Abstract
The regulation of adhesion and growth is important for epithelial function and dysfunction. β-catenin (armadillo in Drosophila) is the prototype of a multifunctional molecule that regulates cell adhesion via adherens junctions and cell signaling via LEF/TCF transcription factors. Desmosomal armadillo proteins comprise plakoglobin and the plakophilins 1, 2, and 3. These proteins are essential for building up the desmosome and linking the desmosomal cadherins to keratin filaments. High expression of plakophilins in desmosomes correlates with strong intercellular cohesion and is essential for tissue integrity under mechanical stress. However, like β-catenin, these proteins have diverse non-desmosomal functions, for example, in regulating actin organization, protein synthesis, and growth control. In line with these functions, their de-regulated expression with up- as well as down-regulation has been connected to cancer and metastasis. Now, recent evidence sheds light on the post-translational regulation and provides an explanation for how de-regulation of plakophilins can contribute to cancer.
INTRODUCTION
Desmosomes are dynamic intercellular junctions that are essential for cell cohesion and tissue integrity. They are composed of transmembrane proteins, the desmosomal cadherins (desmogleins and desmocollins), and intracellular plaque proteins that link the transmembrane proteins to the intermediate filament network. The desmosomal cadherins interact with molecules of the armadillo family such as plakoglobin (PG) and plakophilins (PKPs) 1–3. This membrane complex provides a platform for association with desmoplakin (DP) (CitationThomason et al., 2010; CitationDubash & Green, 2011). DP couples the keratin network to sites of desmosomal adhesion. In recent years, it has been recognized that the desmosomal armadillo proteins are multifunctional: When located at the desmosomes they promote cell adhesion, but in the cytoplasm or nucleus they participate in cell signaling.
When desmosomal adhesion is compromised, cells lose cohesiveness leading to severe conditions affecting primarily tissues that are subjected to mechanical stress, such as the heart and the skin (CitationBazzi & Christiano, 2007; CitationLai-Cheong et al., 2007; CitationThomason et al., 2010; CitationBrooke et al., 2012; CitationAl-Jassar et al., 2013). Symptoms typically include epidermal fragility and blistering, thickened skin on the palm or soles (palmoplantar keratoderma), and/or cardiomyopathy. Of the armadillo-related desmosomal plaque proteins, PG and PKP2 are essential and their ablation in the mouse leads to embryonic death (CitationRuiz et al., 1996; CitationGallicano et al., 1998; CitationRuiz & Birchmeier, 1998; CitationBierkamp et al., 1999). In humans, mutations have been detected in PG, PKP1, and PKP2 leading to heart or skin diseases or a combination of both (CitationBazzi and Christiano, 2007; CitationMcGrath & Mellerio, 2010; CitationBrooke et al., 2012; CitationAl-Jassar et al., 2013).
In this review, we will summarize the structural and signaling functions of the PKP family of armadillo proteins in epithelia and discuss how these proteins are regulated. Finally, we will evaluate the potential role of these proteins in cancer. For a discussion of PKPs in genetic diseases of the skin or the heart, the reader is referred to other reviews in this issue.
PKPS ARE EVOLUTIONARILY CONSERVED PROTEINS
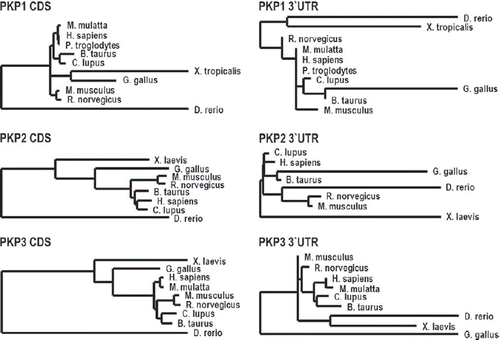
PKPs are members of a subfamily of armadillo proteins named according to the founder member the p120-catenin (p120ctn) family of armadillo proteins. This family comprises seven members, four of which are found primarily in adherens junctions (p120ctn, NPRAP/ δ-catenin, ARVCF (Armadillo-Repeat gene deleted in Velo-Cardio-Facial syndrome) and p0071/PKP4), whereas PKPs1–3 localize primarily at desmosomes. Several family members with homology to p120ctn are found in vertebrates, although invertebrates such as C. elegans and Drosophila express only one representative of this family. Ablation of the sole p120ctn in these species is not lethal, whereas a knockout of p120ctn in mice leads to early embryonic death, indicating that these proteins have acquired essential functions in higher organisms. In contrast to the p120ctn family, PKPs are vertebrate-specific and PKPs1–3 are found in zebrafish, Xenopus, chicken, and mammals (CitationTewari et al., 2010). Whereas their coding sequence is highly conserved between the species, the untranslated region (UTR) reveals considerable divergence in length and sequence, suggesting some heterogeneity in their post-transcriptional regulation ().
Figure 1. Sequence conservation of PKPs.
Phylogenetic analysis of the PKPs is based on cDNA-sequences of the respective coding sequences (CDS) or 3´-UTR. The phylogenetic tree was created using the “One click” mode of the Phylogeny.fr platform (CitationCastresana, 2000; CitationGuindon and Gascuel, 2003; CitationEdgar, 2004; CitationAnisimova and Gascuel, 2006; CitationChevenet et al., 2006; CitationDereeper et al., 2008; CitationDereeper et al., 2010). The length of the horizontal connections correlates with the degree of nucleotide substitution.
THE STRUCTURE OF PKPS DETERMINES THEIR INTERACTIONS
How can PKPs combine adhesive and signaling activities and interact with numerous binding partners? PKPs consist of a central domain of nine armadillo (arm) repeat motifs that are flanked by rather long N- and very short C-terminal domains, respectively. Each arm repeat comprises approximately 42 amino acids that form three short α-helices arranged in a triangular shape (CitationChoi & Weis, 2005). β-catenin and PG contain 12 arm repeats flanked by rather short N- and C-terminal domains. In β-catenin, these arm repeats form a superhelix with a long, positively charged groove which serves as an interaction platform for many binding partners and is therefore considered as a scaffold for protein complex formation (CitationHuber et al., 1997). Since most protein interactions involve the same region of β-catenin/armadillo the spatial segregation of these binding partners in the cell appears to be an important regulatory mechanism. The PKP arm domain is interrupted between repeats five and six by a sequence that introduces a kink into the overall structure (CitationChoi & Weis, 2005).
Whereas β-catenin binds to many distinct proteins by its arm domain, PKPs interact with binding partners by their N-terminal domains and interactions that depend on their arm repeat region have so far not been described. The N-terminal domains of PKPs are much larger than in β-catenin and are devoid of any known secondary structures with the exception of a short conserved α-helical stretch close to the N-terminus. Therefore, they are considered as largely unfolded. The function of natively disordered proteins has been related to their flexibility and their ability to adopt suitable conformations upon interacting with their binding partners. Binding to distinct partners can lead to distinct structures that correlate with multiple functions (CitationZhuang et al., 2011). Thus, the N-terminal domains of PKPs may adopt distinct structures when associated with various desmosomal, cytoplasmic or nuclear binding partners. Such protein interactions may be required to induce folding and protect PKPs from degradation. Post-translational modifications could serve a similar purpose by inducing conformations suitable for the interaction with a specific binding partner. In this context, the high number of phosphorylation sites in the N-terminal domains of PKPs is striking and suggests that these modifications play a role in determining the structure–function relationships. A third option to induce a secondary structure could be an association of the N-terminal domain with the arm repeat region either in cis or in trans within a dimer.
FUNCTION OF PKPS IN REGULATING ADHESION
PKPs were first described as desmosomal proteins that interact with many other desmosomal proteins including the desmosomal cadherins, PG, and DP. They exhibit differential but overlapping tissue distribution patterns (CitationSchmidt & Jager, 2005). PKP1, originally described as band 6 protein (CitationHatzfeld et al., 1994; CitationHeid et al., 1994) is found in desmosomes of stratified epithelia and localizes primarily to desmosomes in suprabasal layers. PKP2 is expressed in all simple, complex, and stratified epithelia but is also found in non-epithelial junctions from cardiac myocytes and germinal centers of lymph node follicles. PKP3 is more widely expressed and was detected in most simple and stratified epithelia except hepatocytes. In the epidermis, no differentiation-associated changes in its distribution have been noted. These overlapping but distinct expression patterns suggest tissue-specific functions but at the same time allow for some redundancy, for example, in facilitating cell cohesion (CitationBass-Zubek et al., 2009). In agreement, loss of function mutations in the PKP1 gene in humans (CitationMcGrath & Mellerio, 2010) or targeted deletion of PKP3 in mice (CitationSklyarova et al., 2008) lead to skin disease with reduced epithelial stability but are not lethal. In contrast, loss of PKP2 which is the only PKP of cardiomyocytes, induces embryonic lethality in a knockout mouse model due to heart failure (CitationGrossmann et al., 2004).
Based on their interaction with many desmosomal proteins PKPs appear to act as scaffolds in desmosome formation to provide a link among the transmembrane cadherins, PG, DP, and keratins. Additionally, they can laterally cluster with DP to increase desmosome size (CitationKowalczyk et al., 1999; CitationHatzfeld et al., 2000; CitationChen et al., 2002; CitationBonne et al., 2003). Although a direct association with keratins was demonstrated in vitro (CitationKapprell et al., 1988; CitationSmith & Fuchs, 1998; CitationHatzfeld et al., 2000; CitationBonne et al., 2003), the in vivo relevance of this interaction has been disputed since PKPs were detected in the outer desmosomal plaque, whereas keratins are anchored at the inner desmosomal plaque (CitationNorth et al., 1999). The distance between the inner and the outer plaque is bridged by DP, which anchors the keratin filaments via its C-terminus and at the same time binds to PKPs via its N-terminal domain. Therefore, PKPs are considered to anchor keratin filaments at the desmosome indirectly via DP.
Although all PKPs localize to desmosomes only PKP1 considerably enhances the number and/or size of cell–cell contacts when overexpressed in cultured cells (CitationKowalczyk et al., 1999; CitationHatzfeld et al., 2000; CitationSouth et al., 2003; CitationSouth, 2004). This correlates with the finding that PKP1 is highly expressed in the suprabasal cells of the epidermis which contain numerous large desmosomes. In agreement, loss-of-function mutations in PKP1 are accompanied by small and fewer desmosomes in the epidermis (CitationMcGrath et al., 1997). Furthermore, PKP1 overexpression transformed desmosome adhesion from a calcium-dependent to a calcium-independent, hyperadhesive state and protected keratinocytes from Pemphigus vulgaris autoantibody-induced loss of cell–cell adhesion (CitationTucker et al., 2013). These data suggest that PKP1 is specifically suited to impart strong intercellular cohesion and protect the epidermis from mechanical and other stresses.
Although PKPs are considered as core desmosomal proteins, they can under certain circumstances associate with proteins from adherens junctions. This is observed for PKP2 in cardiomyocytes which form mixed junctions termed “area composita” (CitationPieperhoff et al., 2008). However, PKP2 has also been shown to co-localize with and co-immunoprecipitate β-catenin when ectopically expressed in cultured keratinocytes (CitationChen et al., 2002). These findings may indicate a role for PKP2 in sorting adherens junctions and desmosome components (CitationBass-Zubek et al., 2009). Moreover, it was suggested that E-cadherin together with PG is required to recruit PKP3 to cell borders to initiate desmosome formation (CitationGosavi et al., 2011). An association of the adherens junction proteins p0071 and p120ctn with desmosomal proteins has also been reported (CitationHatzfeld et al., 2003; CitationKanno et al., 2008a, Citation2008b). Taken together, these findings suggest that the armadillo proteins, although restricted to either adherens junctions or desmosomes in the mature cell–cell contacts, can transiently associate with the “wrong” type of junctional proteins and may participate in the segregation of junction complexes or in limiting the size of the individual junctions.
SIGNALING FUNCTIONS
Rho-signaling
Although the function of p120ctn and its direct homologs in regulating the actin cytoskeleton via small Rho- GTPases is well established (CitationWolf et al., 2006; CitationAnastasiadis, 2007; CitationKeil et al., 2007; CitationDohn et al., 2009; CitationSchackmann et al., 2013), much less is known about the role of PKPs in organizing actin filaments and regulating Rho-GTPases. Keratinocyte cell–cell adhesion and differentiation depends on calcium-mediated and calcium-sensing receptor-mediated RhoA-signaling (CitationTu & You, 2013). Calcium stimulates formation of filopodia that embed into neighboring cells in a Rho/ROCK-dependent way. E-cadherin localizes at filopodia tips generating a zipper of E-cadherin positive puncta. The contacts achieved by interdigitating filopodia induced adherens junction initiation and maturation and subsequently desmosome assembly at flanking sites (CitationVasioukhin et al., 2000). This suggests that RhoA is involved in regulating not only adherens junctions but also in desmosome assembly. PKP2 was shown to regulate cortical actin rearrangement during junction formation (CitationGodsel et al., 2010). These authors showed that in PKP2 knockdown cells activated RhoA failed to localize at intercellular interfaces after the cell–cell contact induction, implicating a role of PKP2 in the spatio-temporal control of RhoA during junction formation (). At the same time, actin stress fibers were increased resulting from elevated levels of cellular RhoA. Consistent with this observation, focal adhesions were larger and more stable in PKP2-deficient cells, and vinculin dynamics were reduced (CitationKoetsier et al., 2013). Based on these data, the authors speculated that PKP2 might play a role in integrating the cell–cell and cell–substrate contact signaling in basal keratinocytes.
How PKPs1 and 3 are involved in Rho-signaling has not been established. PKP1 associates with actin filaments when ectopically expressed in cells with little or no desmosomes such as HeLa or NIH3T3 cells (CitationHatzfeld et al., 2000). In keratinocytes, an association with actin was observed in filopodia not connected to neighboring cells. Moreover, ectopic expression of the arm repeat domain induced the formation of filopodia and long cellular protrusions strongly suggesting a de-regulation of Rho-GTPases. Waschke et al. provided evidence that pemphigus vulgaris autoantibodies interfere with RhoA activation, thereby inducing loss of desmosomal adhesion (CitationWaschke et al., 2006). Specific activation of RhoA abolished all pemphigus-triggered effects, including cell dissociation. Since the overexpression of PKP1 also abolished cell dissociation the control of Rho-signaling by PKP1 might participate in this phenomenon. However, how exactly PKP1 influences actin organization and how this correlates with a role in regulating Rho-GTPases remains to be determined.
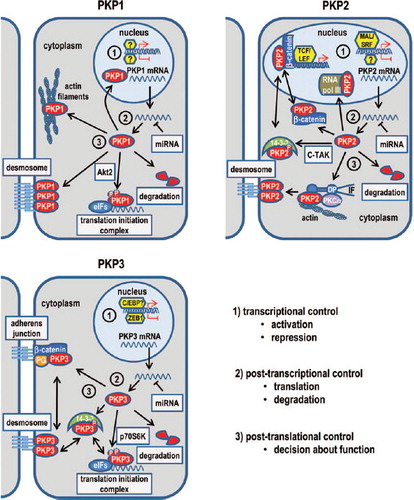
PKP functions in RNA metabolism, translation and proliferation
PKPs1 and 3, but not PKP2, have been shown to associate with RNA-binding proteins (RNA-BPs) and modulate RNA metabolism. Upon cell stress, translation of housekeeping genes is arrested and translation becomes restricted to targets that are directly required for cell survival. However, mRNAs are not degraded under these conditions but instead are stored in stalled translation initiation complexes called stress granules together with many RNA-BPs. Application of oxidative stress to cultured cells induced the accumulation of PKPs1 and 3 in such stress granules, whereas PKP2 was excluded from these structures suggesting distinct functions of the PKPs in this context (CitationHofmann et al., 2006; CitationWolf and Hatzfeld, 2010; CitationWolf et al., 2010). In an attempt to characterize the role of PKP3 in RNA metabolism, CitationHofmann et al. (2006) analyzed proteins that co-purified together with PKP3 in immunoprecipitation experiments. This approach led to the identification of several RNA-BPs, Polyadenylate-binding protein 1 (PABPC1), Fragile X mental retardation-related protein 1 (FXR1), and GAP-SH3-binding protein (G3BP) as PKP3-interacting proteins. The functional relevance of these interactions has so far not been analyzed. PABPC1 is a component of the translation initiation complex. However, whether the PKP3–PABPC1 interaction facilitates translation remains to be determined. FXR1 is a member of the mammalian Fragile X protein family that comprises three members, Fragile X Mental Retardation Protein (FRMP), FXR1 and FXR2, respectively. These RNA-BPs are involved in controlling mRNA translation (CitationZarnescu & Gregorio, 2013). Loss of FMRP is the cause for the most common form of inherited intellectual disability, Fragile X syndrome (reviewed in [CitationKim & Ceman, 2012]). FMRP family proteins appear to regulate distinct sets of target mRNAs (CitationDarnell et al., 2011). Although FXR1 is typically considered as a negative regulator of translation, it can also act as a translational activator by binding AU-rich elements (AREs) of the tumor necrosis factor alpha (TNFα) mRNA (CitationVasudevan & Steitz, 2007). AREs are one of the most common determinants of RNA stability and are found in many mediators of the inflammatory response. They usually target the mRNA for degradation. Binding of RNA-BPs can stabilize these mRNAs (CitationVasudevan & Steitz, 2007). Based on the finding that the targeted deletion of the PKP3 gene was associated with an increase in the inflammatory response (CitationSklyarova et al., 2008) it is tempting to speculate that PKP3 might contribute to limiting the inflammatory response via its interaction with FXR1. Alternatively, the PKP3–FXR1 interaction could play a role in controlling desmosomal adhesion. In cardiomyocytes, the translation of DP was shown to be controlled by FXR1, which binds the DP–mRNA and inhibits its translation (CitationWhitman et al., 2011). Thus, by associating with FXR1–PKP3 could also control desmosomal adhesion. Interestingly, DP levels were elevated in the PKP3 knockout mice (CitationSklyarova et al., 2008). In order to understand, the relevance of the PKP3-RNA-BP associations it will be necessary to determine under which circumstances PKP3 interacts with these proteins and how it modulates their activities.
PKPs1 and 3 have been shown to stimulate translation in vitro as well as in cells (CitationWolf et al., 2010). Moreover, both proteins associated with the mRNA-cap- binding complex that consists of the eukaryotic translation initiation factors (eIFs) 4A, 4B, 4G, 4E, and PABPC. PKP1 directly interacted with and stimulated the enzymatic activity of eIF4A, a helicase that has been implicated in the unwinding of secondary structures in the 5’-UTRs of mRNAs to facilitate scanning for the start codon and translation initiation. This leads to an overall up-regulation of translation in PKP1 overexpressing cells, whereas the knockdown of PKP1 reduced protein biosynthesis. Since proliferation depends on protein synthesis the function of PKPs1 and 3 in controlling translation correlated with a function in regulating proliferation and cell size.
PKP functions in the nucleus
All PKPs have been observed in the nucleus. Nuclear localization of PKP1 is most prominent and endogenous as well as overexpressed PKP1 have been detected in the nucleus. Two splice variants that differ by an insertion in the arm repeat domain of PKP1 have been described with distinct subcellular localization patterns: PKP1a appears able to associate either with desmosomes or localize to the nucleus, whereas the minor variant PKP1b has been reported to be exclusively nuclear, suggesting that splicing is involved in regulating PKP1 localization and function (CitationSchmidt et al., 1997).
DNAse I digestion of chromatin resulted in loss of PKP1 from the nucleus of cultured cells suggesting an interaction with DNA. In agreement, the N-terminal domain of PKPs1 and 2 was reported to bind to single-stranded DNA in vitro. This was correlated with a putative function in the DNA damage response. Induction of DNA damage induced a partial redistribution of PKP1 to the nucleolus, and depletion of PKP1 resulted in increased survival in response to DNA damage (CitationHatzfeld, 2010; CitationSobolik-Delmaire et al., 2010). However, protein-binding partners of PKP1 that would link PKP1 to the DNA damage response and provide some mechanistic insight have not been reported.
PKP2 was found to co-immunoprecipitate with a subunit of RNA polymerase (pol) III (CitationMertens et al., 2001). Pol-III plays a pivotal role in the regulation of protein synthesis and growth control as it catalyzes the synthesis of a variety of short, untranslated RNA molecules, many of which have essential functions in cellular metabolism including tRNA and 5S rRNA (CitationHatzfeld, 2007; CitationBass-Zubek et al., 2009). Pol-III transcription is regulated by nutrient availability, cell stress, and cell cycle stage and is elevated in tumor cells. However, the role of PKP2 in the pol-III complex remains elusive. Another nuclear function of PKP2 might be in transcriptional control. PKP2 can indirectly regulate transcription by stimulating β-catenin/TCF-mediated transcriptional activity implicating a role in Wnt signaling (CitationChen et al., 2002). The functions of PKPs reported to date are summarized in .
PKP FUNCTION IN CANCER: LOSS OR GAIN OF FUNCTION?
In contrast to the well-documented role of adherens junctions in tumorigenesis, the contribution of desmosomes to cancer is not well understood. The loss of E-cadherin expression is a hallmark in carcinogenesis and activation of the signaling function of β-catenin promotes proliferation by stimulating the expression of pro-tumorigenic factors such as myc and this is frequently observed in colon and other cancers (CitationBirchmeier et al., 1995; CitationConacci-Sorrell et al., 2002; CitationGloushankova, 2008).
Since desmosomes have traditionally been viewed as static protein complexes that reinforce adhesion between epithelial cells, it was expected that a loss of desmosomes would facilitate migration and proliferation and thus tumor growth and/or metastasis. Many descriptive studies have been published addressing the expression of desmosomal proteins including PKPs in cancer and trying to correlate the expression pattern with tumor progression (CitationDusek & Attardi, 2011). However, the results of these studies are contradictory with up-regulation, down-regulation, or maintenance of desmosome components being reported. While the expression of PKPs1–3 was increased in certain cancers of various origin (CitationFurukawa et al., 2005; CitationCheung et al., 2007; CitationDemirag et al., 2012; CitationTakahashi et al., 2012), the loss or reduction of PKPs1–3 was observed in the development and/or progression of the same or other cancers (CitationSobolik-Delmaire et al., 2007; CitationNarayana et al., 2010; CitationDemirag et al., 2011; CitationTakahashi et al., 2012). Both conditions were correlated with advanced tumor grade, increased metastasis and/or poor prognosis. summarizes PKP expression in cancer of various origins. PKPs1 and 2 appear up- or down-regulated with similar frequencies (PKP1 up-regulation described in 41, down-regulation in 32 studies; PKP2: up-regulation described in 30, down-regulation in 23 studies; ), whereas an up-regulation of PKP3 was more frequently described than its down-regulation (53 vs. 18 reports). Finally, a number of analyses found no changes in PKP-expression during cancer progression (CitationKurzen et al., 2003; CitationDemirag et al., 2011; CitationDemirag et al., 2012). In a few studies, immunohistological or immunofluorescence data suggested that changes in intracellular localization rather than expression level might be important (CitationKurzen et al., 2003; Sobolik- Delmaire et al., 2007; CitationGomez-Morales et al., 2013). Studies in cultured tumor-derived cell lines were likewise confusing. In some cases, the overexpression of a PKP suppressed tumor-promoting behavior such as invasion and anchorage-independent growth (CitationSouth et al., 2003), whereas in other experiments the overexpression promoted proliferation and increased invasion (CitationKundu et al., 2008; CitationWolf and Hatzfeld, 2010; CitationWolf et al., 2010). In light of the multiple functions of PKPs in cell adhesion and signaling, their contribution to cancer might be context-dependent and determined by the status of distinct signaling pathways that affect PKP functions.
Figure 3. Changes in PKP expression in various tumors.
The NextBio Disease Atlas (CitationKupershmidt et al., 2010) was used to identify studies showing significantly altered expression of the PKPs in various cancers compared to the corresponding normal tissues. Red bars correspond to the number of studies showing up-regulation, green bars indicate the number of studies showing a down-regulation of PKP-mRNA levels.
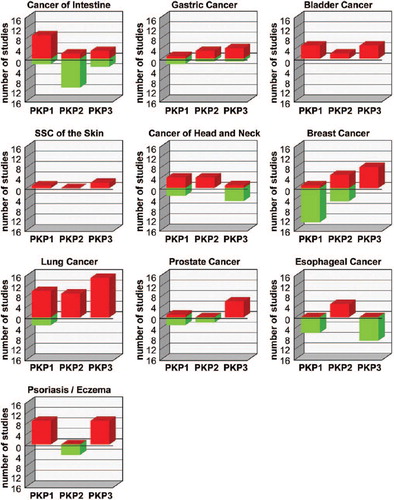
Table 1. PKP expression in cancer.
An up-regulation of PKPs1 and 3 has also been reported in prevalent skin diseases associated with inflammation and hyperproliferation such as psoriasis, whereas PKP2 was not affected in this condition (Figure 3, ).
REGULATION OF MULTIFUNCTIONAL PKPS BY PHOSPHORYLATION
The spatio-temporal control of many multifunctional proteins including PKPs during cell and tissue differentiation remains a challenging topic. As discussed before, PKPs can interact with various binding partners in specific cellular compartments where they have distinct and partially opposing functions. Thus the decision on where PKPs localize and with which proteins they interact has a major impact on cell fate and an imbalance in PKP regulation may lead to disease such as cancer. Post-translational modifications can modulate protein interactions and as a consequence control protein stability, transport, localization, and finally determine protein function. Phosphorylation represents the most frequent form of modification, and serine/threonine or tyrosine kinases are activated by signaling pathways to control the functions of their targets. PKPs contain numerous phosphorylation sites, which are highly clustered in their N-terminal domains. Many of these sites have been detected as being phosphorylated in large-scale mass spectrometry approaches. PKP1 contains 52 serine/threonine and 15 tyrosine residues that were found to be phosphorylated, in PKP2 43 serine/threonine- and 23 tyrosine-phosphorylated sites have been identified, and PKP3 contains 32 phosphorylated serine/threonine and 11 phosphorylated tyrosine residues (http://www.phosphosite.org/). Moreover, methylation has been detected in all three PKPs, an acetylation in PKP3 and 15 ubiquitinated lysine residues were reported in PKP2 (CitationKim et al., 2011; CitationWagner et al., 2012). This wealth of modifications needs further characterization to distinguish between “basal” modifications that could, for example, serve to stabilize the unfolded head domain and those that are induced by specific signals and thus depend on the context of the cell or tissue. Moreover, the functional consequences with respect to PKP associations, localization, and functions need to be determined.
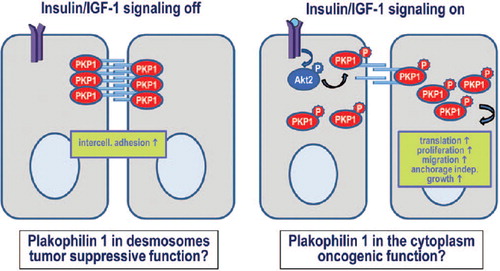
A recent report shows how phosphorylation of PKP1 controls the distribution between its desmosomal and cytoplasmic pools and shifts the balance from an adhesion promoting function of un-phosphorylated PKP1 to a translation and proliferation promoting function of the phosphorylated form (, [CitationWolf et al., 2013]). Insulin/IGF-1-signaling via the PI3K–AKT pathway induced the phosphorylation of the PKP1 head domain. This led to the stabilization of the cytoplasmic pool which in turn correlated with an increase in translation rates and as a consequence an increase in proliferation. At the same time intercellular cohesion was impaired. This regulation was highly specific and mediated by the AKT2 isoform of AKT kinases that interacted directly and specifically with PKP1, but not PKP2 or 3. Moreover, EGF was much less efficient in stimulating PKP1 phosphorylation and re-localization compared to insulin/IGF-1. Four phosphorylation motifs in the N-terminal domain were identified. Interestingly, the expression of a phospho-mimetic mutant of PKP1 not only stimulated translation and proliferation but also conferred the capacity of anchorage-independent growth. This suggests that the AKT2-mediated modification might play a role in tumorigenesis. In support of this, AKT2 is associated with tumors with a much higher frequency than AKT1 (CitationCohen, 2013). Moreover, several of the AKT2 phosphorylation sites of PKP1 were identified in a mouse model for squamous cell carcinoma (CitationZanivan et al., 2013). Precisely how phosphorylated PKP1 is protected from degradation remains to be determined. 14-3-3 proteins bind specifically to phosphorylated target proteins and modulate their localization, stability, and functions. Seven isoforms of 14-3-3 in humans and several hundreds of 14-3-3 binding partners have been identified to date. These proteins are involved in diverse processes like regulation of the cytoskeleton, GTPase function, membrane signaling, cell fate determination, response to insulin and TNFα, cell cycle progression, and apoptosis (CitationUhart & Bustos, 2013). Interestingly, two of the motifs phosphorylated by AKT2 are putative 14-3-3 binding sites raising the possibility that an interaction with 14-3-3 might be involved in regulating PKP1.
PKP2 is a target of Cdc25C-associated kinase 1 (C-TAK1) also called microtubule affinity-regulating kinase 3 (MAP/MARK3). Its phosphorylation by C-TAK1 was shown to generate a 14-3-3 binding site (CitationMuller et al., 2003). Mutagenesis experiments identified S82 as a phosphorylation site and PKP2 mutated at this site (S82A) exhibited increased nuclear accumulation, suggesting that C-TAK1 phosphorylation and subsequent interaction with 14-3-3 prevents PKP2 nuclear localization. Whether this affects junction formation and stability has not been analyzed. PKP2 appears to be able to interact with all 14-3-3 isoforms, since all isoforms were detected as interaction partners in large-scale interaction screens (e.g., http://www.ebi.ac.uk/intact/pages/interactions/interactions.xhtml? query = Q99959*). Whether the isoforms have differential effects on PKP2 localization and function and whether they all recognize the same motif or interact with different phosphorylation sites have not been analyzed so far. Moreover, it is not known whether C-TAK1 is the only kinase generating a 14-3-3 binding site in PKP2 or any other kinases could be involved. Taken together these data indicate that PKP2, like PKP1, is regulated by specific signaling pathways that affect the translocation of cytoplasmic PKP2 to the nucleus, where it may act to stimulate β-catenin/ TCF-mediated transcriptional activity or have additional so far-unknown functions.
PKP3 was recently shown to co-purify with 14-3- 3σ/stratifin. This interaction was specific for PKP3 and not detected for PKP1 or 2 in a yeast-2-hybrid approach. Moreover, the interaction was lost in a PKP3 mutant carrying a S285A exchange, suggesting that phosphorylation of S285 in the N-terminal domain is required for this interaction (CitationRoberts et al., 2013). Stratifin interacted with cytoplasmic PKP3 and limited the exchange of cytoplasmic PKP3 with its desmosomal pool. Accordingly, decreased stratifin expression lead to increased exchange rates resulting in reduced desmosomal adhesion and increased cell migration. Phospho-S285 has so far only been detected in mouse, but not in human tissues (CitationZanivan et al., 2008; CitationHuttlin et al., 2010), and a kinase or signaling pathway that would induce the phosphorylation of PKP3 at S285 has not been reported.
Tyrosine phosphorylation of PKPs, although frequently detected in large-scale mass spectrometry analyses, has not been systematically studied so far. However, a co-immunoprecipitation experiment and a tandem affinity purification approach identified the EGF- receptor and ERBB2 as putative PKP2 interaction partners (CitationThelemann et al., 2005). Whether these receptors phosphorylate PKP2 directly or via downstream tyrosine kinases remains elusive.
Other post-translational modifications such as methylation and acetylation are likely to play additional roles in the regulation of PKPs. The functional consequences of these modifications or their interplay with phosphorylation have not been characterized.
Several large-scale screens revealed that PKP2 is regulated by ubiquitination and a total of 15 ubiquitinated lysine residues have been identified (CitationKim et al., 2011; CitationWagner et al., 2012). Protein ubiquitination is a regulatory post-translational modification that controls numerous biological processes including proteasomal degradation of proteins. In a systematic proteomics approach, Bennett et al. identified cullin 3, a core component of E3 ubiquitin-protein ligase complexes which mediate poly-ubiquitination and subsequent proteasomal degradation of specific protein substrates, as a putative PKP2-interacting protein (CitationBennett et al., 2010). It will be interesting to unravel the pathways that regulate PKP2 ubiquitination and protein stability.
TRANSCRIPTIONAL CONTROL OF PKPS
Since an up-regulation or a down-regulation of PKP-mRNAs has been associated with disease such as cancer and psoriasis, it is important to understand not only how post-translational modifications influence PKP functions and stability but also how their expression is controlled at the transcriptional and post-transcriptional levels. For all three PKPs, splice variants have been described. The PKP1 variants 1a and 1b differ by exon 7 as the alternatively used exon in the 1b isoform. This exon codes for 21 amino acids inserted at position 412 within arm repeat 4. A differential intracellular localization was reported for the two splice variants (CitationSchmidt et al., 1997). However, neither the functional consequences nor the expression patterns and regulation of these alternative splice variants have been characterized so far. Moreover, two PKP1-mRNAs that differ in their 3’-UTR are generated (CitationSchmidt et al., 1997). PKP2b differs from the canonical isoform 2a by an extra exon encoding 44 amino acids inserted at position 460 within arm repeat 3. No information is available on differential expression patterns, distinct functions, or the regulation of the splice variants. For PKP3, a splice variant has been detected very recently. PKP3b differs from the canonical PKP3a by an alternative amino- terminal exon which results in an altered N-terminal amino acid sequence (CitationMuhmer et al., 2013). Whereas PKP3a is broadly expressed in epithelial cells, PKP3b was predominantly found in desmosomes of stratified epithelia, but absent from simple epithelial cells. Tissue-specific expression was validated using a reporter assay showing that a fragment upstream of the alternative exon 1b promoted transcription only in HaCaT keratinocytes but not in CaCo2 simple epithelial cells. Therefore, the authors speculated that the two isoforms are controlled by distinct promoters. Finally, by using electromobility shift assays, they found a potential binding site in the PKP3b promoter for transcription factor C/EBP (CCAAT/enhancer binding protein) although the identity of nuclear factors that bind to the PKP3 promoter has not been validated (CitationMuhmer et al., 2013). C/EBP proteins are a family of basic leucine zipper (bZIP) transcription factors that includes six members with related functions. C/EBPα and β are highly expressed in the skin in a differentiation-dependent manner. C/EBP proteins function as transcriptional activators but can also repress transcription of certain target genes.
Epithelial–mesenchymal transition (EMT) is a process whereby epithelial cells lose apicobasal polarity and cell–cell contacts and gain mesenchymal phenotypes with increased migratory and invasive capabilities. Several transcription factors have been identified as master regulators of EMT, including the Snail, ZEB, and Twist families of E-box transcription factors. In invasive, de-differentiated MDA-MB-231 breast cancer cells, PKP3 was transcriptionally repressed by the ZEB1 (zinc finger E-box binding homeobox 1) transcription factor which also represses E-cadherin (CitationAigner et al., 2007). ZEB1 associated with two conserved E-box elements in the PKP3 promoter and partially repressed the activity of PKP3 promoter fragments in reporter assays. Since ZEB1 is up-regulated in invasive cancer cells, it was speculated that PKP3 repression by ZEB1 contributes to the disintegration of intercellular adhesion and EMT.
In breast cancer, estrogen receptor alpha (ERα) expression is associated with differentiated tumors and a lower metastasis risk. Estrogens were shown to increase the formation of desmosomes in normal and malignant cells, and several desmosomal proteins including PKP3 were up-regulated in response to estrogens (CitationMaynadier et al., 2012). Whether PKP3 is directly regulated by estrogens or indirectly via other desmosomal proteins required for its stabilization remains to be determined. Interestingly, two ERα binding sites are predicted in the PKP3 promoter region.
Serum response factor (SRF) is a transcription factor that regulates gene expression to control growth, differentiation, and cytoskeletal integrity in different cell types. Epidermal SRF-loss resulted in an abnormal epidermal architecture with hyperplasia but without visible skin lesions or erosions. SRF can be regulated through monomeric actin which inhibits its transcriptional co-activator MAL/MRTF. PKP2 was identified as one of the actin-regulated MAL targets and MAL/SRF were recruited to cis-regulatory elements of the PKP2 gene suggesting a direct regulation of PKP2 by MAL/SRF (CitationLeitner et al., 2011).
The transcriptional regulation of PKP1 has not been characterized to date. PKP1 promoter methylation resulting in a loss of protein expression was observed in Barrett's esophagus and may facilitate the progression of Barrett's esophagus to esophageal adenocarcinoma in a subset of patients via decreased desmosome assembly and increased cell motility (CitationKaz et al., 2012).
FUTURE DIRECTIONS—IS IT ALL A QUESTION OF CONTEXT?
Like β-catenin, PKPs are multifunctional molecules and we are only just beginning to unravel their diverse roles and to grasp the complexities of their distinct functions. The situation is even more complex than in the case of β-catenin since we deal with three related proteins which are often co-expressed and have unique as well as partially shared functions. Moreover, there is very little information available on the differential expression patterns or functions of the identified PKP splice variants. While all three proteins play a role in mediating desmosomal adhesion how they act in concert and how the balance of their expression influences cell cohesion remains to be determined. With regard to translation, we find overlapping functions in stimulating protein biosynthesis for PKPs1 and 3, whereas PKP2 appears to be excluded from translation initiation complexes. Least is known about the nuclear functions of PKPs and it remains one of the future challenges to identify interaction partners and functions of these proteins in the nucleus. Another unresolved issue is the role of PKP3 in limiting the inflammatory response which was deduced from the phenotype observed in PKP3 knockout mice.
As outlined in this review, part of the solution will be found in dissecting the roles of (a) the proteins that interact with PKPs and (b) the proteins that regulate PKPs. By introducing a variety of post-translational modifications, a plethora of proteins with distinct characteristics can be generated that are optimally suited for specific interactions while preventing other interactions. Large-scale screens have revealed that PKPs are the targets of numerous modifications and these modifications can act either alone or in combination to regulate nearly all aspects of their functions. Thus, deciphering the post-translational modifications of PKPs and how they are coordinately regulated is of fundamental importance to understanding their functions and the interplay of PKPs with their binding partners. Identifying the signals that induce PKP-modifications and characterizing their impact on protein function will finally allow us to understand the role of PKPs and their de-regulation in disease.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
The work in our group is funded by grants from the DFG (Ha 1791/7-1, Ha1791/8-1, GRK 1591) and the BMBF (ProNet T3) to MH.
REFERENCES
- Abba M, Laufs S, Aghajany M, Korn B, Benner A, Allgayer H (2012). Look who's talking: deregulated signaling in colorectal cancer. Cancer Genomics Proteomics. 9: 15–25.
- Aigner K, Descovich L, Mikula M, Sultan A, Dampier B, Bonne S, Van Roy F, Mikulits W, Schreiber M, Brabletz T, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A (2007). The transcription factor ZEB1 (deltaEF1) represses Plakophilin 3 during human cancer progression. FEBS Lett. 581: 1617–1624.
- Al-Jassar C, Bikker H, Overduin M, Chidgey M (2013). Mechanistic Basis of Desmosome-Targeted Diseases. J Mol Biol. 425: 4006–4022.
- Anastasiadis PZ (2007). p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 1773: 34–46.
- Ancona N, Maglietta R, Piepoli A, D'addabbo A, Cotugno R, Savino M, Liuni S, Carella M, Pesole G, Perri F (2006). On the statistical assessment of classifiers using DNA microarray data. BMC Bioinformatics. 7: 387.
- Anisimova M, Gascuel O (2006). Approximate likelihood- ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol. 55: 539–552.
- April C, Klotzle B, Royce T, Wickham-Garcia E, Boyaniwsky T, Izzo J, Cox D, Jones W, Rubio R, Holton K, Matulonis U, Quackenbush J, Fan JB (2009). Whole-genome gene expression profiling of formalin-fixed, paraffin-embedded tissue samples. PLoS One. 4: e8162.
- Arencibia JM, Martin S, Perez-Rodriguez FJ, Bonnin A (2009). Gene expression profiling reveals overexpression of TSPAN13 in prostate cancer. Int J Oncol. 34: 457–463.
- Bass-Zubek AE, Godsel LM, Delmar M, Green KJ (2009). Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol. 21: 708–716.
- Bazzi H, Christiano AM (2007). Broken hearts, woolly hair, and tattered skin: when desmosomal adhesion goes awry. Curr Opin Cell Biol. 19: 515–520.
- Bennett EJ, Rush J, Gygi SP, Harper JW (2010). Dynamics of cullin-RING ubiquitin ligase network revealed by systematic quantitative proteomics. Cell. 143: 951–965.
- Bierkamp C, Schwarz H, Huber O, Kemler R (1999). Desmosomal localization of beta-catenin in the skin of plakoglobin null-mutant mice. Development. 126: 371–381.
- Bigler J, Rand HA, Kerkof K, Timour M, Russell CB (2013). Cross-study homogeneity of psoriasis gene expression in skin across a large expression range. PLoS One. 8: e52242.
- Birchmeier W, Hulsken J, Behrens J (1995). Adherens junction proteins in tumour progression. Cancer Surv. 24: 129–140.
- Bonne S, Gilbert B, Hatzfeld M, Chen X, Green KJ, Van Roy F (2003). Defining desmosomal plakophilin-3 interactions. J Cell Biol. 161: 403–416.
- Bose S, Yap LF, Fung M, Starzcynski J, Saleh A, Morgan S, Dawson C, Chukwuma MB, Maina E, Buettner M, Wei W, Arrand J, Lim PV, Young LS, Teo SH, Stankovic T, Woodman CB & Murray PG (2009). The ATM tumour suppressor gene is down-regulated in EBV-associated nasopharyngeal carcinoma. J Pathol. 217: 345–352.
- Brooke MA, Nitoiu D, Kelsell DP (2012). Cell-cell connectivity: desmosomes and disease. J Pathol. 226: 158–171.
- Callari M, Dugo M, Musella V, Marchesi E, Chiorino G, Grand MM, Pierotti MA, Daidone MG, Canevari S, De Cecco L (2012). Comparison of microarray platforms for measuring differential microRNA expression in paired normal/cancer colon tissues. PLoS One. 7: e45105.
- Castresana J (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 17: 540–552.
- Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W, Michalopoulos G, Becich M, Monzon FA (2007). Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 7: 64.
- Chen C, Mendez E, Houck J, Fan W, Lohavanichbutr P, Doody D, Yueh B, Futran ND, Upton M, Farwell DG, Schwartz SM, Zhao LP (2008). Gene expression profiling identifies genes predictive of oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 17: 2152–2162.
- Chen DT, Nasir A, Culhane A, Venkataramu C, Fulp W, Rubio R, Wang T, Agrawal D, Mccarthy SM, Gruidl M, Bloom G, Anderson T, White J, Quackenbush J, Yeatman T (2010). Proliferative genes dominate malignancy-risk gene signature in histologically-normal breast tissue. Breast Cancer Res Treat. 119: 335–346.
- Chen X, Bonne S, Hatzfeld M, Van Roy F, Green KJ (2002). Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta-catenin signaling.J Biol Chem. 277: 10512–10522.
- Chen X, Leung SY, Yuen ST, Chu KM, Ji J, Li R, Chan AS, Law S, Troyanskaya OG, Wong J, So S, Botstein D, Brown PO (2003). Variation in gene expression patterns in human gastric cancers. Mol Biol Cell. 14: 3208–3215.
- Cheng AS, Culhane AC, Chan MW, Venkataramu CR, Ehrich M, Nasir A, Rodriguez BA, Liu J, Yan PS, Quackenbush J, Nephew KP, Yeatman TJ, Huang TH (2008). Epithelial progeny of estrogen-exposed breast progenitor cells display a cancer-like methylome. Cancer Res. 68: 1786–1796.
- Cheung IY, Feng Y, Danis K, Shukla N, Meyers P, Ladanyi M, Cheung NK (2007). Novel markers of subclinical disease for Ewing family tumors from gene expression profiling. Clin Cancer Res. 13: 6978–6983.
- Chevenet F, Brun C, Banuls AL, Jacq B, Christen R (2006). TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 7: 439.
- Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, Noh SH, Park ES, Chu IS, Hong WK, Ajani JA, Lee JS (2011). Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 17: 1850–1857.
- Choi HJ, Weis WI (2005). Structure of the armadillo repeat domain of plakophilin 1. J Mol Biol. 346: 367–376.
- Cohen MM Jr. (2013). The AKT genes and their roles in various disorders. Am J Med Genet A. 161: 2931–2937.
- Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A (2002). The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 109: 987–991.
- Cromer A, Carles A, Millon R, Ganguli G, Chalmel F, Lemaire F, Young J, Dembele D, Thibault C, Muller D, Poch O, Abecassis J, Wasylyk B (2004). Identification of genes associated with tumorigenesis and metastatic potential of hypopharyngeal cancer by microarray analysis. Oncogene. 23: 2484–2498.
- D’Errico M, De Rinaldis E, Blasi MF, Viti V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D, Palombo F, Giuliani A, Dogliotti E (2009). Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur J Cancer. 45: 461–469.
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 146: 247–261.
- Dehan E, Ben-Dor A, Liao W, Lipson D, Frimer H, Rienstein S, Simansky D, Krupsky M, Yaron P, Friedman E, Rechavi G, Perlman M, Aviram-Goldring A, Izraeli S, Bittner M, Yakhini Z, Kaminski N (2007). Chromosomal aberrations and gene expression profiles in non-small cell lung cancer. Lung Cancer. 56: 175–184.
- Demirag GG, Sullu Y, Gurgenyatagi D, Okumus NO, Yucel I (2011). Expression of plakophilins (PKP1, PKP2, and PKP3) in gastric cancers. Diagn Pathol. 6: 1.
- Demirag GG, Sullu Y, Yucel I (2012). Expression of Plakophilins (PKP1, PKP2, and PKP3) in breast cancers. Med Oncol. 29: 1518–1522.
- Dereeper A, Audic S, Claverie JM, Blanc G (2010). BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol. 10: 8.
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36: W465–469.
- Dodd LE, Sengupta S, Chen IH, Den Boon JA, Cheng YJ, Westra W, Newton MA, Mittl BF, Mcshane L, Chen CJ, Ahlquist P, Hildesheim A (2006). Genes involved in DNA repair and nitrosamine metabolism and those located on chromosome 14q32 are dysregulated in nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 15: 2216–2225.
- Dohn MR, Brown MV, Reynolds AB (2009). An essential role for p120-catenin in Src- and Rac1-mediated anchorage-independent cell growth. J Cell Biol. 184: 437–450.
- Dubash AD, Green KJ (2011). Desmosomes. Curr Biol. 21: R529–531.
- Dusek RL, Attardi LD (2011). Desmosomes: new perpetrators in tumour suppression. Nat Rev Cancer. 11: 317–323.
- Dyrskjot L, Kruhoffer M, Thykjaer T, Marcussen N, Jensen JL, Moller K, Orntoft TF (2004). Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 64: 4040–4048.
- Edgar RC (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797.
- Emery LA, Tripathi A, King C, Kavanah M, Mendez J, Stone MD, De Las Morenas A, Sebastiani P, Rosenberg CL (2009). Early dysregulation of cell adhesion and extracellular matrix pathways in breast cancer progression. Am J Pathol. 175: 1292–1302.
- Estilo CL, O-Charoenrat P, Talbot S, Socci ND, Carlson DL, Ghossein R, Williams T, Yonekawa Y, Ramanathan Y, Boyle JO, Kraus DH, Patel S, Shaha AR, Wong RJ, Huryn JM, Shah JP, Singh B (2009). Oral tongue cancer gene expression profiling: identification of novel potential prognosticators by oligonucleotide microarray analysis. BMC Cancer. 9: 11.
- Furukawa C, Daigo Y, Ishikawa N, Kato T, Ito T, Tsuchiya E, Sone S, Nakamura Y (2005). Plakophilin 3 oncogene as prognostic marker and therapeutic target for lung cancer. Cancer Res. 65: 7102–7110.
- Gaedcke J, Grade M, Jung K, Camps J, Jo P, Emons G, Gehoff A, Sax U, Schirmer M, Becker H, Beissbarth T, Ried T, Ghadimi BM (2010). Mutated KRAS results in overexpression of DUSP4, a MAP-kinase phosphatase, and SMYD3, a histone methyltransferase, in rectal carcinomas. Genes Chromosomes Cancer. 49: 1024–1034.
- Galamb O, Sipos F, Solymosi N, Spisak S, Krenacs T, Toth K, Tulassay Z, Molnar B (2008). Diagnostic mRNA expression patterns of inflamed, benign, and malignant colorectal biopsy specimen and their correlation with peripheral blood results. Cancer Epidemiol Biomarkers Prev. 17: 2835–2845.
- Galamb O, Spisak S, Sipos F, Toth K, Solymosi N, Wichmann B, Krenacs T, Valcz G, Tulassay Z, Molnar B (2010). Reversal of gene expression changes in the colorectal normal- adenoma pathway by NS398 selective COX2 inhibitor. Br J Cancer. 102: 765–773.
- Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, Fuchs E (1998). Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol. 143: 2009–2022.
- Gloushankova NA (2008). Changes in regulation of cell-cell adhesion during tumor transformation. Biochemistry (Mosc). 73: 742–750.
- Godsel LM, Dubash AD, Bass-Zubek AE, Amargo EV, Klessner JL, Hobbs RP, Chen X, Green KJ (2010). Plakophilin 2 couples actomyosin remodeling to desmosomal plaque assembly via RhoA. Mol Biol Cell. 21: 2844–2859.
- Gomez-Morales M, Camara-Pulido M, Miranda-Leon MT, Sanchez-Palencia A, Boyero L, Gomez-Capilla JA, Farez-Vidal ME (2013). Differential immunohistochemical localization of desmosomal plaque-related proteins in non-small-cell lung cancer. Histopathology. 63: 103–113.
- Gosavi P, Kundu ST, Khapare N, Sehgal L, Karkhanis MS, Dalal SN (2011). E-cadherin and plakoglobin recruit plakophilin3 to the cell border to initiate desmosome assembly. Cell Mol Life Sci. 68: 1439–1454.
- Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W (2004). Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol. 167: 149–160.
- Grotenhuis BA, Van Lanschot JJ, Dinjens WN, Wijnhoven BP (2010). The pathogenesis of Barrett's metaplasia and the progression to esophageal adenocarcinoma. Recent Results Cancer Res. 182: 39–63.
- Guindon S, Gascuel O (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 52: 696–704.
- Hao Y, Triadafilopoulos G, Sahbaie P, Young HS, Omary MB, Lowe AW (2006). Gene expression profiling reveals stromal genes expressed in common between Barrett's esophagus and adenocarcinoma. Gastroenterology. 131: 925–933.
- Harvell DM, Kim J, O'brien J, Tan AC, Borges VF, Schedin P, Jacobsen BM, Horwitz KB (2013). Genomic signatures of pregnancy-associated breast cancer epithelia and stroma and their regulation by estrogens and progesterone. Horm Cancer. 4: 140–153.
- Hatzfeld M (2007). Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesion?Biochim Biophys Acta. 1773: 69–77.
- Hatzfeld M (2010). A nuclear function for plakophilin-1 in the DNA damage response?J Invest Dermatol. 130: 2538–2540.
- Hatzfeld M, Green KJ, Sauter H (2003). Targeting of p0071 to desmosomes and adherens junctions is mediated by different protein domains. J Cell Sci. 116: 1219–1233.
- Hatzfeld M, Haffner C, Schulze K, Vinzens U (2000). The function of plakophilin 1 in desmosome assembly and actin filament organization. J Cell Biol. 149: 209–222.
- Hatzfeld M, Kristjansson GI, Plessmann U, Weber K (1994). Band 6 protein, a major constituent of desmosomes from stratified epithelia, is a novel member of the armadillo multigene family. J Cell Sci. 107: 2259–2270.
- Hawthorn L, Luce J, Stein L, Rothschild J (2010). Integration of transcript expression, copy number and LOH analysis of infiltrating ductal carcinoma of the breast. BMC Cancer. 10: 460.
- Heid HW, Schmidt A, Zimbelmann R, Schafer S, Winter-Simanowski S, Stumpp S, Keith M, Figge U, Schnolzer M, Franke WW (1994). Cell type-specific desmosomal plaque proteins of the plakoglobin family: plakophilin 1 (band 6 protein). Differentiation. 58: 113–131.
- Hinoue T, Weisenberger DJ, Lange CP, Shen H, Byun HM, Van Den Berg D, Malik S, Pan F, Noushmehr H, Van Dijk CM, Tollenaar RA, Laird PW (2012). Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome Res. 22: 271–282.
- Hofmann I, Casella M, Schnolzer M, Schlechter T, Spring H, Franke WW (2006). Identification of the junctional plaque protein plakophilin 3 in cytoplasmic particles containing RNA-binding proteins and the recruitment of plakophilins 1 and 3 to stress granules. Mol Biol Cell. 17: 1388–1398.
- Hong Y, Ho KS, Eu KW, Cheah PY (2007). A susceptibility gene set for early onset colorectal cancer that integrates diverse signaling pathways: implication for tumorigenesis. Clin Cancer Res. 13: 1107–1114.
- Hou J, Aerts J, Den Hamer B, Van Ijcken W, Den Bakker M, Riegman P, Van Der Leest C, Van Der Spek P, Foekens JA, Hoogsteden HC, Grosveld F, Philipsen S (2010). Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One. 5: e10312.
- Hu Z, Fan C, Livasy C, He X, Oh DS, Ewend MG, Carey LA, Subramanian S, West R, Ikpatt F, Olopade OI, Van De Rijn M, Perou CM (2009). A compact VEGF signature associated with distant metastases and poor outcomes. BMC Med. 7: 9.
- Huber AH, Nelson WJ, Weis WI (1997). Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 92: 871–882.
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP (2010). A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 143: 1174–1189.
- Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, Ji H, Potash JB, Sabunciyan S, Feinberg AP (2009). The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 41: 178–186.
- Johnson-Huang LM, Pensabene CA, Shah KR, Pierson KC, Kikuchi T, Lentini T, Gilleaudeau P, Sullivan-Whalen M, Cueto I, Khatcherian A, Hyder LA, Suarez-Farinas M, Krueger JG, Lowes MA (2012). Post-therapeutic relapse of psoriasis after CD11a blockade is associated with T cells and inflammatory myeloid DCs. PLoS One. 7: e30308.
- Jones MH, Virtanen C, Honjoh D, Miyoshi T, Satoh Y, Okumura S, Nakagawa K, Nomura H, Ishikawa Y (2004). Two prognostically significant subtypes of high-grade lung neuroendocrine tumours independent of small-cell and large-cell neuroendocrine carcinomas identified by gene expression profiles. Lancet. 363: 775–781.
- Kanno M, Aoyama Y, Isa Y, Yamamoto Y, Kitajima Y (2008a). P120 catenin is associated with desmogleins when desmosomes are assembled in high-Ca2 + medium but not when disassembled in low-Ca2 + medium in DJM-1 cells. J Dermatol. 35: 317–324.
- Kanno M, Isa Y, Aoyama Y, Yamamoto Y, Nagai M, Ozawa M, Kitajima Y (2008b). P120-catenin is a novel desmoglein 3 interacting partner: identification of the p120-catenin association site of desmoglein 3. Exp Cell Res. 314: 1683–1692.
- Kapprell HP, Owaribe K, Franke WW (1988). Identification of a basic protein of Mr 75,000 as an accessory desmosomal plaque protein in stratified and complex epithelia. J Cell Biol. 106: 1679–1691.
- Kaz AM, Luo Y, Dzieciatkowski S, Chak A, Willis JE, Upton MP, Leidner RS, Grady WM (2012). Aberrantly methylated PKP1 in the progression of Barrett's esophagus to esophageal adenocarcinoma. Genes Chromosomes Cancer. 51: 384–393.
- Keil R, Wolf A, Huttelmaier S, Hatzfeld M (2007). Beyond regulation of cell adhesion: local control of RhoA at the cleavage furrow by the p0071 catenin. Cell Cycle. 6: 122–127.
- Kim IJ, Quigley D, To MD, Pham P, Lin K, Jo B, Jen KY, Raz D, Kim J, Mao JH, Jablons D, Balmain A (2013a). Rewiring of human lung cell lineage and mitotic networks in lung adenocarcinomas. Nat Commun. 4: 1701.
- Kim M, Ceman S (2012). Fragile X mental retardation protein: past, present and future. Curr Protein Pept Sci. 13: 358–371.
- Kim SM, Park YY, Park ES, Cho JY, Izzo JG, Zhang D, Kim SB, Lee JH, Bhutani MS, Swisher SG, Wu X, Coombes KR, Maru D, Wang KK, Buttar NS, Ajani JA, Lee JS (2010). Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS One. 5: e15074.
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW, Gygi SP (2011). Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 44: 325–340.
- Kim YJ, Yoon HY, Kim JS, Kang HW, Min BD, Kim SK, Ha YS, Kim IY, Ryu KH, Lee SC, Kim WJ (2013b). HOXA9, ISL1 and ALDH1A3 methylation patterns as prognostic markers for nonmuscle invasive bladder cancer: array- based DNA methylation and expression profiling. Int J Cancer. 133: 1135–1142.
- Kimchi ET, Posner MC, Park JO, Darga TE, Kocherginsky M, Karrison T, Hart J, Smith KD, Mezhir JJ, Weichselbaum RR, Khodarev NN (2005). Progression of Barrett's metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res. 65: 3146–3154.
- Koetsier JL, Amargo EV, Todorovic V, Green KJ, Godsel LM (2013). Plakophilin 2 affects cell migration by modulating focal adhesion dynamics and integrin protein expression. J Invest Dermatol. [Epub] Advance online publication, doi:10.1038/jid.2013.266.
- Kowalczyk AP, Hatzfeld M, Bornslaeger EA, Kopp DS, Borgwardt JE, Corcoran CM, Settler A, Green KJ (1999). The head domain of plakophilin-1 binds to desmoplakin and enhances its recruitment to desmosomes. Implications for cutaneous disease. J Biol Chem. 274: 18145–18148.
- Kulski JK, Kenworthy W, Bellgard M, Taplin R, Okamoto K, Oka A, Mabuchi T, Ozawa A, Tamiya G, Inoko H (2005). Gene expression profiling of Japanese psoriatic skin reveals an increased activity in molecular stress and immune response signals. J Mol Med (Berl). 83: 964–975.
- Kundu ST, Gosavi P, Khapare N, Patel R, Hosing AS, Maru GB, Ingle A, Decaprio JA, Dalal SN (2008). Plakophilin3 downregulation leads to a decrease in cell adhesion and promotes metastasis. Int J Cancer. 123: 2303–2314.
- Kuner R, Falth M, Pressinotti NC, Brase JC, Puig SB, Metzger J, Gade S, Schafer G, Bartsch G, Steiner E, Klocker H, Sultmann H (2013). The maternal embryonic leucine zipper kinase (MELK) is upregulated in high-grade prostate cancer. J Mol Med (Berl). 91: 237–248.
- Kupershmidt I, Su QJ, Grewal A, Sundaresh S, Halperin I, Flynn J, Shekar M, Wang H, Park J, Cui W, Wall GD, Wisotzkey R, Alag S, Akhtari S, Ronaghi M (2010). Ontology-based meta-analysis of global collections of high-throughput public data. PLoS One. 5: e10.1371
- Kurzen H, Munzing I, Hartschuh W (2003). Expression of desmosomal proteins in squamous cell carcinomas of the skin. J Cutan Pathol. 30: 621–630.
- Lai-Cheong JE, Arita K, McGrath JA (2007). Genetic diseases of junctions. J Invest Dermatol. 127: 2713–2725.
- Lapointe J, Li C, Higgins JP, Van De Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, Ekman P, Demarzo AM, Tibshirani R, Botstein D, Brown PO, Brooks JD, Pollack JR (2004). Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 101: 811–816.
- LaTulippe E, Satagopan J, Smith A, Scher H, Scardino P, Reuter V, Gerald WL (2002). Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 62: 4499–4506.
- Leitner L, Shaposhnikov D, Mengel A, Descot A, Julien S, Hoffmann R, Posern G (2011). MAL/MRTF-A controls migration of non-invasive cells by upregulation of cytoskeleton-associated proteins. J Cell Sci. 124: 4318–4331.
- Leja J, Essaghir A, Essand M, Wester K, Oberg K, Totterman TH, Lloyd R, Vasmatzis G, Demoulin JB, Giandomenico V (2009). Novel markers for enterochromaffin cells and gastrointestinal neuroendocrine carcinomas. Mod Pathol. 22: 261–272.
- Lin G, He X, Ji H, Shi L, Davis RW, Zhong S (2006). Reproducibility Probability Score–incorporating measurement variability across laboratories for gene selection. Nat Biotechnol. 24: 1476–1477.
- Lohavanichbutr P, Mendez E, Holsinger FC, Rue TC, Zhang Y, Houck J, Upton MP, Futran N, Schwartz SM, Wang P, Chen C (2013). A 13-gene signature prognostic of HPV-negative OSCC: discovery and external validation. Clin Cancer Res. 19: 1197–1203.
- Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC, Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC, Chuang EY (2010). Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol Biomarkers Prev. 19: 2590–2597.
- Maynadier M, Chambon M, Basile I, Gleizes M, Nirde P, Gary-Bobo M, Garcia M (2012). Estrogens promote cell-cell adhesion of normal and malignant mammary cells through increased desmosome formation. Mol Cell Endocrinol. 364: 126–133.
- McGrath JA, Mcmillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, Garrod DR, Eady RA (1997). Mutations in the plakophilin 1 gene result in ectodermal dysplasia/ skin fragility syndrome. Nat Genet. 17: 240–244.
- McGrath JA, Mellerio JE (2010). Ectodermal dysplasia- skin fragility syndrome. Dermatol Clin. 28: 125–129.
- Mengual L, Burset M, Ars E, Lozano JJ, Villavicencio H, Ribal MJ, Alcaraz A (2009). DNA microarray expression profiling of bladder cancer allows identification of noninvasive diagnostic markers. J Urol. 182: 741–748.
- Mertens C, Hofmann I, Wang Z, Teichmann M, Sepehri Chong S, Schnolzer M, Franke WW (2001). Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc Natl Acad Sci U S A. 98: 7795–7800.
- Mitsui H, Suarez-Farinas M, Belkin DA, Levenkova N, Fuentes-Duculan J, Coats I, Fujita H, Krueger JG (2012). Combined use of laser capture microdissection and cDNA microarray analysis identifies locally expressed disease-related genes in focal regions of psoriasis vulgaris skin lesions. J Invest Dermatol. 132: 1615–1626.
- Moriya Y, Iyoda A, Kasai Y, Sugimoto T, Hashida J, Nimura Y, Kato M, Takiguchi M, Fujisawa T, Seki N, Yoshino I (2009). Prediction of lymph node metastasis by gene expression profiling in patients with primary resected lung cancer. Lung Cancer. 64: 86–91.
- Muhmer M, Ditthardt D, Jakel J, Wischmann V, Moll R, Schmidt A (2013). An alternative promoter of the human plakophilin-3 gene controls the expression of the new isoform PKP3b. Cell Tissue Res. [Epub] Advance online publication, doi:10.1007/s00441-013-1736-1.
- Muller J, Ritt DA, Copeland TD, Morrison DK (2003). Functional analysis of C-TAK1 substrate binding and identification of PKP2 as a new C-TAK1 substrate. EMBO J. 22: 4431–4442.
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok PY, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR (2009). Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 41: 199–204.
- Narayana N, Gist J, Smith T, Tylka D, Trogdon G, Wahl JK (2010). Desmosomal component expression in normal, dysplastic, and oral squamous cell carcinoma. Dermatol Res Pract. 2010: 649731. doi: 10.1155/2010/649731. Epub 2010 Mar 18.
- Nindl I, Dang C, Forschner T, Kuban RJ, Meyer T, Sterry W, Stockfleth E (2006). Identification of differentially expressed genes in cutaneous squamous cell carcinoma by microarray expression profiling. Mol Cancer. 5: 30.
- North AJ, Bardsley WG, Hyam J, Bornslaeger EA, Cordingley HC, Trinnaman B, Hatzfeld M, Green KJ, Magee AI, Garrod DR (1999). Molecular map of the desmosomal plaque. J Cell Sci. 112: 4325–4336.
- Novak P, Jensen T, Oshiro MM, Wozniak RJ, Nouzova M, Watts GS, Klimecki WT, Kim C, Futscher BW (2006). Epigenetic inactivation of the HOXA gene cluster in breast cancer. Cancer Res. 66: 10664–10670.
- Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S, Watanabe S, Sakamoto H, Kumamoto K, Takenoshita S, Gotoh N, Mizuno H, Sarai A, Kawano S, Yamaguchi R, Miyano S, Yokota J (2012). Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 72: 100–111.
- Olsson M, Broberg A, Jernas M, Carlsson L, Rudemo M, Suurkula M, Svensson PA, Benson M (2006). Increased expression of aquaporin 3 in atopic eczema. Allergy. 61: 1132–1137.
- Pascal LE, Goo YA, Vencio RZ, Page LS, Chambers AA, Liebeskind ES, Takayama TK, True LD, Liu AY (2009). Gene expression down-regulation in CD90 + prostate tumor-associated stromal cells involves potential organ-specific genes. BMC Cancer. 9: 317.
- Pedraza V, Gomez-Capilla JA, Escaramis G, Gomez C, Torne P, Rivera JM, Gil A, Araque P, Olea N, Estivill X, Farez-Vidal ME (2010). Gene expression signatures in breast cancer distinguish phenotype characteristics, histologic subtypes, and tumor invasiveness. Cancer. 116: 486–496.
- Peng Z, Wei D, Wang L, Tang H, Zhang J, Le X, Jia Z, Li Q, Xie K (2006). RUNX3 inhibits the expression of vascular endothelial growth factor and reduces the angiogenesis, growth, and metastasis of human gastric cancer. Clin Cancer Res. 12: 6386–6394.
- Pieperhoff S, Schumacher H, Franke WW (2008). The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur J Cell Biol. 87, 399–411.
- Ramaswamy S, Tamayo P, Rifkin R, Mukherjee S, Yeang CH, Angelo M, Ladd C, Reich M, Latulippe E, Mesirov JP, Poggio T, Gerald W, Loda M, Lander ES, Golub TR (2001). Multiclass cancer diagnosis using tumor gene expression signatures. Proc Natl Acad Sci U S A. 98: 15149–15154.
- Reischl J, Schwenke S, Beekman JM, Mrowietz U, Sturzebecher S, Heubach JF (2007). Increased expression of Wnt5a in psoriatic plaques. J Invest Dermatol. 127: 163–169.
- Rentoft M, Coates PJ, Laurell G, Nylander K (2012). Transcriptional profiling of formalin fixed paraffin embedded tissue: pitfalls and recommendations for identifying biologically relevant changes. PLoS One. 7: e35276.
- Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S (2006). X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 9: 121–132.
- Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, Shevde LA, Li W, Eschrich S, Daud A, Ju J, Matta J (2008). The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 1: 13.
- Roberts BJ, Reddy R, Wahl JK III (2013). Stratifin (14-3-3 sigma) Limits plakophilin-3 exchange with the desmosomal plaque. PLoS One. 8: e77012.
- Rousseaux S, Debernardi A, Jacquiau B, Vitte AL, Vesin A, Nagy-Mignotte H, Moro-Sibilot D, Brichon PY, Lantuejoul S, Hainaut P, Laffaire J, De Reynies A, Beer DG, Timsit JF, Brambilla C, Brambilla E, Khochbin S (2013). Ectopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancers. Sci Transl Med. 5: 186ra66.
- Ruiz P, Birchmeier W (1998). The plakoglobin knock-out mouse: a paradigm for the molecular analysis of cardiac cell junction formation. Trends Cardiovasc Med. 8: 97–101.
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Gunthert U, Franke WW, Birchmeier W (1996). Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol. 135: 215–225.
- Sabatier R, Finetti P, Cervera N, Lambaudie E, Esterni B, Mamessier E, Tallet A, Chabannon C, Extra JM, Jacquemier J, Viens P, Birnbaum D, Bertucci F (2011). A gene expression signature identifies two prognostic subgroups of basal breast cancer. Breast Cancer Res Treat. 126: 407–420.
- Sanchez-Palencia A, Gomez-Morales M, Gomez-Capilla JA, Pedraza V, Boyero L, Rosell R, Farez-Vidal ME (2011). Gene expression profiling reveals novel biomarkers in nonsmall cell lung cancer. Int J Cancer. 129: 355–364.
- Schackmann RC, Tenhagen M, Van De Ven RA, Derksen PW (2013). p120-catenin in cancer - mechanisms, models and opportunities for intervention. J Cell Sci. 126: 3515–3525.
- Schmidt A, Jager S (2005). Plakophilins–hard work in the desmosome, recreation in the nucleus?Eur J Cell Biol. 84: 189–204.
- Schmidt A, Langbein L, Rode M, Pratzel S, Zimbelmann R, Franke WW (1997). Plakophilins 1a and 1b: widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque components. Cell Tissue Res. 290: 481–499.
- Sengupta S, Den Boon JA, Chen IH, Newton MA, Dahl DB, Chen M, Cheng YJ, Westra WH, Chen CJ, Hildesheim A, Sugden B, Ahlquist P (2006). Genome-wide expression profiling reveals EBV-associated inhibition of MHC class I expression in nasopharyngeal carcinoma. Cancer Res. 66: 7999–8006.
- Sheffer M, Bacolod MD, Zuk O, Giardina SF, Pincas H, Barany F, Paty PB, Gerald WL, Notterman DA, Domany E (2009). Association of survival and disease progression with chromosomal instability: a genomic exploration of colorectal cancer. Proc Natl Acad Sci U S A. 106: 7131–7136.
- Sircoulomb F, Bekhouche I, Finetti P, Adelaide J, Ben Hamida A, Bonansea J, Raynaud S, Innocenti C, Charafe-Jauffret E, Tarpin C, Ben Ayed F, Viens P, Jacquemier J, Bertucci F, Birnbaum D, Chaffanet M (2010). Genome profiling of ERBB2-amplified breast cancers. BMC Cancer. 10: 539.
- Sklyarova T, Bonne S, D'hooge P, Denecker G, Goossens S, De Rycke R, Borgonie G, Bosl M, Van Roy F, Van Hengel J (2008). Plakophilin-3-deficient mice develop hair coat abnormalities and are prone to cutaneous inflammation. J Invest Dermatol. 128: 1375–1385.
- Skrzypczak M, Goryca K, Rubel T, Paziewska A, Mikula M, Jarosz D, Pachlewski J, Oledzki J, Ostrowski J (2010). Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One. 5.
- Smith EA, Fuchs E (1998). Defining the interactions between intermediate filaments and desmosomes. J Cell Biol. 141: 1229–1241.
- Sobolik-Delmaire T, Katafiasz D, Keim SA, Mahoney MG, Wahl JK III (2007). Decreased plakophilin-1 expression promotes increased motility in head and neck squamous cell carcinoma cells. Cell Commun Adhes. 14: 99–109.
- Sobolik-Delmaire T, Reddy R, Pashaj A, Roberts BJ, Wahl JK III (2010). Plakophilin-1 localizes to the nucleus and interacts with single-stranded DNA. J Invest Dermatol. 130: 2638–2646.
- South AP (2004). Plakophilin 1: an important stabilizer of desmosomes. Clin Exp Dermatol. 29: 161–167.
- South AP, Wan H, Stone MG, Dopping-Hepenstal PJ, Purkis PE, Marshall JF, Leigh IM, Eady RA, Hart IR, McGrath JA (2003). Lack of plakophilin 1 increases keratinocyte migration and reduces desmosome stability. J Cell Sci. 116: 3303–3314.
- Stearman RS, Dwyer-Nield L, Zerbe L, Blaine SA, Chan Z, Bunn PA, Jr., Johnson GL, Hirsch FR, Merrick DT, Franklin WA, Baron AE, Keith RL, Nemenoff RA, Malkinson AM, Geraci MW (2005). Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am J Pathol. 167: 1763–1775.
- Su H, Hu N, Yang HH, Wang C, Takikita M, Wang QH, Giffen C, Clifford R, Hewitt SM, Shou JZ, Goldstein AM, Lee MP, Taylor PR (2011). Global gene expression profiling and validation in esophageal squamous cell carcinoma and its association with clinical phenotypes. Clin Cancer Res. 17: 2955–2966.
- Su LJ, Chang CW, Wu YC, Chen KC, Lin CJ, Liang SC, Lin CH, Whang-Peng J, Hsu SL, Chen CH, Huang CY (2007). Selection of DDX5 as a novel internal control for Q-RT-PCR from microarray data using a block bootstrap re-sampling scheme. BMC Genomics. 8: 140.
- Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG (2012). Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 132: 2552–2564.
- Swindell WR, Johnston A, Carbajal S, Han G, Wohn C, Lu J, Xing X, Nair RP, Voorhees JJ, Elder JT, Wang XJ, Sano S, Prens EP, Digiovanni J, Pittelkow MR, Ward NL, Gudjonsson JE (2011). Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS One. 6: e18266.
- Takahashi H, Nakatsuji H, Takahashi M, Avirmed S, Fukawa T, Takemura M, Fukumori T, Kanayama H (2012). Up-regulation of plakophilin-2 and Down-regulation of plakophilin-3 are correlated with invasiveness in bladder cancer. Urology. 79: 240.e1–8.
- Tewari R, Bailes E, Bunting KA, Coates JC (2010). Armadillo-repeat protein functions: questions for little creatures. Trends Cell Biol. 20: 470–481.
- Thelemann A, Petti F, Griffin G, Iwata K, Hunt T, Settinari T, Fenyo D, Gibson N, Haley JD (2005). Phosphotyrosine signaling networks in epidermal growth factor receptor overexpressing squamous carcinoma cells. Mol Cell Proteomics. 4: 356–376.
- Thomason HA, Scothern A, Mcharg S, Garrod DR (2010). Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 429: 419–433.
- Toruner GA, Ulger C, Alkan M, Galante AT, Rinaggio J, Wilk R, Tian B, Soteropoulos P, Hameed MR, Schwalb MN, Dermody JJ (2004). Association between gene expression profile and tumor invasion in oral squamous cell carcinoma. Cancer Genet Cytogenet. 154: 27–35.
- Tu CL, You M (2013). Obligatory roles of filamin A in E-cadherin-mediated cell-cell adhesion in epidermal keratinocytes. J Dermatol Sci. [Epub] Advance online publication, doi:10.1016/j.jdermsci.2013.09.007.
- Tucker DK, Stahley SN, Kowalczyk AP (2013). Plakophilin-1 protects keratinocytes from pemphigus vulgaris IgG by forming calcium-independent desmosomes. J Invest Dermatol. [Epub] Advance online publication, doi:10.1038/jid.2013.401.
- Uddin S, Ahmed M, Hussain A, Abubaker J, Al-Sanea N, Abduljabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Jehan Z, Bavi P, Siraj AK, Al-Kuraya KS (2011). Genome-wide expression analysis of Middle Eastern colorectal cancer reveals FOXM1 as a novel target for cancer therapy. Am J Pathol. 178: 537–547.
- Uhart M, Bustos DM (2013). Human 14-3-3 paralogs differences uncovered by cross-talk of phosphorylation and lysine acetylation. PLoS One. 8: e55703.
- Vasioukhin V, Bauer C, Yin M, Fuchs E (2000). Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 100: 209–219.
- Vasudevan S, Steitz JA (2007). AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 128: 1105–1118.
- Wachi S, Yoneda K, Wu R (2005). Interactome-transcriptome analysis reveals the high centrality of genes differentially expressed in lung cancer tissues. Bioinformatics. 21: 4205–4208.
- Wagner SA, Beli P, Weinert BT, Scholz C, Kelstrup CD, Young C, Nielsen ML, Olsen JV, Brakebusch C, Choudhary C (2012). Proteomic analyses reveal divergent ubiquitylation site patterns in murine tissues. Mol Cell Proteomics. 11: 1578–1585.
- Waschke J, Spindler V, Bruggeman P, Zillikens D, Schmidt G, Drenckhahn D (2006). Inhibition of Rho A activity causes pemphigus skin blistering. J Cell Biol. 175: 721–727.
- Wei TY, Juan CC, Hisa JY, Su LJ, Lee YC, Chou HY, Chen JM, Wu YC, Chiu SC, Hsu CP, Liu KL, Yu CT (2012). Protein arginine methyltransferase 5 is a potential oncoprotein that upregulates G1 cyclins/cyclin-dependent kinases and the phosphoinositide 3-kinase/AKT signaling cascade. Cancer Sci. 103: 1640–1650.
- Whitman SA, Cover C, Yu L, Nelson DL, Zarnescu DC, Gregorio CC (2011). Desmoplakin and talin2 are novel mRNA targets of fragile X-related protein-1 in cardiac muscle. Circ Res. 109: 262–271.
- Wolf A, Hatzfeld M (2010). A role of plakophilins in the regulation of translation. Cell Cycle. 9: 2973–2978.
- Wolf A, Keil R, Gotzl O, Mun A, Schwarze K, Lederer M, Huttelmaier S, Hatzfeld M (2006). The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat Cell Biol. 8: 1432–1440.
- Wolf A, Krause-Gruszczynska M, Birkenmeier O, Ostareck-Lederer A, Huttelmaier S, Hatzfeld M (2010). Plakophilin 1 stimulates translation by promoting eIF4A1 activity. J Cell Biol. 188: 463–471.
- Wolf A, Rietscher K, Glass M, Huttelmaier S, Schutkowski M, Ihling C, Sinz A, Wingenfeld A, Mun A, Hatzfeld M (2013). Insulin signaling via Akt2 switches plakophilin 1 function from stabilizing cell adhesion to promoting cell proliferation. J Cell Sci. 126: 1832–1844.
- Wrage M, Ruosaari S, Eijk PP, Kaifi JT, Hollmen J, Yekebas EF, Izbicki JR, Brakenhoff RH, Streichert T, Riethdorf S, Glatzel M, Ylstra B, Pantel K, Wikman H (2009). Genomic profiles associated with early micrometastasis in lung cancer: relevance of 4q deletion. Clin Cancer Res. 15: 1566–1574.
- Wu Y, Grabsch H, Ivanova T, Tan IB, Murray J, Ooi CH, Wright AI, West NP, Hutchins GG, Wu J, Lee M, Lee J, Koo JH, Yeoh KG, Van Grieken N, Ylstra B, Rha SY, Ajani JA, Cheong JH, Noh SH, Lim KH, Boussioutas A, Lee JS, Tan P (2013). Comprehensive genomic meta- analysis identifies intra-tumoural stroma as a predictor of survival in patients with gastric cancer. Gut. 62: 1100–1111.
- Yan W, Shih JH, Rodriguez-Canales J, Tangrea MA, Ylaya K, Hipp J, Player A, Hu N, Goldstein AM, Taylor PR, Emmert-Buck MR, Erickson HS (2012). Identification of unique expression signatures and therapeutic targets in esophageal squamous cell carcinoma. BMC Res Notes. 5: 73.
- Yao Y, Richman L, Morehouse C, De Los Reyes M, Higgs BW, Boutrin A, White B, Coyle A, Krueger J, Kiener PA, Jallal B (2008). Type I interferon: potential therapeutic target for psoriasis?PLoS One. 3: e2737.
- Yu K, Ganesan K, Tan LK, Laban M, Wu J, Zhao XD, Li H, Leung CH, Zhu Y, Wei CL, Hooi SC, Miller L, Tan P (2008). A precisely regulated gene expression cassette potently modulates metastasis and survival in multiple solid cancers. PLoS Genet. 4: e1000129.
- Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, Mcdonald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo JH (2004). Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 22: 2790–2799.
- Zanivan S, Gnad F, Wickstrom SA, Geiger T, Macek B, Cox J, Fassler R, Mann M (2008). Solid tumor proteome and phosphoproteome analysis by high resolution mass spectrometry. J Proteome Res. 7: 5314–5326.
- Zanivan S, Meves A, Behrendt K, Schoof EM, Neilson LJ, Cox J, Tang HR, Kalna G, Van Ree JH, Van Deursen JM, Trempus CS, Machesky LM, Linding R, Wickstrom SA, Fassler R, Mann M (2013). In vivo SILAC-based proteomics reveals phosphoproteome changes during mouse skin carcinogenesis. Cell Rep. 3: 552–566.
- Zarnescu DC, Gregorio CC (2013). Fragile hearts: New insights into translational control in cardiac muscle. Trends Cardiovasc Med. 23: 275–281.
- Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B (2012). Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 148: 259–272.
- Zhao H, Langerod A, Ji Y, Nowels KW, Nesland JM, Tibshirani R, Bukholm IK, Karesen R, Botstein D, Borresen-Dale AL, Jeffrey SS (2004). Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 15: 2523–2536.
- Zhou N, Singh K, Mir MC, Parker Y, Lindner D, Dreicer R, Ecsedy JA, Zhang Z, Teh BT, Almasan A, Hansel DE (2013). The investigational Aurora kinase A inhibitor MLN8237 induces defects in cell viability and cell-cycle progression in malignant bladder cancer cells in vitro and in vivo. Clin Cancer Res. 19: 1717–1728.
- Zhuang Z, Jewett AI, Kuttimalai S, Bellesia G, Gnanakaran S, Shea JE (2011). Assisted peptide folding by surface pattern recognition. Biophys J. 100: 1306–1315.