18 (alpha L, M, X/beta 2) integrins have an important role in subsequent steps of leukocyte migration into tissues (CitationPetri 2006).
Apparently, cell adhesion molecules provide the foundation for cell communication, trafficking, and immune surveillance central to host defence. These soluble adhesion molecules (selectins, integrins, CD44, and members of the Ig superfamily), provide a recognition system between leukocytes, endothelial cells and matrix molecules. The activation and increased expression of these adhesion glycoproteins have been attributed to excessive production of cytokines and oxidants (CitationTailor 2000). Additionally, the adherence phenomena depend on a process that is strictly controlled by the cytokines and enable intervention of cell-cell reactions and cell-protein recognition of the extra-cellular matrix. Cytokines play a key role in control of the expression and/or avidity of membrane receptors for ligands (CitationMousa 2008). Deregulation of these adhesion and signal transduction pathways can contribute to continued recruitment and persistent leukocyte activation with unresolved inflammation.
Infective Endocarditis
Experimental data and pathologic observations support the assumption that endothelial cells play a fundamental role in the development of inflammatory processes and various stimuli result in endothelial activation and endothelial leukocyte interactions including adhesion and extravasation. These interactions are mediated by augmented expression of adhesion molecules, such as E-selectin, ICAM-1, and VCAM-1 (CitationGearing 1992, CitationLeewenberg 1992, CitationNewman 1993, CitationPigot 1992, CitationRadi 2001). The attachment of pathogenic microorganisms to vascular endothelial cells (EC) or sites of vascular injury is considered a critical initiating event for many types of intravascular infections.
In bacterial endocarditis (BE), the microbial infection is localized on the endocardial surface of the heart and, depending on the bacterial species, may cause an inflammatory reaction that in most cases affects the mural endocardium and the mitral and aortic valves (CitationBayer 2000). Providing a brief definition, infective endocarditis is a microbial infection of the endothelial surface of the heart. The characteristic lesion, the vegetation, is a variably sized amorphous mass of platelets and fibrin in which abundant microorganisms and moderate inflammatory cells are enmeshed. Acute infective endocarditis is caused typically, although not exclusively, by staphylococcus aureus, whereas the subacute syndrome is more likely to be caused by viridans streptococci, enterococci, coagulase-negative staphylococci, or gram negative coccobacilli. Endothelial activation contributes significantly to the systemic inflammatory response to bacteraemia. Increased expression and release of soluble endothelial markers into the circulation have been demonstrated. Elevated plasma levels of E-selectin have been reported in bacteraemic patients. It has also been proposed that the release of E-selectin is related to the degree of vascular or endothelial injury caused by the sepsis (CitationCowley 1994, CitationSessler 1995). Increased plasma and serum levels of VCAM-1 and ICAM-1 have been shown in bacteremic patients. E-selectin as well as ICAM-1 has also been found to be associated with multiple organ dysfunction, septic shock and death (CitationVeltrop 2001). It has been proposed that infection of endothelial cells with Staphylococcus aureus, Streptococcus sanguis, or Staphylococcus epidermidis induces surface expression of intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) and monocyte adhesion (CitationNorris 1991).
Several studies have demonstrated that during endocarditis the formation of circulating immune complexes which are identified to contain bacterial components, may contribute directly or indirectly to the stimulation of endothelial cells. Expression of adhesion molecules in vivo can be maintained for several days after stimulation (CitationCowley 1994, CitationSessler 1995, CitationVeltrop 2001, CitationNorris 1991, CitationSoderquist 1999, CitationMuller 2000). It has been suggested in the literature that patients with Staphylococcus aureus bacteraemia and endocarditis, which represents a sustained endothelial involvement, showed significantly higher E-selectin and VCAM-1 concentrations on admission than those with Staphylococcus aureus bacteraemia but without endocarditis which might reflect a more extensive activation of endothelial cells (CitationSoderquist 1999, CitationMarshal 2002, CitationWeber 2003). It is known that in BE intravascular infection with Staphylococcus aureus, Streptococcus sanguis, or Staphylococcus epidermidis can lead to formation of a fibrin clot on the inner surface of the heart and cause heart dysfunction. In addition, the same study was demonstrated that infection of endothelial cells with these three pathogens induces surface expression of ICAM-1 and VCAM-1 as well as monocyte adhesion (CitationVeltrop 2001). Furthermore, by using immunohistochemistry, the CAM expression of endothelial cells on degenerative, mostly calcified heart valves and on heart valves with florid endocarditis was characterized. As expected, the constitutively expressed molecules (ICAM-1, CD34, CD31) were found both on degenerative and on inflamed valves. Furthermore, marked expression of E-selectin and VCAM-1 was found not only on inflamed valves but also on larger portions of the degenerative valves with no morphological evidence of inflammation. This striking finding might help to explain why patients with fibrotic heart valves are susceptible to recurrent endocarditis (CitationKorkmarz 2001). Another perspective was given from a relatively recent study regarding the role of soluble adhesion molecules E- and P- selectin. Specifically, the mean plasma concentrations of P-selectin were elevated in patients with embolic events as compared to both patients without embolic events and control subjects. Similarly, the patients with embolic events had increased plasma levels of E-selectin compared to those without embolic events and the control group (CitationWoodruff 1980). This assumption reflected enhanced platelet activation, which have a direct impact to thrombus generation. Moreover, the increased expression of endothelial activation markers E-selectin and VCAM-1 on degenerative heart, the CAM expression of endothelial cells on degenerative, mostly calcified heart valves and on heart valves with florid endocarditis as well as the constitutively expressed molecules (ICAM-1, CD34, CD31) both on degenerative and on inflamed valves suggest that adhesion molecule mediated leukocyte recruitment or activation of endothelium may constitute a critical role in the pathogenesis of endocarditis and in the manifestation of its major complications such as thromboembolism (CitationSoderquist 1999, CitationMuller 2000, CitationKoekmaz 2001). However, it remains to be clarified by future studies whether the elevated adhesion molecule levels result from focal release of adhesion molecules at the site of endocardial involvement or from the systemic effects of severe bacteraemic disease. Although, potential clinical value of establishing the diagnosis of endocarditis by measuring serum adhesion molecule concentrations is diminished by the presence of an overlap between the groups of bacteraemic patients with and without endocarditis (CitationSoderquist 1999). CAMs nevertheless constitute relevant diagnostic targets and after additional elaborated studies might be considered as future diagnostic criteria of endocarditis in the future.
CONCLUSION
Scientific evidence is rapidly accumulating to support the view that leukocyte- and endothelial cell-associated CAM are critical participants in the vascular dysfunction and tissue injury that is associated with a wide variety of inflammatory and cardiovascular diseases. In addition, Advancements in this field of investigation have largely resulted from the marriage of novel immunologic and molecular biological approaches to traditional experimental strategies in cardiovascular physiology. Leukocyte extravasation is a multistep process, mediated by several cell adhesion molecules including selectins (P-, E- and L-), integrins and members of the Ig superfamily (ICAM-1, VCAM-1). These molecules can be targeted for imaging purposes (e.g. to identify atherosclerotic plaques). Furthermore, cell adhesion molecules can serve as drugable targets to prevent leukocyte extravasation where warranted to decrease inflammatory tissue damage (e.g. reperfusion injury). Current techniques involve blocking of binding sites, targeted drug delivery using liposomes and polymeric particles as carriers or imaging of inflammation sites using labeled cells or antibodies. By understanding the cellular and molecular events in leukocyte endothelial cell interaction, strategies on therapies may develop having a specific clinical benefit in the future to overcome tissue damage induced by inflammation process.
Declaration of interest: The authors report no declarations of interest.
REFERENCES
- Eidelman GM and Crossin KL (1991). Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem 60: 155–190.
- Charalabopoulos K, Pignatelli M (2001). Adhesion molecule and cancer. Arch Hell Med 18: 16–19.
- Golias CH, Charalabopoulos A, Peschos D, Maritsi D, Charalabopoulos K, Batistatou A (2005). Adhesion molecules in cancer invasion and metastasis. Hippokratia 9:106–114.
- Skubitz AP (2002). Adhesion molecules. Cancer Treat Res 107: 305–29.
- Charalabopoulos K, Gogali A, Kostoula OK, Constantopoulos SH (2004). Cadherin superfamily of adhesion molecules in primary lung cancer. Exp Oncol 26(4):256–260.
- Mousa SA (2008). Cell adhesion molecules: potential therapeutic & diagnostic implications. Mol Biotechnol 38(1):33–40.
- Pozzi A, Zent R (2003). Integrins: sensors of extracellular matrix and modulators of cell function. Nephron Exp Nephrol 94(3): e77–84.
- El-Hariry, Pignatelli M (1997). Adhesion molecules: opportunities for modulation and a paradigm for novel therapeutic approaches in cancer. Expert Opin Investig Drugs 6:1465–1478.
- Golias C, Tsoutsi E, Matziridis A, Makridis P, Batistatou A, Charalabopoulos K (2007). Review. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo 21(5):757–69.
- Rojas AI, Ahmed AR (1999). Adhesion receptors in health and disease. Crit RevOral Biol Med 10(3):337–58.
- Jaitovich A, Etcheverry GJ (2004). Adhesion molecules. Their role in cardiovascular physiopathology. Medicina (B Aires) 64(5):455–462.
- Charalabopoulos K, Binolis J, Karkabounas S (2002). Adhesion molecules in carcinogenesis. Exp Oncol 24:249–257.
- Batistatou A, Makrydimas G, Zagoriannakou N, Zagoriannakou P, Nakanishi Y, Agnantis N, Hirohashi S, Charalabopoulos K (2006). Expression of dysadherin and E-cadherin in trophoblastic tissue in normal and abnormal pregnancies. Placenta 28(5-6):590–592.
- Krieglstein C.F., Granger D.N (2001). Adhesion molecules and their role in vascular disease. American Journal of Hypertension.
- Ohene-Abuakwa Y, Pignatelli M (2000). Adhesion molecules as diagnostic tools in tumor pathology. Int J Surg Pathol 8:191–200.
- Pafilis J, Batistatou A, Iliopoulou A, Tsanou E, Bakogiannis A, Dassopoulos D, Charalabopoulos K (2007). Expression of adhesion molecules during the normal pregnancy. Cell Tissue Res 329(1):1–11.
- Batistatou A, Charalabopoulos A, Scopa C, Nakanishi Y, Kappas A, Hirohashi S, Agnantis NJ, Charalabopoulos K (2006). Expression patterns of dysadherin and E-cadherin in lymph node metastases of colorectal carcinoma. Virchows Archiv 448(6):763–767.
- Kyzas P, Batistatou A, Stefanou D, Nakanishi Y, Agnantis NJ, Hirohashi S, Charalabopoulos K (2006). Dysadherin expression in head and neck squamous cell carcinoma: association with lymphagiogenesis and prognostic significance. Am J Surg Pathol 30:185–193.
- Horvathova M, Ferencik M (2000). The role of adhesion molecules in the immune system. Bratisl Lek Listy 101(3):138–145.
- Borowska K, Jedrych B, Czerny K, Zabielski S (2006). The role of integrins in the physiologic and pathogenic processes. Pol Merkur Lekarski 21(124):362–366.
- Hope SA, Meredith IT (2003). Cellular adhesion molecules and cardiovascular disease. Part I. Their expression and role in atherogenesis. Intern Med J 33(8):380–386.
- Georgolios A, Batistatou A, Charalabopoulos K (2005). Integrins in head and neck squamous cell carcinoma. A review article of the current literature. Cell Adhesion Commun 12–18.
- Georgolios A, Batistatou A, Charalabopoulos A, Manolopoulos L, Charalabopoulos K (2006). The role of CD44 adhesion molecule in oral cavity cancer. Exp Oncol 28(2):94–98.
- Belohlavkova S, Simak J (1999). Adhesion receptors of the vascular endothelium and their role in acute inflammation. Cesk Fysiol 48(2):51
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B (2007). Functional atlas of the integrin adhesome. Nat Cell Biol 9(8):858–867.
- Paris L, Bazzoni G (2008). The protein interaction network of the epithelial junctional complex: a system-level analysis. Mol Biol Cell 19(12):5409–5421
- Cirillo N, Prime SS (2009). Desmosomal interactome in keratinocytes: a systems biology approach leading to an understanding of the pathogenesis of skin disease. CellMol Life Sci 66(21):3517–3533.
- Reddy KV, Mangale SS (2003). Integrin receptors: the dynamic modulators of endometrial function. Tissue Cell 35(4):260–273.
- Van den Vieren M (1995). A novel leukointegrin, αdβ2, binds preferentially to ICAM-3. Immunity 3: 683–690.
- McEver RP, Zhu C (2007). A catch to integrin activation. Nat Immunol 8(10):1035–1037.
- Zaidel-Bar R, Geiger B (2010). The switchable integrin adhesome. J Cell Sci 123(Pt 9):1385–1388.
- Arnaout MA, Goodman SL, Xiong JP (2002). Coming to grips with integrin binding to ligands. Curr Opin Cell Biol 14(5):641–651.
- Hogg N (1986). The p 150, 95 molecules is a marker of human mononuclear phagocytes: comparison with expression of class II molecules. Eur J Immunol 16: 240–248.
- Pignatelli M (1998). Integrins, cadherins, and catenins: molecular cross talk in cancer cells. J Pathol 186:1–2.
- McEver RP and Zhu C (2009). Rolling Adhesion. Annu Rev Cell Dev Biol.
- Bluchbern BK and Gadek TR 1993. Glycoprotein IIbIIIa antagonists. Annu RepMed Chem 28: 79–87.
- McKay CR, Imhof BA (1993). Cell adhesion in the immune system. Immunology Today 14: 99–102.
- Anderson DC, Springer TA (1987). Leukocyte adhesion deficiency: an inherrited defect in the Mac-1, LFA-1 and p150, 95 glycoproteins. Ann Rev Med 38:175–194.
- Elices MJ, Osborn L, Takada Y (1990). VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at site distinct from VLA-4/fibronectin binding site. Cell 60: 577–584.
- Springer TA (1990). Adhesion receptors on the immune system. Nature 346:425–434.
- Bechter OE, Eisterer W, Dirnhoter S (1999). Expression of LFA-1 identifies different prognostic subgroups in patients with advanced follicle center lymphoma (FCL). Leuk Res 23(5):483–488.
- Sumpio BE, Riley JT, Dardik A (2002). Cells in focus: endothelial cell. Int JBiochem Cell Biol 34(12):1508–1512.
- Holness C, Simmons D.L (1994). Structural motifs for recognition and adhesion in members of the Immunoglobulin superfamily. J Cell Sci 107: 2065–2070.
- Klein RM, Breuer R, Mundhenke M, Schwartzkopff B, Strauer BE (1998). Circulating adhesion molecules (cICAM-1, lcVCAM-1) in patients with suspected inflammatory heart muscle disease. Z Kardiol 87(2):84–93.
- Zimmermann G.A, Prescott SM, McIntyre TM (1992). Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today 13(3):93–100.
- Pignatelli M, Durbin H, Bodmer WF (1990). Carcinoembryonic antigen functions as an accessory adhesion molecule mediating colon epithelial cell-collagen interactions. Proc Natl Acad Sci USA 87:1541–1545.
- Shimizu Y (1992). Lymphocyte interactions with endothelial cells. Immunol Today 13:106–112.
- Dustin M.L, Rothlein R, Bhan A.F, Dinarello C.A, Springer T.A (1986). A natural adherence molecule (ICAM-1): induction by IL-1 and IFN-, tissue distribution, biochemistry, and function. J Immunol 137: 245–254.
- Panés J, Perry M.A, Anderson D.C, Manning A, Leone B, Cepinskas G, Rosenbloom C.L, Miyasaka M, Kvietys P.R, Granger D.N (1995). Regional differences in constitutive and induced ICAM-1 expression in vivo. Am JPhysiol 269: H1955–H1964.
- Henninger D.D, Panés J., Eppihimer M, Russell J., Gerritsen M, Anderson D.C, Granger D.N (1997). Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol 158: 1825–1832.
- Gearing AJH (1992). Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann NY Acad Sci 667: 324–331.
- Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW (2007). Endothelial-depndent mechanisms of leukocyte recruitment to the vascular wall. Circ Res 101(3):234–247.
- Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G (1992). A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci USA 89(21):9991–9995.
- Leone M, Garcin F, Chaabane W, Boutière-Albanèse B, Albanèse J, Dignat-Georges F, Martin C (2003). Activation of adhesion molecules in patients with septic shock . Ann Fr Anesth Reanim 22(8):721–729.
- Mundhekar AN, Bullard DC, Kucik DF (2006). Intracellular heterogeneity in adhesiveness of endothelium affects early steps in leukocyte adhesion. Am JPhysiol Cell Physiol 291(1):C130–137.
- Wong D, Prameya R, Dorovini-Zis K (2007). Adhesion and migration of polymorphonuclear leukocytes across human brain microvessel endothelial cells are differentially regulated by endothelial cell adhesion molecules and modulate monolayer permeability. J Neuroimmunol 184(1-2):136–148.
- Nortamo P, Renkonen R., Li, R, Timonen T, Pieta J, Patarroyo M, Gahmberg C.G (1991). The expression of human intercellular adhesion molecule 2 is refractory to inflammatory cytokines. Eur J Immunol 21: 2629–2632.
- De Fougerolles A.R, Stacker S.A, Schwarting R, Springer T.A (1991). Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med 174: 253–267.
- Cartwright JE, Whitley GS, Johnstone A (1995). The expression and release of adhesion molecules by human endothelial cell lines and their consequent binding of lymphocytes. Exp Cell Res 217:329–335.
- Verhamme P, Hoylaerts MF (2006). The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin Belg 61(5):213–219.
- Holness C, Bates PA, Little AJ, Buckley CD, McDowall A, Bossy D, Hogg N, Simmons DL (1995). Analysis of the binding site on ICAM-3 for the leukocyte integrin LFA-1. J Biol Chem 220: 877–884.
- Acevedo A, del Pozo MA, Arroyo AG, Sanchez-Mateos P, Gonzalez-Amaro R, Sanchez-Madrid F (1993). Distribution of ICAM-3-bearing cells in normal human tissues. Expression of a novel counter-receptor for LFA-1 in epidermal Langerhans cells. Am J Pathol 143(3):774–783.
- Campanero MR, del Pozo MA, Arroyo AG, Sanchez-Mateos P, Hernandez-Caselles T, Craig A, Pulido R, Sanchez-Madrid F (1993). ICAM-3 interacts with LFA-1 and regulates the LFA-1/ICAM-1 cell adhesion pathway. J Cell Biol 123(4):1007–1016.
- Doussis-Anagnostopoulou I, Kaklamanis L, Cordell J, Jones M, Turley H, Pulford K, Simmons D, Mason D, Gatter K (1993). ICAM-3 expression on endothelium in lymphoid malignancy. Am J Pathol 143(4):1040–1043.
- Steeber DA, Tedder TF (2000). Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res 22(2-3):299–317.
- Albelda S.M, Muller W.A, Buck C.A, Newman P.J (1991). Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol 114: 1059–1068.
- Muller WA, Weigl SA, Deng X, Phillips DM (2003). PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 178(2):449–460.
- Vaporciyan A (1993). Involvement of PECAM-1 in neutrophil recruitment in vivo. Science 262:1580–1582.
- De Lisser H.M, Newman P.J, Albelda S.M (1994). Molecular and functional aspects of PECAM-1/CD31. Immunol Today 15:490–495.
- Streete P.R, Berg E.L, Rouse B.T.N, Bargatze R.F, Butcher E.C (1988). A tissue-specific endothelial cell molecule involved in leukocyte homing. Nature 331: 41–46.
- Briskin M.J, McEvoy LM, Butcher EC (1993). MadCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA. Nature 363:461–464.
- Buckley CD, Doyonnas R, Newtop JP, Blystone SD, Brown EJ, Watt SM, Simmons DL (1996). Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci 109(Pt2):436–445.
- Aoki J, Umeda M, Takio K, Titani K, Utsumi H, Sasaki M, Inoue K (1991). Neural cell adhesion molecule mediated contact dependent inhibition of growth of near diploid mouse fibroblast cell line m5S/1m. J Cell Biol 115: 1751–1761.
- Thomson JA, Grunert F, Zimmerman W (1991). Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal 5: 344–348.
- Molenaar WM, Muntinghe FL (1998). Expression of neural cell adhesion molecules and neurofilament protein isoforms in skeletal muscle tumors. Hum Pathol 29(11):1290–1293.
- Gold P, Freedman SO (1965). Demonstration of tumor specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. JExp Med 121: 439–443.
- Paxton RJ, Mooser G, Pandle H (1987). Sequence analysis of carcinoembryonic antigen: Identification of glycosylation sites and homology with the immunoglobulin superfamily. Proc Natl Acad Sci USA 84: 920–924.
- Levin LV, Griffin TW (1991). Specific adhesion of carcinoembryonic antigen-bearing colorectal cancer cells to immobilized carcinoembryonic antigen. Cancer Lett 60(2):143–152.
- Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzier KW (1990). Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 47: 247–256.
- Pierceall WE, Cho KR, Getzenberg RH, Reale MA, Hedrick L, Vogelstein B, Fearon ER (1994). NIH3T3 cells expressing the deleted in colorectal cancer tumor suppressor gene product stimulate neurite outgrowth in rat PCIZ pheochromocytoma cells. J Cell Biol 124(6):1017–1027.
- Figarella-Bronger D, Nedelec J, Pellisier JF, Boucrant J, Bianco N, Rougon G (1990). Expression of various isoforms of neural cell adhesive molecules and their highly polysialylated comiter parts in diseased human muscles. J Neurol Sci 98(1):21–36.
- Chavakis T, Orlova V. The role of junctional adhesion molecules in interactions between vascular cells. Methods Mol Biol 341:37–50, 2006.
- Petri B, Bixel MG (2006). Molecular events during leukocyte diapedesis. FEBS J 273(19):4399–407.
- Tailor A, Granger DN (2000). Role of adhesion molecules in vascular regulation and damage. Curr Hypertens Rep 2(1):78–83.
- Barthel SR, Gavino JD, Descheny L, Dimitroff CJ (2007). Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets 11(11):1473–91.
- Tedder T.F, Steeber DA, Chen A, Engel P (1995). The selectins: vascular adhesion molecules. FASEB 9:866–873.
- Von Adrian U.H, Berger EM, Ramezani L, Chambers JD, Ochs HD, Harlan JM, Paulson JC, Etzioni A, Arfors KE (1993). In vivo behavior of neutrophils from two patients with distinct inherited LAD syndromes. J Clin Invest 91:2893–2897.
- Gearing A.J, Newman W (1993). Circulating adhesion molecules in disease. Immunol Today 14: 506–512.
- van Gils JM, Zwaginga JJ, Hordijk PL (2009). Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol 85(2):195–204.
- Fang Y, Wu J, McEver RP, Zhu C (2009). Bending rigidities of cell surface molecules P-selectin and PSGL-1. J Biomech 42(3):303–307.
- McEver RP (2004). Interactions of selectins with PSGL-1 and other ligands. Ernst Schering Res Found Workshop (44):137–147.
- Gearing A.J.H, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ (1992). Soluble forms of vascular adhesion molecules, E-selectin, ICAM -1, and VCAM-1: pathological significance. Annals N Y Acad Sci 667:324–331.
- Madri JA, Graesser D (2000). Cell migration in the immune system: the evolving inter-related roles of adhesion molecules and proteinases. Dev Immunol 7(2-4):103–116.
- Fries J.W, Williams A.J, Atkins R.C, Newman W, Lipscomb M.F, Collins T (1993). Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am J Pathol 143: 725–737.
- Walcheck B, Kahn J, Fisher JM (1996). Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature 380: 720–723.
- Hallahan DE, Kuchibhotla J, Wyble C (1997). Sialyl Lewis X mimetics attenuate E-selectin-mediated adhesion of leukocytes to irradiated human endothelial cells. Radiat Res 147(1):41–47.
- Patel K.D, Moore K.L, Nollert M.U, McEver R.P (1995). Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest 96: 1887–1896.
- Lawrence MB, Springer TA (1991). Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65:859–873.
- Calder PC (2006). Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids 75(3):197–202.
- Eriksson E.E, Wer Jr Guo, Thoren P, Lindbom L (2000). Direct observations in vivo on the role of endothelial selectins and alpha(4) integrin in cytokine-induced leukocyte-endothelium interactions in the mouse aorta. Circ Res 86: 526–533.
- Gearing AJH, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ (1992). Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathologic significance. Ann NY Acad Sci 667:324–331.
- Leewenberg JFM, Smeets EF, Neefjes JShaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA (1992). E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. J Immunol 77:543–549.
- Newman W, Beall LD, Carson CW, Hunder GG, Graben N, Randhawa ZI, Gopal TV, Wiener-Kronish J, Matthay M (1993). Soluble E-selectin is found in supernatants of activated endothelial cells and is elevated in the serum of patients with septic shock. J Immunol 150:644–654.
- Pigott R, Dillon LP, Hemingway IH, Gearing AJH (1992). Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine-activated endothelial cells. Biochem Biophys Res Commun 187:584–589.
- Radi ZA, Kehrli ME Jr, Ackermann MR (2001). Cell adhesion molecules, leukocyte trafficking, and strategies to reduce leukocyte infiltration. J Vet Intern Med 15(6):516–529.
- Bayer AS, Scheld WM (2000). Endocarditis and intravascular infections. Mandell GL, Bennett JE, Dolin M.. Mandell, Douglas, and Bennett's principals and practices of infectious diseases. 5th. New York: Churchill Livingstone 857–902.
- Cowley HC, Heney D, Gearing AJH, Hemingway I, Webster NR (1994). Increased circulating adhesion molecule concentrations in patients with systemic inflammatory response syndrome: a prospective cohort study. Crit Care Med 22:651–657.
- Sessler CN, Windsor AC, Schwartz M (1995). Circulating ICAM-1 is increased in septic shock. Am J Respir Crit Care Med 151:1420–1427.
- Veltrop MH, Thompson J, Beekhuizen H (2001). Monocytes augment bacterial species- and strain-dependent induction of tissue factor activity in bacterium-infected human vascular endothelial cells. Infect Immun 69 (5):2797–2807.
- Norris P, Poston RN, Thomas DS, Thornhill M, Hawk J, Haskard DO (1991). The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol 96:763–770.
- Söderquist B, Sundqvist KG, Vikerfors T (1999). Adhesion molecules (E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)) in sera from patients with Staphylococcus aureus bacteraemia with or without endocarditis. Clin Exp Immunol 118 (3):408–411.
- Muller AM, Cronen C, Kupferwasser LI, Oelert H, Muller KM, Kirkpatrick CJ (2000). Expression of endothelial cell adhesion molecules on heart valves: up-regulation in degeneration as well as acute endocarditis. J Pathol 191 (1):54–60.
- Marshall D, Haskard DO (2002). Clinical overview of leukocyte adhesion and migration: where are we now? Semin Immunol 14(2):133–140.
- Weber C (2003). Novel mechanistic concepts for the control of leukocyte transmigration: specialization of integrins, chemokines, and junctional molecules. J Mol Med 81(1):4–19.
- Korkmaz S, Ileri M, Hisar I, Yetkin E, Kosar F (2001). Increased levels of soluble adhesion molecules, E-selectin and P-selectin, in patients with infective endocarditis and embolic events. Eur Heart J 22(10):811–812.
- Woodruff JF (1980). Viral myocarditis: A review. Am J Pathol 101:425.