Abstract
Skeletal muscle satellite cells, a postulated multipotential stem cell population, play an essential role in the postnatal replenishment of skeletal muscles. In the present research, the skeletal muscle satellite cells were isolated from the pectorals of 15-day-old Beijing Fatty Chicken embryos using combined enzymatic digestion of 0.1% collagenase 1 and 0.25% trypsin. Myogenic markers such as MyoD, Pax7 and demin were detected, indicating their skeletal muscle satellite cell identity. Karyotype analysis showed that these in vitro cultured cells were genetically stable. Being exposed to bone morphogen and adipogenic factors, it was proved that they differentiated into osteocytes and adipocytes correspondingly.
INTRODUCTION
Skeletal muscle satellite cells, which are responsible for the postnatal growth, repair and maintenance of skeletal muscles, are tissue-specific stem cells. They were first found in frog muscle by Mauro and were later identified in adult avian and mammalian muscle (CitationMauro, 1961). Satellite cells in adult skeletal muscle are normally mitotically quiescent, but are activated in response to stress induced by weight-bearing exercise or trauma. Then satellite cells enter the cell cycle, express muscle regulatory factors (MRFs) such as MyoD and Myf-5, and execute the myogenic program, eventually restore muscle structure and function (CitationYablonka-Reuveni & Rivera, 1994; CitationZammit, 2008).
Pax3 and Pax7 are specific markers of skeletal muscle satellite cells. Both the proteins probably act through the recruitment of a histone methyltransferase complexin, promoting expansion of the activated pool (CitationMcKinnell et al., 2008; CitationCollins et al., 2009), but repressing premature differentiation of satellite cells by inducing proteins which are able to keep myogenesis at bay, such as the myogenic factor inhibitors Id2 or Id3 (CitationAltarsha et al., 2009). Whereas all satellite cells express and require Pax7, which acts as a survival factor of the adult lineage, only a subset of the satellite cells express Pax3 (CitationRelaix et al., 2006; CitationKuang et al., 2006). MyoD is also a very important protein expressed in skeletal muscle satellite cells; nearly all satellite cells (98%–100%, depending on the muscle types) express MyoD, even at a very early stage of the satellite cell lineage. When satellite cells are activated, all the cells express MyoD before entering into a MRF-negative, quiescent state when muscles are repaired (CitationZammit et al., 2004).
The traditional view is that the skeletal muscle satellite cells are myogenic cells but not the pluripotent cells. However, this view is being challenged. There are some data implying that Pax3/7+, MRF− cells which occupy the satellite cell position in fetal muscle and likely represent satellite cell progenitors are not strictly committed to the myogenic program (CitationKassar-Duchossoy et al., 2005; CitationRelaix et al., 2005). In the previous study, the in vitro cultured skeletal muscle satellite cells can differentiate into osteocytes or adipocytes following treatment with bone morphogenetic proteins (BMPs) or adipogenic inducers respectively (CitationAsakura et al., 2001). Consistent with this, several groups have reported that satellite cells from adult muscles can spontaneously adopt adipogenic, osteogenic and fibroblastic fates in culture (CitationCsete et al., 2001; CitationShefer et al., 2004; CitationBrack et al., 2007).
Beijing Fatty Chicken is a unique breed in China and has a history of 300 years. It is a superior dual-purpose native chicken breed which produces good meat and eggs. Beijing Fatty Chicken owns some notable properties, such as the special appearance (e.g. crested, hair legs and beard mouth), meticulous and tasty meat, good egg quality, strong viability and genetic stability.
In this study, we have explored the methods for isolation, culture and identification of Beijing Fatty Chicken skeletal muscle satellite cells. Meanwhile, these cells were induced to differentiate into osteocytes and adipocytes following treatment with BMPs or adipogenic inducers, respectively. Through this experiment, we established the technology platform for further studies on skeletal muscle development mechanism and the role of Pax7 gene in satellite cells of Beijing Fatty Chicken.
MATERIALS AND METHODS
Cell culture
Primary cultures of Beijing Fatty Chicken muscle satellite cells were prepared as described by CitationMcFarland et al. (1993). Skeletal muscles were isolated from 15-day old chicken embryos and cut into about 1 mM3 pieces using ophthalmic scissors. The comminuted tissues were disaggregated by combinatorial digestion with 0.1% collagenase I for 30 min and 0.25% trypsin for 1 h at 37°C. Then DMEM containing 20% FBS (Biochrom, Germany) was added to terminate the reaction. The cell suspension was centrifuged at 1,500 rpm for 8 min with the supernatant discarded, whereafter the cells were resuspended with complete medium (DMEM/F12 + 20% FBS + 2.5 ng/ml bFGF) and seeded in cell culture bottles. Cells were cultured in 5% CO2 incubator at 37°C for 2h, and then the cell suspension was transferred to 24-well plates, and continued to culture at 37°C, in 5% CO2 (CitationQu et al., 1998). When the cells reached 80%–90% confluence, add 0.25% trypsin and 0.02% EDTA were added to digest cells and subculture at a ratio of 1:1.
When subcultured to passage 3 and at about 80%–90% confluence, the cells were digested conventionally and centrifuged, the supernatant was discarded, then an appropriate amount of freezing medium (50% FBS + 10% DMSO + 40% DMEM) was added. The suspension was dispensed into freezing tubes. After refrigerating at −80°C overnight, the tubes were transferred into a liquid nitrogen tank for long-term preservation. For the cells to be recovered, the freezinging tube was take out from the liquid nitrogen and rapidly put it into 42°C water bath. When the contents melted, the complete medium was added and the cells were cultured at 37.5°C, 5% CO2 incubator.
The localization of chicken skeletal muscle satellite cells in muscle tissues
The muscle tissues from 15-day old chicken embryos were sectioned using Leica cryostat microtome (Leica CM 1850), subsequently fixed in 4% paraformaldehyde for 30 min at room temperature and then washed three times with PBS (each 5 min). The section was incubated in 3% H2O2 for 15 min to eliminate endogenous peroxidase activity and then washed three times with PBS (each 5 min). In order to block the combining sites of non-specific antibodies, the section was incubated with 4% BSA at room temperature for 30 min. Then monoclonal anti-pax7 antibody (1:50, Santa Cruz Technology) and monoclonal anti-MyoD antibody (1:60, Abcam) were added and incubated for 1.5 h at room temperature. The primary antibody was removed and the cells were washed three times with PBS (each 5 min). FITC-conjugated goat anti-mouse secondary antibody (1:100, Zhongshan Golden Bridge, Beijing) was added and incubated at room temperature in a darkroom for 1.5 h. The secondary antibody solution was decanted and the sample was washed three times with PBS (each 5 min) in dark. Eventually, the cells were incubated in 1 μg/ml DAPI (DNA stain) for 15 min and washed three times with PBS and photos were taken with laser scanning confocal microscope.
Identification of skeletal muscle satellite cells
Immunofluorescence
The third passage cells were cultured on glass coverslips coated with poly-lysine. When the cells proliferated to 70% confluence, they were washed three times with PBS. The cells were then fixed in 4% paraformaldehyde for 15 min at room temperature and were washed three times with ice-cold PBS (each 5 min). For the cells to permeabilize, the samples were incubated for 10 min with PBS containing 0.25% Triton X-100 and were washed three times with PBS (each 5 min). In order to close the combining sites of non-specific antibodies, the samples were incubated with 4% BSA at room temperature for 30 min. Then monoclonal anti-pax7 (1:50, Santa Cruz Technology), monoclonal anti-MyoD (1:60, Abcam), monoclonal anti-demin (1:20, Abcam), and monoclonal anti-myosin heavy chain(1:80, Develop-mental Studies Hybridoma Bank) were added and incubated overnight at 4°C. The primary antibody was removed and the cells were washed three times with PBS(each 5 min). FITC-goat anti-mouse secondary antibody (1:100, Zhongshan Golden Bridge, Beijing) was added and incubated at room temperature in a darkroom for 1 h. The secondary antibody solution was decanted and washed three times with PBS (each 5 min) in dark. Eventually, the cells were incubated in 1 μg/ml DAPI (DNA stain) for 15 min and washed three times with PBS and take photos were taken with laser scanning confocal microscope.
RT-PCR
Cells of passage 3 were collected and total RNA was extracted with Trizol (Invitrogen). Total RNA was reverse transcribed followed by 35 PCR cycles using RNA PCR kit ver 3.0 (TARAKA, China). Information of gene specific primer pairs was listed in . PCR was performed in a 50 μl mixture containing 10μl 5 × PCR Buffer (TARAKA, China), 28.5 μl ddH2O, 0.25 μl Ex-Taq (TARAKA, China), 0.5 μl forward and reverse primers, and 1.5 μl template cDNA. The cycling conditions consisted of initial 2 min at 94°C one cycle and then followed by 30 cycles of 30 s at 94°C (for denaturation), 30 s at 50–60°C (for annealing), 2 min at 72°C ( for extension). PCR products were detected by 2.5% agarose gel electrophoresis.
Table 1. Primer sequences used in RT-PCR assay.
Karyotype analysis
Chromosomes were prepared, fixed and stained following standard methods (CitationQu et al., 1998). After Gimesa staining, the chromosome numbers per spread were counted for 100 metaphase spreads under an oil immersion objective. Relative length, arm ratio and centromeric index were calculated according to the protocol of CitationSun et al. (2006) and CitationKawarai et al. (2006).
In vitro directional differentiation of skeletal muscle satellite cells
Skeletal muscle satellite cells of passage 3 were induced to differentiate into muscle cells by culturing in DMEM with 5% horse serum for ten days, and differentiated into osteocytes by culturing in DMEM supplemented with 5% horse serum and 200 ng/ml human recombinant BMP7 (invitrogen) for six days with the induction medium changed every two days (CitationKatagiri et al., 1994). For adipogenic differentiation cells were exposed to DMEM supplemented with 5% horse serum, MDI-I cocktail, 0.5 mM methyl-isobutylxanthine (Sigma-Aldrich), 1 mM dexamethasone (Sigma-Aldrich), 100 mM indomethacin (Sigma-Aldrich) and 10 mg/ml insulin(Sigma-Aldrich) for two days, followed by culturing in 10 mg/ml insulin for one day as described previously (CitationPittenger et al., 1999). This treatment cycle was performed two times (six days in total). Alizarin red staining was used for detection of osteocytes. Oil Red-O staining was used for detection of accumulated oil droplets. The expression of specific genes was determined by RT-PCR. Information of gene specific primer pairs was listed in . For each group, 10 microscopic fields were taken to calculate the positive efficiencies, the ratio of positive cells to the total cells in the field.
RESULTS
Morphology of skeletal muscle satellite cells
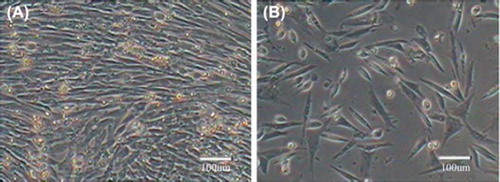
Under the inverted microscope, it was found that skeletal muscle satellite cells before adhering were round with strong refraction (). The cells began to adhere after 24 h and were spindle-shaped or fusoid (). With the increase of cell density, cells became arranged regularly in parallel (). When the cells reached 70%–80% confluence, they were subcultured using 0.25% trypsin and 0.02% EDTA. The passage cells began to adhere after 1 h and completely adhered after 24 h (–F). The cells were subcultured to passage 15, and displayed such representative senescent appearance as vacuole and karyopyknosis in most cells (). Eventually, when the passage number increased, the cells would detach from the plates.
Figure 1. Morphology of in vitro cultured skeletal muscle satellite cells A. Satellite cells before adhere were round, refract sexual strong; B. Adherent cells were spindle-shaped or fusoid; C. With the increase of the cell density, cells become regularly arranged in parallel; D–I. Chicken satellite cells, passage 2 (D), passage 4 (E), passage 6 (F), passage 8 (G), passage 10 (H) and passage 12 (I).
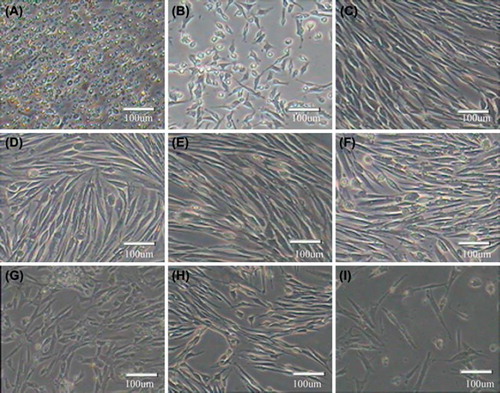
After recovery, the cells began to adhere after 1 h and grew rapidly in good health. There was no significant difference between cells before freezing and after recovery in growth and morphology. In addition, cell viabilities before freezing and after thawing were 98%±0.04% and 97.7%±0.02, respectively. Thus it was known that the freezing storage conditions were appropriate and caused little damage to the cells. The genetic resources of Beijing Fatty Chicken could be effectively preserved by this way. Cell morphology before freezing and after recovery was shown in .
The localization of chicken skeletal muscle satellite cells in muscle tissues
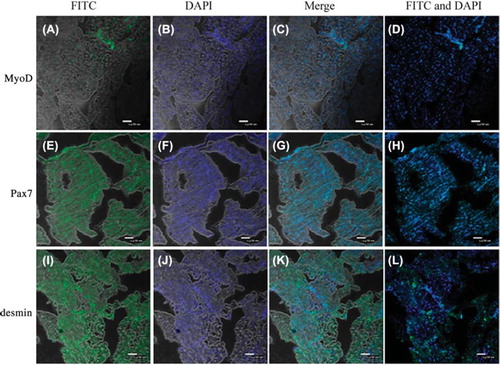
Demin and MyoD are special markers of skeletal muscle satellite cells, and are used to distinguish skeletal muscle satellite cells from mature muscle cells ( and L). Pax7, expressed in nucleus, is a special marker for muscle cell line and embryonic fibroblasts ().
Figure 3. The localization of chicken skeletal muscle satellite cells on the muscle tissue by Pax7, MyoD and desmin detection. MyoD and desmin are characteristic markers for skeletal muscle satellite cells different from those of muscle cells. Pax7 is one of the key factors for development, growth and regeneration of skeletal muscle cell. A. Skeletal muscle satellite cells expressed MyoD in muscle tissues; B. Using DAPI to stain the nuclei of muscle tissues; C. A and B Merge; D. DAPI and FITC merge; E. Skeletal muscle cells expressed Pax7 in the tissue; F. Using DAPI to stain the nuclei of muscle tissue; G. E and F Merge; H. DAPI and FITC merge; I. Skeletal muscle cells expressed desmin in muscle tissues; J. Using DAPI to stain the nuclei of muscle tissues; K. I and J Merge; L. DAPI and FITC merge.
Identification of skeletal muscle satellite cells
Immunofluorescence
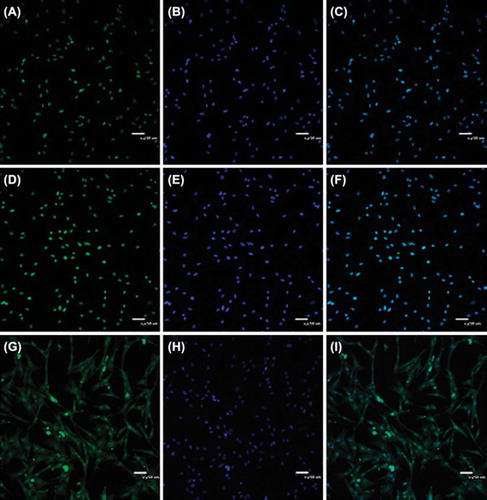
Pax7, Demin and MyoD are the specific markers of skeletal muscle satellite cells. In this experiment, we examined the expression of these specific markers in Beijing Fatty Chicken skeletal muscle satellite cells and found that all the cells expressed the three makers (). Pax7 and MyoD distributed in nucleus and Demin in cytoplasm.
Figure 4. Pax7, desmin and MyoD expression in chicken skeletal muscle satellite cells. A. Skeletal muscle satellite cells expressed Pax7 in nucleus; B. E. Using DAPI to stain the nuclei of chicken skeletal muscle satellite cells; D. Skeletal muscle satellite cells expressed MyoD in nucleus; G. Skeletal muscle satellite cells expressed desmin in cytoplasm; H. Using DAPI to stain the nuclei of chicken skeletal muscle satellite cells. C. F. I. The stacked images.
RT-PCR
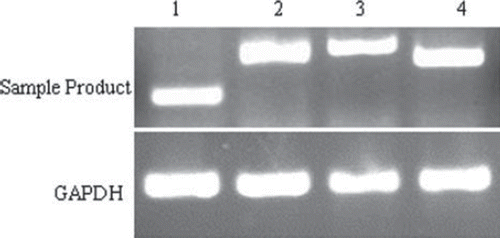
We analyzed four gene expressions of the skeletal muscle satellite cells by RT-PCR. The four genes, included c-met, Pax7, MyoD and Desmin were all specific makers of satellite cells. Experimental results showed that the chicken skeletal muscle satellite cells were positive for c-met, Pax7, MyoD and Desmin (). GADPH was used as an internal control.
Karyotype analysis
The chicken skeletal muscle satellite cells were diploid (2n = 78), containing 9 pairs of macrochromosomes and 30 pairs of microchromosomes (). The sex chromosome type is ZZ/ZW. The parameters of relative length, centromere index and kinetochore type were shown in .
Figure 6. Chromosomes at metaphase (left) and karyotype (right) of chicken skeletal muscle satellite cells (1000 ×). The chromosome number of chicken was 2n = 78, comprising 9 pairs of macrochromosomes and 30 pairs of microchromosomes, whilst the sex chromosome type was ZW.
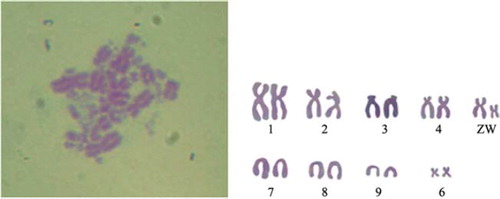
Table 2. Chromosome parameters of the chicken.
Myogenic, osteogenic and adipogenic differenciation
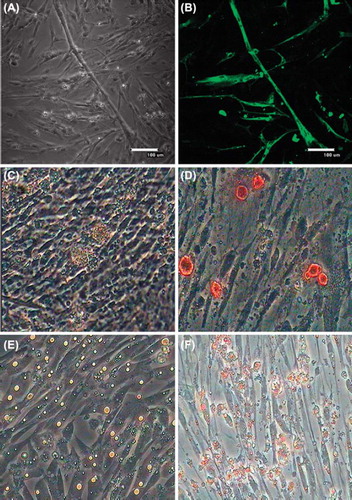
When cultured in myogenic inducing medium, cells proliferated slowly and anastomosis among cells began to generate. On the seventh day multinucleated myotubes were observed (). Immunofluorescence result showed that myosin heavy chain (MHC) was expressed in the differentiated cells (). In osteogenic differentiation when treated with human recombinant BMP7 for 3 days, skeletal muscle satellite cells began to become round from spindle and then formed calcified nodules on the sixth day (). Alizarin red staining was used to detect calcium nodules and the positive ratio was 71.24 ± 1.32%. When cultured in adipogenic-inducing medium, cells began to become round on the second day and there appeared a small amount of lipid droplets in cells on the third day and a large number of lipid droplets on the sixth day (). After staining with Oil-Red O, large amounts of lipid droplets in salmon pink color were observed in the differentiated cells and the positive ratio was observed to be 91.74 ± 0.93%.
Figure 7. Skeletal muscle satellite cells differentiate into myogenic, osteogenic and adipogenic cells. A. Skeletal muscle satellite cells initiated terminal differentiation to form multinucleated myotubes; B. MHC was expressed in the differentiated cells; C. Skeletal muscle satellite cells produced calcified nodules on the sixth day; D. Alizarin red staining was used to to detect calcium nodules; E. A large number of lipid droplets formed in differentiating cells on day 6; F. lipid droplets were stained with Oil-Red O.
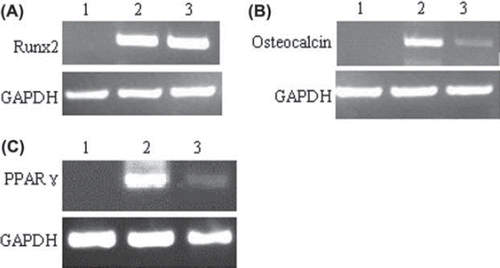
RT-PCR was also used to detect gene expression in differentiated cells. The myogenic maker myogenin was up-regulated when treated with DMEM and 5% horse serum for 10 days. Osteogenic makers runx2 and osteocalcin were up-regulated when treated with BMP7 for 6 days (). Adipogenic maker PPARγ was also up-regulated when treated with adipogenic inducing medium for 6 days ().
Figure 8. Analysis of runx2, osteocalcin and PPAR? expression of differentiating cells by RT-PCR. Runx2 is necessary transcription factor for osteogenic differentiation. Osteocalcin is expressed restrictedly in the bone tissue, which symbolizes the bone formation and bone development. PPAR? is a key factor for adipogenic differentiation and regulate fat differentiation through the regulation of specific genes. A. Lane 1. Negative control (skeletal muscle satellite cells); Lane 2. Runx2 was up-regulated in differentiated cells treated with BMP7 on day 6; Lane3. Positive control (osseous tissue). B. Lane 1. Negative control (skeletal muscle satellite cells); Lane 2. Osteocalcin was up-regulated in differentiated cells treated with BMP7 on day 6; Lane3. Positive control (osseous tissue). C. Llane 1. Negative control (skeletal muscle satellite cells); Lane 2. PPAR? was up-regulated in differentiated cells treated with MDI-I cocktail and insulin on day 6; Lane 3. Positive control (adipose tissue).
DISCUSSION
Skeletal muscle satellite cells are located between the muscle cell membrane and basement membrane, so it is diffucult to isolate the cells. In the experiment, Beijing Fatty Chicken skeletal muscle satellite cells were isolated from the pectorals of the 15-day-old Beijing Fatty Chicken embryos using combined enzymatic digestion of 0.1% collagenase I and 0.25% trypsin. Muscle fiber can be isolated by collagenase I and trypsin which make satellite cells out from the muscle cell membrane and basement membrane. Chicken skeletal muscle satellite cells were purified by the method of differential adherence, because fibroblasts adhered to the culture bottle wall more readily than skeletal muscle satellite cells (CitationLee et al., 2000). Many factors, such as dearth of genetic information and concurrent progress in other fields, unawareness and negligence of its therapeutic potentials, unwillingness to investigate animals other than model species, the delicate avian cytoarchitecture, which is not so easy to operate and so forth, are all responsible for the research involving avian skeletal muscle cells to lag behind when compared with that research regarding mammals. Therefore, this research is performed, which showed that chicken skeletal muscle satellite cells could be cultured and in vitro for at least 15 passages.
The molecular markers for identifying quiescent satellite cells include VCAM-1 (CitationTeboul et al., 1995), c-Met (CitationRoss et al., 2000), Pax7 (CitationLadjali-Mohammedi et al., 1999), and M-cadherin while the molecular markers Myf5, MyoD and Desmin (CitationPittenger et al., 1999) can be used to identify proliferating skeletal muscle satellite cells. In this study, we detected the expression of Pax7, c-Met, Demin and MyoD to identify chicken skeletal muscle satellite cells by immunofluorescence and RT-PCR. And all the results showed that the chicken skeletal muscle satellite cells were c-Met, Pax7, MyoD and Demin positive.
Cells possess a characteristic chromosome number, shape and structure, which remain very stable in the normal cells. Therefore, karyotype analysis is a major method for distinguishing normal cells from variants. The chicken skeletal muscle satellite cells cultured in this study were all normal diploids. The International Poultry Karyotype Criterion defines poultry genome as comprising 8 pairs of macrochromosomes and 30 pairs of microchromosomes. In our experiment, we found that the chicken skeletal muscle satellite cells were diploid (2n = 78), containing 9 pairs of macrochromosomes and 30 pairs of microchromosomes. This proved that the genetic property of the cells we cultured was stable.
Previous work has demonstrated that myogenic cells such as C2C12 myoblasts, or clonal myogenic cells isolated from adult skeletal muscles display osteogenicor adipogenic (CitationLaBarge & Blau, 2002) differentiation capability following treatment with BMPs or adipogenic inducers, respectively. In addition, blocking the Wnt signaling pathway also induces adipogenic differentiation of C2C12 cells (CitationRoss et al., 2000). However, it remains unclear whether chicken skeletal muscle satellite cells possess the similar differentiation capabilities. In this work, we clearly demonstrated that chicken skeletal muscle satellite cells could give rise to osteocytes and adipocytes as well as skeletal myocytes.
In conclusion, this study established an optimal method for isolation and culture of chicken skeletal muscle satellite cells, and identified cells in cell morphology and molecular expression. We also induced the satellite cells to muscle, osteogenic and adipogenic cells, which proved that they were multipotential stem cells. Through this study we have not only provided a technology platform for establishment of chicken skeletal muscle satellite cell bank, but also opened a new way to preserve the valuable genetic resources of Beijing Fatty Chicken.
Declaration of interest: The authors report no declarations of interest. The authors are responsible for the content and writing of the paper.
This research was supported by the Ministry of Agriculture of China for Transgenic Research Program (2011ZX08009-003-006, 2011ZX08012-002-06), the project of National Infrastructure of Animal Germplasm Resources (2012 year) and the central level, scientific research institutes for R & D special fund business (2011cj-9, 2012zl072).
REFERENCES
- Altarsha M, Benighaus T, Kumar D, Thiel W (2009). How is the reactivity of cytochrome P450cam affected by Thr252X mutation? A QM/MM study for X = serine, valine, alanine, glycine. J Am Chem Soc. 131: 4755–4763.
- Asakura A, Komaki M, Rudnicki M (2001). Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 68: 245–253.
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA (2007). Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 317: 807–810.
- Collins CA, Gnocchi VF, White RB, Boldrin L, Perez-Ruiz A, Relaix F, Morgan JE, Zammit PS (2009). Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PloS One. 4: e4475.
- Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Wold B (2001). Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 189: 189–196.
- Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S (2005)Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 19: 1426–1431.
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T (1994). Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 127: 1755–1766.
- Kawarai S, Hashizaki K, Kitao S, Nagano S, Madarame H, Neo S, Ishikawa T, Furuichi M, Hisasue M, Tsuchiya R, Tsujimoto H, Yamada T (2006). Establishment and characterization of primary canine hepatocellular carcinoma cell lines producing alpha-fetoprotein. Vet Immunol Immunopathol. 113:30–36.
- Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA (2006). Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 172: 103–113.
- LaBarge MA, Blau HM (2002). Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 111: 589–601.
- Ladjali-Mohammedi K, Bitgood JJ, Tixier-Boichard M, Ponce De Leon FA (1999). International system for standardized avian karyotypes (ISSAK): standardized banded karyotypes of the domestic fowl (Gallus domesticus). Cytogenet Cell Genet. 86: 271–276.
- Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J (2000). Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 150: 1085–1100.
- Mauro A (1961). Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 9: 493–495.
- McFarland DC, Pesall JE, Gilkerson KK, Swenning TA (1993). Comparison of the proliferation and differentiation of myogenic satellite cells derived from Merriam's and commercial varieties of turkeys. Comp Biochem Physiol Comp Physiol. 104: 455–460.
- McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA (2008). Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 10: 77–84.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999). Multilineage potential of adult human mesenchymal stem cells. Science. 284: 143–147.
- Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J (1998). Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 142: 1257–1267.
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M (2006). Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 172:91–102.
- Relaix F, Rocancourt D, Mansouri A, Buckingham M (2005). A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 435: 948–953.
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA (2000). Inhibition of adipogenesis by Wnt signaling. Science. 289: 950–953.
- Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z (2004). Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 117: 5393–5404.
- Sun CC, Su Pang JH, Cheng CY, Cheng HF, Lee YS, Ku WC, Hsiao CH, Chen JK, Yang CM (2006). Interleukin-1 receptor antagonist (IL-1RA) prevents apoptosis in ex vivo expansion of human limbal epithelial cells cultivated on human amniotic membrane. Stem Cells. 24: 2130–2139.
- Teboul L, Gaillard D, Staccini L, Inadera H, Amri EZ, Grimaldi PA (1995). Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. J Biol Chem. 270: 28183–28187.
- Yablonka-Reuveni Z, Rivera AJ (1994). Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 164: 588–603.
- Zammit PS, Carvajal JJ, Golding JP, Morgan JE, Summerbell D, Zolnerciks J, Partridge TA, Rigby PW, Beauchamp JR (2004). Myf5 expression in satellite cells and spindles in adult muscle is controlled by separate genetic elements. Dev Biol. 273: 454–465.
- Zammit PS (2008). All muscle satellite cells are equal, but are some more equal than others? J Cell Sci. 121: 2975–2982.