Abstract
Multimodal tumor therapies should aim not only to kill the tumor cells, but also to stimulate a specific immune response to keep residual tumor (stem) cells and metastases under control. Apoptotic cells are mostly noninflammatory or even anti-inflammatory while necrotic cells stimulate the immune system. Whether the immunogenicity of apoptotic tumor cells can be increased by interfering with their swift and phosphatidylserine (PS)-dependent clearance by macrophages was examined. AnnexinA5 (AnxA5) is a naturally occurring highly specific ligand for PS. Proteins of the annexin family are characterized by a selective affinity for phospholipids in the presence of Ca2+ ions. The phagocytosis by macrophages of irradiated, apoptotic tumor cells (ITC) was partially inhibited when the ITC were preincubated with AnxA5. Activated macrophages secreted higher amounts of TNFα and IL-1β after contact with ITC plus AnxA5 in comparison with ITC alone, while the amount of TGF-β was decreased. Macrophages of AnxA5-deficient mice showed an increased phagocytosis of dead cells. Wild-type mice, where endogenous AnxA5 is present, displayed a significantly faster decline in size of allogeneic tumors in comparison with AnxA5-deficient animals. The addition of AnxA5 to ITC vaccines increased the percentage of tumor-free mice in syngeneic tumor protection and tumor cure assays. AnxA5 alone led to a retard of syngeneic tumor growth that was, however, less pronounced in comparison to treatment of the tumor with ionizing irradiation. In conclusion, AnxA5 disturbs the PS-dependent clearance by macrophages of dying tumor cells, leading to the accumulation of the latter, to the occurrence of secondary necrotic cells, and to an increased uptake of the dead cells by dendritic cells. Tumor cure appendages with dead tumor cells should be performed with AnxA5 as an immune stimulator and could be combined with irradiation, chemotherapy, and hyperthermia to induce immunogenic cell death forms in vivo or ex vivo.
Introduction
Radiation immunobiologists are becoming more and more interested in the meaning of death and how dying and dead cells modulate the immune system. The two extreme forms of cell death are apoptosis and primary necrosis (Kroemer et al., Citation2009). Apoptotic cells maintain their membrane integrity for a certain time. During these phases of apoptosis, these cells are swiftly recognized and phagocytosed by macrophages. A plethora of adaptor molecules and receptors have been shown to contribute to the recognition and uptake, the so-called clearance, of apoptotic cells (Lauber et al., Citation2004). The exposure of phosphatidylserine (PS) on the outer cellular membrane represents an evolutionarily-conserved and important recognition signal for apoptotic cells. The latter are capable of modulating the immune system. Apoptotic cells induce the secretion of anti-inflammatory cytokines by activating cytokines (Voll et al., Citation1997). Therefore, apoptotic tumor cells may lead to immune suppression, thereby promoting further tumorigenesis (Stach et al., Citation2000).
When the rate of apoptosis is high and/or the clearance process is defective, apoptotic cells eventually lose their membrane integrity and proceed to late stages of apoptosis becoming, finally, secondarily necrotic. These forms of cell death influence the microenvironment by resulting in leakage of potential autoantigens, noxious contents, and danger signals from the dying cells (summarized in Krysko and Vandenabeele, Citation2008). Primary necrotic cells also induce inflammation by releasing danger signals like HMGB1 (Bianchi and Manfredi, Citation2004), ATP (Hanley et al., Citation2004), or uric acid (Shi et al., Citation2003). Taken together, the clearance of apoptotic cells is non-inflammatory or anti-inflammatory, while secondary and primary necrotic cells can induce inflammation. However, early apoptotic cells can also lead to immune activation by exposing “eat-me” signals (like calreticulin) for dendritic cells (DC) (Obeid et al., Citation2007). Nevertheless, a common concept arises, namely, shifting the clearance of dying and dead cells from macrophages to DC that results in the induction of specific immunity against antigens of apoptotic and necrotic cells.
In the chronic autoimmune disease systemic lupus erythematosus (SLE), apoptotic cells accumulate in the lymph nodes and skin of some patients due to impaired clearance capabilities (summarized in Gaipl et al., Citation2006). The resulting secondary necrotic cells, which are rarely found in the body under healthy conditions, induce inflammation. Autoantigens get accessible to DC that in turn, together with further co-stimulation, activate autoreactive T- and B-cells (CitationBondanza et al., 2004a). It is hypothesized that blocking of the swift clearance of tumor cells by macrophages may also lead to immune activation and increased immunogenicity of the dying cells.
As mentioned earlier, PS exposition at the outer membrane leaflet is a hallmark of apoptotic cells. One of the main biochemical characteristics of annexins is binding anionic phospholipids in a Ca2+-dependent manner. Annexins represent a huge family of evolutionarily-related proteins and are detected in most eukaryotic phylae. AnnexinA5 (AnxA5) was identified as a placental anticoagulant protein (Grundmann et al., Citation1988) and also inhibits blood coagulation (Reutelingsperger et al., Citation1985). The high-affinity binding of labeled AnxA5 to PS has been extensively used for identifying apoptotic cells (Vermes et al., Citation1995).
Unlabeled AnxA5 was used to interfere with the specific recognition and removal of dying cells. It was also examined whether AnxA5 influences the secretion of inflammatory and anti-inflammatory cytokines by activated macrophages after contact with apoptotic tumor cells. Furthermore, tumor protection and cure assays were performed with apoptotic tumor cells with or without AnxA5. To gain further insight into the immunomodulatory properties of the endogenously present low levels of AnxA5, AnxA5 knockout (KO) mice were compared with wild-type (WT) mice with regard to the macrophages’ phagocytic capabilities and their immune reactions against allogeneic tumors.
Materials and methods
Mice and cells
C57BL/6 mice were obtained from Charles River Laboratories (Sulzfeld, Germany) and used for the phagocytosis assays as well as for the tumor vaccination and cure assays. Balb/c mice, which were also obtained from Charles River Laboratories, were used for the syngeneic tumor growth retardation experiments which were also obtained from Charles River Laboratories. The AnxA5-deficient (KO) mice were generated (by our cooperating partners Drs. Brachvogel and Pöschl) by homologous recombination containing a LacZ reporter gene cassette fused in frame with exon 3 of the AnxA5 gene (Brachvogel et al., Citation2003). Thereafter, 8- to 12-week-old AnxA5-deficient and WT mice displaying mixed genetic background of C57/BL6x129/SvJ were used for the experiments.
All mice were provided Purina chow diet ad libitum, and were kept individually in well-ventilated cages under standard conditions of humidity (55 ± 5%), temperature (22 ± 2°C), and light (12/12 hr light-dark cycles). Animal studies were conducted according to the principles in the guidelines for the care and use of laboratory animals of the “Regierung von Mittelfranken,” under the supervision of the Institute Animal Care and Use Committee (IACUC). This IACUC reviewed and approved all study protocols related to the use of animals to make sure that they adhered to internationally accepted standards for the ethical use of animals in research.
To test whether AnxA5 heightens the immunogenicity of apoptotic tumor cells, the highly tumorigenic H-2b RMA lymphoma cell line (syngeneic to C57BL/6 mice) displaying a loss of in vivo immunogenicity after induction of apoptosis (Ronchetti et al., Citation1999) was used. To analyze the allogeneic immune response toward tumor cells in AnxA5 KO and WT mice with a C57BL/6 background, CT26 murine colon cancer cells (syngeneic to BALB/c and allogeneic to C57BL/6 mice) were used and tumor regression was then analyzed. The murine colorectal tumor cell line CT26 was obtained from the American Type Culture Collection (Rockville, MD). The H-2b RMA lymphoma cell line was provided by V. Cerundolo (John Radcliff Hospital, Oxford, England). The murine B-cell line WEHI 231 was provided by Dr. Dirk Mielenz (Division of Molecular Immunology, Erlangen, Germany) and used as standardized prey in in vivo phagocytosis assays.
Induction of cell death
To induce apoptosis, H-2b RMA lymphoma cells were irradiated for 60 sec with ultraviolet (UV)-B light (2.0 mW/cm2) and then cultured for 24 hr in RPMI medium containing 10% fetal calf serum, 100 U penicillin/mL, 100 mg streptomycin/mL, and 200 mM L-glutamine (referred to hereafter as R10). Apoptotic RMA cells are referred to as irradiated tumor cells (ITC) hereafter as well. The WEHI 231 cells for the in vivo phagocytosis assays in AnxA5 KO and the respective WT mice were heated at 56°C for 30 min. This procedure leads to primary necrotic cells that are a standardized prey of dead cells for use in phagocytosis assays (Bottcher et al., Citation2006). Before injection into the peritoneum, necrosis was verified by staining with AnxA5-FITC/propidium iodide and with trypan blue. In each individual experiment >90% of the cells stained positive for PI, AnxA5, and trypan blue.
Phagocytosis assays
In vitro
Macrophages (Mϕ) of normal C57BL/6 mice were isolated from thioglycollate-elicited peritoneal lavages by plastic adherence. DC of normal C57BL/6 mice were derived from precursors in the bone marrow. The precursors were propagated for 7 d in R10 medium containing 1000 U recombinant murine GM-CSF/mL (Biomol, Hamburg, Germany) and 5 ng recombinant murine IL-4/mL (BD Biosciences, San Jose, CA).
The Mϕ or DC were stained with green fluorescent PKH67-GL dye in accordance with the instructions of the manufacturer (Sigma-Aldrich, Munich, Germany). RMA lymphoma cells were labeled before irradiation with UV-B light with the alphatic red fluorescent dye PKH26-GL (Sigma) and co-cultured with the stained Mϕ at 37°C for 2 h. Afterward, double positive cells—representing Mϕ or DC that had taken up apoptotic RMA lymphoma cells—were detected and enumerated using flow cytometry.
In vivo
Mϕ were recruited in the peritoneum of the C57BL/6 mice by injection of thioglycollate or amylum (2% in phosphate-buffered saline [PBS]) 4 d before the injection of the labeled necrotic WEHI 231 cells. The latter were labeled with 5-(and 6-)carboxyfluorescein-diacetate succinimidyl ester (Molecular Probes, Leiden, the Netherlands) before the induction of necrosis. Before injection into the peritoneum, the cells were washed with EDTA (5 mM in PBS) to remove potentially intrinsic bound AnxA5. For injection, the labeled necrotic cells (2 × 106 cells/mL) were resuspended in 500 μL Ringer’s solution. Mice were killed 3.5 hr after the injection of the necrotic cell suspension. The lavage of the peritoneum was performed with R10 medium; the obtained cell suspension was filtered through a 70-μm filter and any erythrocytes present were removed by hypotonic lysis. For the detection of phagocytosis by flow cytometry, protocols previously used in our lab were employed (Frey et al., Citation2009). Specifically, the cells were stained with F4/80-PE (Caltag; Invitrogen, Karlsruhe, Germany) to identify Mϕ. Two-color flow cytometry was then applied to quantify the extent of phagocytosis of the green-labeled necrotic WEHI 231 cells by F4/80 positive peritoneal macrophages.
Cytokine secretion
The supernatants of the thioglycollate-elicited peritoneal C57BL/6 Mϕ incubated with ITC for 24 hr were used for the analyses. The ITC:Mϕ ratio was 5:1. The release of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-10, and transforming growth factor (TGF)-β from the Mϕ was then quantified using ELISA (DuoSet ELISA; R&D Systems, Minneapolis, MN).
Immunization and cure experiments
The allogeneic immune response toward CT26 cells in AnxA5 KO and WT mice with C57BL/6 background was analyzed by measuring the tumor regression. CT26 cells (1 × 106) were injected subcutaneously into the flank of the mice. The tumor diameter was determined with an electronic caliper until 3 wk after the injection.
For the syngeneic tumor protection assays in C57BL/6 mice, the hosts were subcutaneously immunized with 5 × 106 apoptotic RMA cells (ITC) in their footpad. These ITC had been preincubated with or without AnxA5. On Day 15, the mice were boosted with a subcutaneous immunization in the right flank. At Day 30, viable RMA cells (2.5 × 104) were injected subcutaneously into the left flank. Mice were thereafter checked for the appearance of tumor at least twice a week. For the tumor cure assays in C57BL/6 mice, 2.5 × 104 viable RMA lymphoma cells were subcutaneously injected into the left flank. At Day 4, the mice were immunized with 5 × 106 apoptotic RMA cells (ITC) that had been incubated with or without AnxA5. One immunization per week was then performed, for up to 3 weeks.
The growth retardation of the Balb/c mice syngeneic CT26 tumor was determined by measuring tumor volume after treatment with AnxA5 and/or ionizing irradiation (X-ray). The 5- to 6-week-old female mice were shaved on the right flank and were administered a subcutaneous injection of CT26 cells (5 × 106 cells/animal) in the right flank after a short-term immobilization with FORENE® inhalation anesthesia (Abbott, Wiesbaden, Germany). When average tumor diameter reached 4–6 mm, the mice were grouped. The irradiation of the mice was performed (under FORENE® inhalation anesthesia) within a linear accelerator unit (Siemens Healthcare, Erlangen, Germany) with 6 MV and a focus skin distance of 1000 mm. The animals were fixed in a Plexiglas box and only the tumor region was tangentially irradiated (beam angle 350°) with an irradiation area of 10 × 10 mm to avoid the irradiation of any organs and bone marrow. During and after the therapy, the diameter of the tumor was measured with a digital caliper with a precision of measurements of 0.1 mm. The AnxA5 was injected 10 times around the tumor (Days 14–23 after injection of the CT26 cells), and the tumor was irradiated ones at Day 14 with 12 Gy.
AnnexinA5
Chicken AnxA5 (responsif GmbH, Germany) was used as a xenologous protein (for mice and humans) that most likely leads to further immune stimulation. For the in vitro as well as the in vivo phagocytosis assays, AnxA5 was used at a final concentration of 100 μg/mL. Bovine serum albumin was used at the same concentration as control peptide. ITC for the tumor protection and cure assays were also preincubated with 100 μg AnxA5/mL for 20 min at room temperature. For the syngeneic tumor growth retardation experiment, AnxA5 (40 μg/100 mm3 tumor volume) was injected (100 μL injection volume, in each of the ten times) around the tumor.
Statistical analysis
Statistical analyses were performed using the heteroscedastic two-tailed Student’s t-test for unpaired data. Results were considered statistically significant at *P < 0.05 and highly significant at **P < 0.01.
Results
AnxA5 blocks the uptake of apoptotic tumor cells by macrophages (Mϕ)
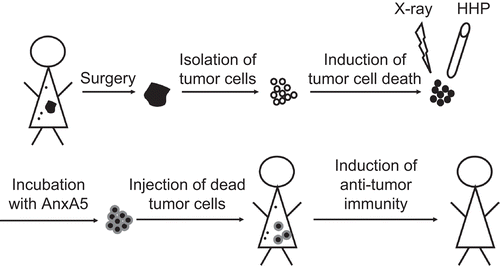
The in vitro uptake of irradiated, apoptotic RMA lymphoma cells (ITC) by peritoneal Mϕ or DC was compared in the presence and absence of AnxA5. As shown in , AnxA5, but not the control peptide BSA, led to a significant increased inhibition of phagocytosis of ITC by macrophages, but not by the DC. The most effective inhibition was to be observed at ratios of ITC:Mϕ of 5:1 and higher.
Figure 1. Phagocytosis of irradiated, apoptotic tumor cells (ITC) by macrophages or dendritic cells (DC) in the presence or absence of AnxA5. Irradiated RMA tumor cells (ITC) were co-incubated at different ratios with thioglycollate-elicited Mϕ (A) or DC (B) from C57BL/6 mice for 2 h. The phagocytosis (analyzed by flow cytometry) at 37°C of ITC by Mϕ or DC in the presence of medium only was set to 100. The percentage of inhibition of phagocytosis in the presence of AnxA5 (100 μg/mL) or BSA (100 μg/mL) is displayed. Results are representative of five independent experiments (each performed in quadruplicate) are expressed as percentage of inhibition (±SD). AnxA5, in contrast to the control peptide BSA, significantly inhibited uptake of ITC by macrophages, but not by DC, at all ratios tested **P < 0.01 vs BSA.
AnxA5 in combination with ITC shifts cytokine secretion profile of macrophages toward inflammation
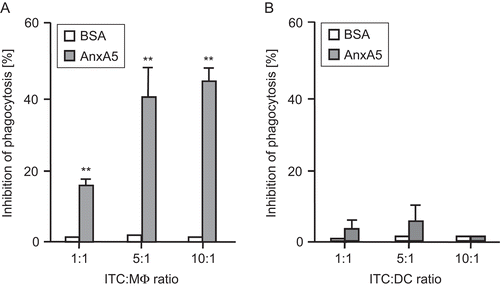
Thioglycollate-activated macrophages from normal C57BL/6 mice were co-cultured with ITC, AnxA5, or ITC plus AnxA5. After 24 h, the cytokine secretion of the macrophages was determined (). In the presence of ITC only, a significantly increased secretion of TNFα, IL-1β, and TGF-β by activated macrophages was observed. AnxA5 alone led to no significant changes in the basal cytokine secretion profile of activated macrophages. The co-incubation of macrophages with AnxA5-preincubated ITC changed the cytokine secretion in comparison with the co-incubation with ITC or AnxA5 only. ITC plus AnxA5 led to a significant increased secretion of TNFα () and IL-1β (). In contrast, the secretion of TGF-β was significantly decreased when ITC plus AnxA5 was added to the macrophages (). The secretion of the IL-10 was not influenced by AnxA5, ITC, or AnxA5 plus ITC ().
Figure 2. AnxA5 modulates the cytokine secretion profile of macrophages after contact with irradiated, apoptotic tumor cells (ITC). Irradiated RMA tumor cells (ITC) were co-incubated, in the presence or absence of AnxA5, at a ratio of 5:1 with thioglycollate-elicited peritoneal Mϕ from C57BL/6 mice for 24 hr at 37°C. The culture of Mϕ in medium (m) alone or in medium supplemented with AnxA5 (100 μg/mL) served as controls. The supernatants were retrieved and the release of TNFα, IL-1β, IL-10, and TGF-β determined using ELISA. The results from one representative experiment (each performed in quadruplicate) are displayed. *P < 0.05; **P < 0.01.
Endogenous AnxA5 blocks the in vivo phagocytosis of dead cells by macrophages
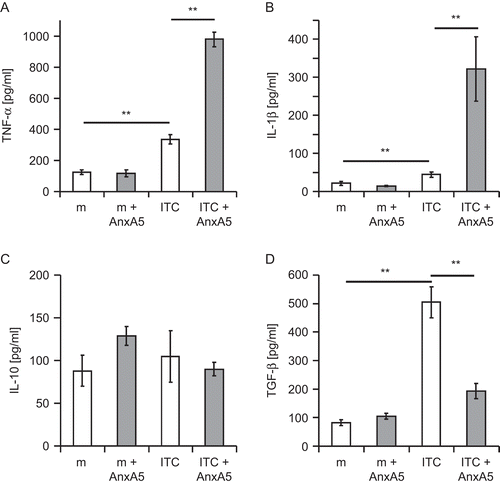
The in vivo uptake of multiple necrotic cells by macrophages in the peritoneum of mice was determined by flow cytometry. displays the percentage of F4/80positive macrophages that had taken up more than one necrotic cell (i.e., highly phagocytosing Mϕ) in the peritoneum of four representative WT and six representative AnxA5 KO mice. Only in one case did the macrophages of an individual WT mouse take up necrotic cells as well as macrophages of an AnxA5 KO mouse. In all other cases, macrophages from AnxA5 KO mice displayed a significantly enhanced in vivo uptake of the necrotic prey.
Figure 3. Influence of endogenous levels of AnxA5 on phagocytosis of necrotic tumor cells and on regression of an allogeneic tumor. Macrophages in the peritoneum of AnxA5 KO and WT mice had contact with necrotic WEHI 231 cells for 3.5 h. Following lavage of the peritoneum, the percentage of highly phagocytosing macrophages (Mϕ) which have taken up more than one necrotic cell was determined using flow cytometry. The results are obtained from lavages of four WT and six AnxA5 KO mice (A). The uptake by macrophages of AnxA5 KO mice of necrotic cells was significantly enhanced (P < 0.01) in comparison to the WT situation. The tumor diameter of the allogeneic CT26 tumor 2 wk after the injection in AnxA5 KO or WT mice is displayed in (B). Note: WT mice showed a significant faster regression of the tumor. One out of three independent experiments (each performed with at least five mice per group) are displayed. **P < 0.01.
Endogenous AnxA5 contributes to the regression of allogeneic tumors
The regression of an allogeneic CT26 colorectal tumor was compared between AnxA5 KO and the respective WT mice. In all cases, the tumor regressed and was rejected after 4 wk (data not shown). However, WT mice, which have endogenous AnxA5, showed a significantly faster regression and final rejection in comparison with AnxA5 KO mice. shows the tumor diameter in WT and AnxA5 KO mice 2 wk after the injection of the allogeneic tumor cells. The diameter of the tumor was significantly lower in WT mice in comparison with that of the tumor in AnxA5 KO hosts (respectively, 3.1 ± 0.8 vs. 4.6 ± 0.7, in mm [±SD]).
AnxA5 enhances the immunogenic properties of syngeneic ITC
The subcutaneous injection of ITC protected 50% and 25% of the mice from tumor growth after the challenge with viable RMA lymphoma cells at Days 20 and 40 after vaccination, respectively (). Adding AnxA5 to ITC led to a rejection of the tumors in 90% of the animals, indicating that AnxA5-coupled ITC are more immunogenic. The cure of growing tumors was also improved in the presence of AnxA5. Specifically, the tumor did not grow in 60% of the animals treated with ITC plus AnxA5. In contrast, the tumor did not grow in only 5% of the animals that were injected with ITC in the absence of AnxA5 (). These differences in outcomes were statistically significant (P < 0.05).
Table 1. Percentage of tumor free-mice after vaccination with irradiated tumor cells (ITC) only or with ITC plus AnnexinA5 (AnxA5).
AnxA5 and ionizing irradiation induce growth delay of syngeneic tumors
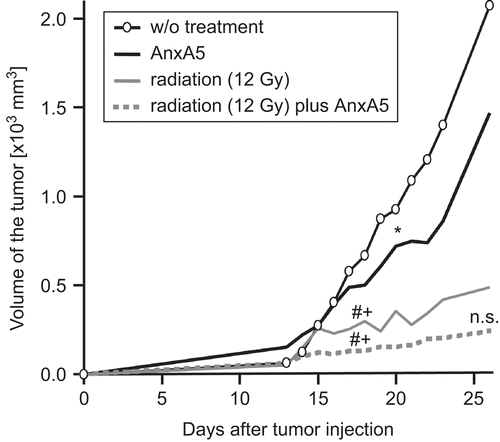
The efficacy of AnxA5 and ionizing irradiation for inhibiting the growth of a syngeneic tumor was tested in Balb/c mice using the CT26 colorectal tumor (). Without treatment, the volume of the tumor steadily increased. The injection of AnxA5 around the tumor during tumor growth significantly retarded the latter in comparison with that seen in nontreated mice. Ionizing irradiation (X-ray) at a single dose of 12 Gy significantly (P < 0.01) reduced the tumor volume in comparison with that in the non–AnxA5- or the AnxA5 only-treated mice. The combined treatment with X-ray plus AnxA5 led to a further, but not significant, improvement in delay of tumor growth in comparison with the effects seen with the irradiation alone.
Figure 4. Growth of a syngeneic tumor is inhibited by ionizing irradiation and by treatment with AnxA5. CT26 tumor cells were subcutaneously injected (on Day 0) into Balb/c mice and tumor growth was monitored thereafter by measures of tumor volume. Irradiation was performed with a single dose of 12 Gy at Day 14, and AnxA5 (40 μg/100 mm3) was injected on Days 14–23. One representative experiment (n = 10) out of three is displayed. The median tumor volume values of tumors of ten different mice are shown. Indications for statistical significance are given for the first timepoint at which the significant difference became evident and remained thereafter, and are marked as follows: *: AnxA5 vs. w/o treatment; #: radiation or radiation plus AnxA5 vs. AnxA5; +: radiation or radiation plus AnxA5 vs. w/o treatment; n.s.: radiation vs. radiation plus AnxA5. *P < 0.05; #P < 0.01; +P < 0.01. n.s.: nonsignificant; w/o: without.
Discussion
Experiments with gentically-modified mice have provided crucial hints that the immune system plays a role in the healing of cancer. The type of cell death and the immune system are important for the effectiveness of chemotherapy since IFNγR1−/−, nu/nu, Rag2−/−, and CD8−/− mice do not properly respond to oxaliplatin treatment, in comparison to WT mice (Tesniere et al., Citation2008). The immunogenicity of dying and dead tumor cells can be increased by fostering the presentation of dying cell-derived antigens by DC. In addition, a maturation signal for DC is needed which is mostly delivered by danger signals released from necrotic cells (Sauter et al., Citation2000).
Normally, apoptotic cells are efficiently and swiftly cleared by professional phagocytes like macrophages. These prestimulated macrophages secrete anti-inflammatory cytokines after contact with apoptotic cells (Voll et al., Citation1997; Fadok et al., Citation1998). It was shown here that the uptake by macrophages of irradiated, apoptotic tumor cells (ITC) can be partially blocked with the PS-binding protein AnxA5. The apoptotic, non-cleared cells accumulate, leading to secondarily necrotic cells and to the uptake of the dying cells by DC. The uptake by DC, in comparison to that by macrophages, is not blocked in vitro (see ) and in vivo by AnxA5 (CitationBondanza et al., 2004b). However, some apoptotic cells are still taken up by macrophages. Nevertheless, in the presence of AnxA5, thioglycollate-pre-activated from C57BL/6 mice secreted an increased amount of the inflammatory cytokines TNFα and IL-1-β and a decreased amount of the anti-inflammatory cytokine TGF-β. In the current studies, the level of IL-10 was not significantly altered when exogenous AnxA5 was added. Only in the complete absence of AnxA5, as it is the case in AnxA5 KO mice, was an increased secretion of IL-10 by activated macrophages after contact with apoptotic and necrotic cells observed (Frey et al., Citation2009). Furthermore, it was demonstrated that the low intrinsic levels of AnxA5 block the phagocytosis of dead cells by macrophages. Xenogeneic mice models revealed that the immunogenicity of apoptotic tumor cells could be increased by adding AnxA5 (Stach et al., Citation2000).
Anti-cancer immune responses can be regarded as a facet of a tissue-specific autoimmune phenomenon specific for cancer tissue (Wang et al., Citation2009). AnxA5 leads to an impaired clearance of apoptotic cells. A scenario of not- properly cleared cells has been observed in the systemic and chronic autoimmune disease SLE (Herrmann et al., Citation1998). Specifically, nuclear material accumulates in the light zone of the germinal centers. Follicular dendritic cells (FDC) then immobilize the nuclear material and autoreactive B-cells (specific for nuclear antigens) may receive a short-term survival signal from FDC loaded with the apoptotic cell-derived nuclear material. The B cells, in turn, specifically interact with autoreactive helper T-cells, obtain further survival signals, permitting their differentiation into autoantibody-secreting plasma cells and memory B-cells (summarized in Gaipl et al., Citation2003). Anti-tumor immunity also evolves dependent on stimulation by danger signals and the uptake and presentation of dead cell-derived peptides by mature DC, as well as their consecutive contact with B- and T-cells (Kono and Rock, Citation2008). These findings indicate that immune responses in chronic autoimmunity, as well as in anti-tumor immunity, are also strongly influenced by the tumor microenvironment.
Proteins of the IL-1 family may also be relevant danger signals similar to HMGB1, ATP, or uric acid for the immune system to develop anti-tumor immunity (Apte and Voronov, Citation2008). Danger signals are released in higher amounts by necrotic cells. The impaired clearance of apoptotic tumor cells induced by AnxA5 may contribute to the immune-activating tumor microenvironment. The tumor protection and cure assays (see ) demonstrated that the percentage of tumor-free mice was increased after vaccination with ITC plus AnxA5, in comparison with the vaccination with ITC alone. Re-challenge of protected animals led to a rejection of the tumor (CitationBondanza et al., 2004b). Even low levels of endogenous AnxA5 contribute to anti-tumor immunity as allogeneic tumors regressed at faster rates in WT mice in comparison with AnxA5 KO mice. Tumor cells have to be sensitized for immune responses by increasing the number of dead cells concomitant with the induction of immunogenic cell death forms (Shanker and Sayers, Citation2007; Green et al., Citation2009).
Combined tumor therapies on the one hand should stop proliferation and kill cancer cells and on the other hand should stimulate an immune response against residual cancer (stem) cells. Immunogenic cell death forms should be induced in vivo by chemotherapeutic agents (Zitvogel et al., Citation2008), ionizing irradiation (Ferrara et al., Citation2009), hyperthermia (Schildkopf et al., Citation2009), and treatment with immune-activating agents like AnxA5 (Gaipl et al., Citation2007). Even very low doses of chemotherapeutic agents (in the range of 1/10–1/20 of the maximal tolerated dose) target tumor cells as well as DC leading to immune activation (Shurin et al., Citation2008). It was demonstrated that irradiation leads to a highly significant reduction of the tumor volume. AnxA5 alone also had positive effects and could further slightly improve the tumor regression induced by X-ray. An important question is at what stage of tumor progression tumor-antigen specific immune responses are effective (Umansky et al., Citation2008)? It is speculated that AnxA5 exerts even more synergistic effects with X-ray at timepoints where the tumor is still small but nevertheless still manifested, and that the AnxA5 application should be combined with clinically-applied fractionated irradiation schemes. Since apoptotic tumor cells could also induce tolerance, approaches to target DC with live PS-labeled tumor cells may also be promising (Shurin et al., Citation2009).
In addition to the in vivo induction of immunogenic tumor cells, dead tumor cells can be applied as a tumor vaccine (). Following surgery, tumor cells are isolated from the solid tumor. For preparation of the vaccine, the cells have to be killed and should result in immunogenic death forms. One safe and clean way to kill tumor cells while preserving their immunogenicity is the application of high hydrostatic pressure (Korn et al., Citation2004). As suggested by the findings reported here, this immunogenicity might then be further increased by coupling AnxA5 to the tumor cells. The AnxA5 bearing dead tumor cells could then be reinjected into the patient and induce a specific anti-tumor immunity, leading to the removal of metastases and residual tumor cells. This application of a whole cell-based autologous tumor vaccine may drive an effective and long-lasting anti-tumor immunity leading to tumor cure and/or control.
Acknowledgments
This work was supported by “Deutsche Forschungsge-meinschaft” SFB 643, by the ELAN program of the Friedrich-Alexander University of Erlangen-Nürnberg, by AIF “Otto von Guerike” e.V. PROINNO II, KF0036801ULA, by the responsif GmbH Erlangen, by the European Commissions [NOTE: (TPA4 FP6) and APOCLEAR (QLK3-CT-2002-02017)], by the Programme Alban, the European Union Program of High Level Scholarships for Latin America, scholarship no. “E04D047956VE” to L. E. M., and by the DFG research training grant GK592.
Declaration of interest: The authors report conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Apte, R. N., and Voronov, E. 2008. Is interleukin-1 a good or bad “guy” in tumor immunobiology and immunotherapy? Immunol. Rev. 222:222–241.
- Bianchi, M. E., and Manfredi, A. 2004. Chromatin and cell death. Biochim. Biophys. Acta 1677:181–186.
- Bondanza, A., Zimmermann, V. S., Dell’Antonio, G., Cin, E. D., Balestrieri, G., Tincani, A., Amoura, Z., Piette, J. C., Sabbadini, M. G., Rovere-Querini, P., and Manfredi, A. A. 2004a. Requirement of dying cells and environmental adjuvants for the induction of autoimmunity. Arthritis Rheum. 50:1549–1560.
- Bondanza, A., Zimmermann, V. S., Rovere-Querini, P., Turnay, J., Dumitriu, I. E., Stach, C. M., Voll, R. E., Gaipl, U. S., Bertling, W., Poschl, E., Kalden, J. R., Manfredi, A. A., and Herrmann, M. 2004b. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J. Exp. Med. 200:1157–1165.
- Bottcher, A., Gaipl, U. S., Furnrohr, B. G., Herrmann, M., Girkontaite, I., Kalden, J. R., and Voll, R. E. 2006. Involvement of phosphatidylserine, αvβ3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum. 54:927–938.
- Brachvogel, B., Dikschas, J., Moch, H., Welzel, H., von der Mark, K., Hofmann, C., and Poschl, E. 2003. Annexin A5 is not essential for skeletal development. Mol. Cell. Biol. 23:2907–2913.
- Fadok, V. A., Bratton, D. L., Konowal, A., Freed, P. W., Westcott, J. Y., and Henson, P. M. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit pro-inflammatory cytokine production through autocrine/paracrine mechanisms involving TGFβ, PGE2, and PAF. J. Clin. Invest. 101:890–898.
- Ferrara, T. A., Hodge, J. W., and Gulley, J. L. 2009. Combining radiation and immunotherapy for synergistic anti-tumor therapy. Curr. Opin. Mol. Ther. 11:37–42.
- Frey, B., Munoz, L. E., Pausch, F., Sieber, R., Franz, S., Brachvogel, B., Poschl, E., Schneider, H., Rodel, F., Sauer, R., Fietkau, R., Herrmann, M., and Gaipl, U. S. 2009. The immune reaction against allogeneic necrotic cells is reduced in AnnexinA5 knock out mice whose macrophages display an anti-inflammatory phenotype. J. Cell. Mol. Med. 13:1391–9.
- Gaipl, U. S., Brunner, J., Beyer, T. D., Voll, R. E., Kalden, J. R., and Herrmann, M. 2003. Disposal of dying cells: a balancing act between infection and autoimmunity. Arthritis Rheum. 48:6–11.
- Gaipl, U. S., Sheriff, A., Franz, S., Munoz, L. E., Voll, R. E., Kalden, J. R., and Herrmann, M. 2006. Inefficient clearance of dying cells and autoreactivity. Curr. Topics Microbiol. Immunol. 305:161–176.
- Gaipl, U. S., Munoz, L. E., Rodel, F., Pausch, F., Frey, B., Brachvogel, B., von der Mark, K., and Poschl, E. 2007. Modulation of the immune system by dying cells and the phosphatidylserine-ligand annexin A5. Autoimmunity 40:254–259.
- Green, D.R., Ferguson, T., Zitvogel, L., and Kroemer, G. 2009. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9:353–363.
- Grundmann, U., Abel, K. J., Bohn, H., Lobermann, H., Lottspeich, F., and Kupper, H. 1988. Characterization of cDNA encoding human placental anticoagulant protein (PP4): homology with the lipocortin family. Proc. Natl. Acad. Sci. USA 85:3708–3712.
- Hanley, P. J., Musset, B., Renigunta, V., Limberg, S. H., Dalpke, A. H., Sus, R., Heeg, K. M., Preisig-Muller, R., and Daut, J. 2004. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc. Natl. Acad. Sci. USA 101:9479–9484.
- Herrmann, M., Voll, R. E., Zoller, O. M., Hagenhofer, M., Ponner, B. B., and Kalden, J. R. 1998. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 41:1241–1250.
- Kono, H., and Rock, K. L. 2008. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8:279–289.
- Korn, A., Frey, B., Sheriff, A., Gaipl, U. S., Franz, S., Meyer-Pittroff, R., Bluemelhuberh, G., and Herrmann, M. 2004. High hydrostatic pressure-inactivated human tumor cells preserve their immunogenicity. Cell. Mol. Biol. (Noisy-le-grand) 50:469–477.
- Kroemer, G., Galluzzi, L., Vandenabeele, P., Abrams, J., Alnemri, E. S., Baehrecke, E. H., Blagosklonny, M. V., El-Deiry, W. S., Golstein, P., Green, D. R., Hengartner, M., Knight, R. A., Kumar, S., Lipton, S.A., Malorni, W., Nunez, G., Peter, M. E., Tschopp, J., Yuan, J., Piacentini, M., Zhivotovsky, B., and Melino, G. 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16:3–11.
- Krysko, D. V., and Vandenabeele, P. 2008. From regulation of dying cell engulfment to development of anti-cancer therapy. Cell Death Differ. 15:29–38.
- Lauber, K., Blumenthal, S. G., Waibel, M., and Wesselborg, S. 2004. Clearance of apoptotic cells: getting rid of the corpses. Mol. Cell. 14:277–287.
- Obeid, M., Panaretakis, T., Joza, N., Tufi, R., Tesniere, A., van Endert, P., Zitvogel, L., and Kroemer, G. 2007. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death Differ. 14:1848–1850.
- Reutelingsperger, C. P., Hornstra, G., and Hemker, H. C. 1985. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur. J. Biochem. 151:625–629.
- Ronchetti, A., Rovere, P., Iezzi, G., Galati, G., Heltai, S., Protti, M.P., Garancini, M.P., Manfredi, A.A., Rugarli, C., and Bellone, M. 1999. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol. 163:130–136.
- Sauter, B., Albert, M. L., Francisco, L., Larsson, M., Somersan, S., and Bhardwaj, N. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423–434.
- Schildkopf, P., Holmer, R., Sieber, R., Ott, O. J., Janko, C., Mantel, F., Frey, B., Fietkau, R., and Gaipl, U. S. 2009. Hyperthermia in combination with X-irradiation induces inflammatory forms of cell death. Autoimmunity 42:311–313.
- Shanker, A., and Sayers, T. 2007. Sensitizing tumor cells to immune-mediated cytotoxicity. Adv. Exp. Med. Biol. 601:163–171.
- Shi, Y., Evans, J. E., and Rock, K. L. 2003. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425:516–521.
- Shurin, G. V., Tourkova, I. L., and Shurin, M. R. 2008. Low-dose chemotherapeutic agents regulate small Rho GTPase activity in dendritic cells. J. Immunother. 31:491–499.
- Shurin, M. R., Potapovich, A. I., Tyurina, Y. Y., Tourkova, I. L., Shurin, G. V., and Kagan, V. E. 2009. Recognition of live phosphatidylserine-labeled tumor cells by dendritic cells: a novel approach to immunotherapy of skin cancer. Cancer Res. 69:2487–2496.
- Stach, C. M., Turnay, X., Voll, R. E., Kern, P. M., Kolowos, W., Beyer, T. D., Kalden, J. R., and Herrmann, M. 2000. Treatment with annexin V increases immunogenicity of apoptotic human T-cells in Balb/c mice. Cell Death Differ. 7:911–915.
- Tesniere, A., Apetoh, L., Ghiringhelli, F., Joza, N., Panaretakis, T., Kepp, O., Schlemmer, F., Zitvogel, L., and Kroemer, G. 2008. Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 20:504–511.
- Umansky, V., Abschuetz, O., Osen, W., Ramacher, M., Zhao, F., Kato, M., and Schadendorf, D. 2008. Melanoma-specific memory T-cells are functionally active in Ret transgenic mice without macroscopic tumors. Cancer Res. 68:9451–9458.
- Vermes, I., Haanen, C., Steffens-Nakken, H., and Reutelingsperger, C. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein-labeled Annexin V. J. Immunol. Meth. 184:39–51.
- Voll, R. E., Herrmann, M., Roth, E. A., Stach, C., Kalden, J. R., and Girkontaite, I. 1997. Immunosuppressive effects of apoptotic cells. Nature 390:350–351.
- Wang, E., Monaco, A., Monsurro, V., Sabatino, M., Pos, Z., Uccellini, L., Wang, J., Worschech, A., Stroncek, D. F., and Marincola, F. M. 2009. Anti-tumor vaccines, immunotherapy and the immunological constant of rejection. IDrugs 12:297–301.
- Zitvogel, L., Apetoh, L., Ghiringhelli, F., Andre, F., Tesniere, A., and Kroemer, G. 2008. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118:1991–2001.