Abstract
Non-human primates (NHPs), primarily macaques, are commonly used as non-rodent species in pre-clinical safety assessment studies. The use of macaques in such studies is increasing largely due to the development of biopharmaceutical and immunomodulatory therapies that necessitates extensive safety testing. Macaques, commonly available for use in such studies, are infected by a rich flora of herpesviruses that cause persistent, latent, life-long infections. Primary infection of immune competent macaques is typically subclinical with very little associated morbidity and mortality only in very rare cases. A life-long consequence of herpesvirus infection is periodic stochastic and frequently asymptomatic recurrences from latency throughout an infected macaque’s lifetime. With immune modulation or suppression, however, immune control of herpesvirus infections can be lost, resulting in significant disease and even death of the affected animals. Since macaques undergo primary infection with herpesviruses starting around 4-6 months-of-age when maternally-derived antibody begins to wane, it is difficult and costly to derive animals that are herpesvirus-free. Further, the herpesvirus flora and prevalence of infection in laboratory macaques mirrors that of the adult human population making the herpesvirus-infected macaque a reasonable model of the general human population. This review is intended to familiarize toxicologists performing preclinical drug safety studies with the basic biology, disease pathogenesis and consequences of immune suppression in herpesvirus-infected laboratory macaques.
Introduction
Viruses of the family Herpesviridae infect a wide variety of animals from bivalves to primates; many animals, when examined closely, are found to be infected by one or more distinct herpesviruses. This family is composed of three subfamilies, Alpha-, Beta-, and Gammaherpesvirinae based upon similar genetic, structural, and biological properties (McGeoch et al., Citation2000). Herpesviruses have a consistent ultrastructural morphology including a core, capsid, tegument, and envelope (). The core contains the linear double-stranded DNA molecule (with a length of 125–230 kb) that is tightly wrapped into a toroid with an inner diameter of 18 nm and an outer diameter of 70 nm. The core is surrounded by a proteinaceous capsid that is composed of 162 capsomeres arranged in icosohedral symmetry (with a diameter of 100–125 nm). Surrounding the capsid is an amorphous-appearing tegument that is within a double layer lipid envelope, giving a mean virion diameter of 180–250 nm.
Figure 1. Schematic diagram of a herpesvirus demonstrating the major structural components including the genomic DNA, virus capsids, tegument, envelope, and envelope glycoproteins.
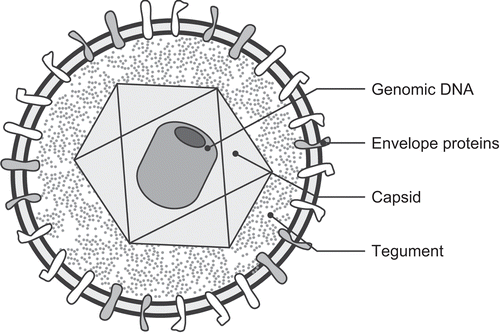
As a group, herpesviruses infect a wide variety of different cell types; however, individual herpesviruses usually have narrow and specific cellular tropisms. When infecting a cell, virus envelope-associated glycoproteins bind to cellular membrane receptors and the virion envelope fuses with the cell’s plasma membrane. Next, the capsid is transported to the nuclear pore and the genome is released into the nucleus where the large and complex genome is transcribed into messenger RNAs and replicated as full-length double-stranded DNA copies. Herpesviruses have large and complex genomes that produce hundreds of proteins and they have acquired genes for a number of proteins that can manipulate the host’s cellular and immunologic responses. Often, the first proteins that are produced begin the process of degrading host cellular mRNAs, while other proteins begin the process of co-opting the cell’s normal cellular functions. Infection of a naïve cell by a herpesvirus can have two potential outcomes: lytic replication or episomal latency. During lytic replication, the herpesvirus genome is packaged into virus capsids in the nucleus, and the capsid buds through the Golgi apparatus or Golgi vesicle membranes to obtain its lipid envelope. Lytic replication results in infectious virion production and cell-to-cell spread or transmission of virus to new hosts. Excess capsid proteins are often produced during lytic infections and stored within the nucleus as inclusion bodies. Thus, intranuclear inclusion bodies are often visible in histopathologic sections taken from herpesvirus-infected cells. During episomal latency, however, the virus genome takes on the form of a closed circular molecule with a restricted subset of latency-associated genes being actively transcribed and intact virions cannot be found within infected cells. The precise mechanisms by which a host cell that is latently infected with a herpesvirus transitions to a productive, lytic, infection is not well understood. An ability to manipulate the host’s cellular and immune responses and episomal latency allow herpesviruses to cause life-long, persistent, infections. Or, put another way, once an animal is infected with a herpesvirus, it is infected forever. Periodic recurrence from latency, and thus active virus production and shedding, may occur at unpredictable time intervals throughout an infected animal’s life. Recurrences from latency are stochastic and, frequently, subclinical events that have been associated with physical or emotional stress, fever, exposure to UV light, tissue damage and immune suppression.
Herpesviruses and their natural hosts have co-evolved with one another over a period of many millions of years (Springer et al., Citation2003; McGeoch et al., Citation2006). Because of this long period of co-evolution, virus and natural host are well adapted to one another. Thus, primary infection is often subclinical and when disease occurs it is often quite mild and transient. Herpesviruses can cause a wide variety of diseases, from neurologic to neoplastic, if their host becomes immune suppressed or otherwise debilitated. While herpesviruses are often host specific they can occasionally infect an aberrant, often closely related, or otherwise permissive host. These aberrant or zoonotic herpesvirus transmissions often result in a significant and rapid disease course for the aberrantly infected host.
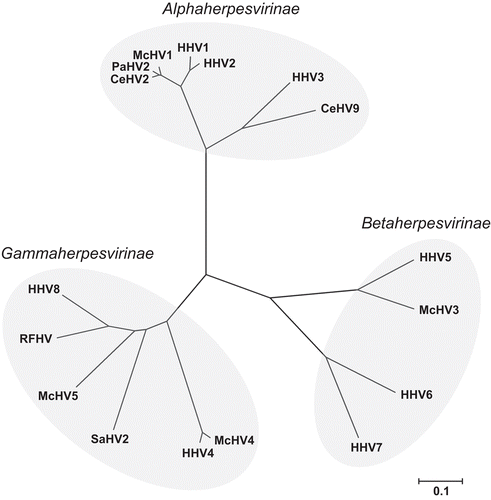
The family Herpesviridae is composed of hundreds of different viruses that infect a wide variety of animal species. Before gene sequencing was widely available, the family was divided into three subfamilies, the Alpha-, Beta-, and Gammaherpesvirinae, based upon biological and morphological characteristics (Roizman et al., Citation1981). With the advent and spread of widely-available sequencing technologies, each of these subfamilies has been further subdivided into genera based upon similarities in genome sequence arrangements and the immunological similarities of important viral proteins (Pellett and Roizman, Citation2007). In addition, with the development of broadly reactive polymerase chain reaction (PCR) primer sets that target conserved genes (such as the viral DNA polymerase), novel herpesviruses are frequently reported, allowing the herpesvirus flora of a wide number of species to be explored (VanDevanter et al., Citation1996; Ehlers et al., Citation1999). Many species of primates, both human and non-human, have a rich herpesvirus flora and often there is a high prevalence of infection with viruses from each of the three subfamilies of the Herpesviridae (). In fact, for most of the herpesviruses that are commonly found in laboratory macaques there is an orthologous human virus () thus the herpesvirus flora and prevalence of humans and macaques often mirror one another.
The International Committee on the Taxonomy of Viruses has recently adopted a series of changes in herpesvirus nomenclature including adopting Herpesvirales as a new virus Order, and subdividing the former family Herpesviridae into three distinct virus subfamilies. Additionally, they have adopted a series of official name changes to a number of the primate herpesviruses resulting in names that more accurately represent the genera of animal that the virus infects (Davison et al., Citation2009). The text and contain both official ICTV and common designations for all herpesviruses that are discussed.
Table 1. Some primate viruses within the order Herpesvirales (Davison et al., Citation2009).
Figure 2. Evolutionary relationships of 17 common primate herpesviruses were inferred from the amino acid sequence of the DNA polymerase protein using the Neighbor-Joining method (Saitou and Nei, Citation1987). The tree is drawn to scale with branch lengths in units of number of amino acid substitutions per site used to infer the phylogenetic tree and evolutionary distances were computed using the Poisson correction method (Zuckerkandl and Pauling, Citation1965). Positions containing gaps and missing data were eliminated from the dataset resulting in a total of 941 positions in the final dataset. Phylogenetic analyses were conducted using MEGA4 (Tamura et al., Citation2007).
Alphaherpesvirinae
Herpesviruses were classified into this subfamily based upon a number of host, virus, and cell culture characteristics. Alphaherpesviruses typically have a variable host range and, most often, establish latency within the nuclei of sensory ganglia, although there are a few exceptions to this neurotropism. In cell culture, they have a very short productive cycle, spread rapidly, and cause distinctive cytopathic effects in infected cell cultures. The Alphaherpesvirinae have been further subdivided to include four established genera, of which two of these genera infect mammals, including humans and a variety of non-human primates: Simplexvirus and Varicellovirus (International Committee on the Taxonomy of Viruses, Citation2006; Pellett and Roizman, Citation2007).
Simplexvirus
The prototypical Simplexvirus (αS) is the human “cold sore virus” herpes simplex virus 1 (HSV-1) and its close relative herpes simplex virus 2 (HSV-2) that causes human oral and genital herpesvirus infections (International Committee on the Taxonomy of Viruses, Citation2006). The generalized pathogenesis of Simplexvirus infections is well-described and is usually initiated when intimate contact occurs between virus-contaminated secretions from an actively-shedding individual and the mucosal or abraded epithelial surfaces of a naïve individual. The virus then replicates and spreads to local naïve epithelial cells. As virions encounter sensory nerves fibers, their envelopes begin to fuse with nerve fiber membranes, depositing their genome-containing capsids within the axons of sensory neurons. Infectious capsids are then transported to the neuron’s cell body by retrograde transport where they can establish a productive infection or latency. When the infection is productive or latent with reactivation, this process is essentially reversed, with infectious virus being transported by anterograde transport to the sensory nerve fibers innervating the epithelial cells in the region of original infection. The epithelial cells are then productively infected, and infectious virions are released to infect other naïve hosts. Oral and genital infections are typically established in the trigeminal nerve (cranial nerve V) and the dorsal root ganglia of the sacral nerve; however, other regional nerves can be infected depending upon where the original epithelial or mucosal infection occurs (Roizman et al., Citation2007). Interestingly, infections can be unilateral or bilateral depending on the site(s) of initial infection. The prevalence of simplexviruses in the human population varies according to a number of features, including gender, age, socioeconomic condition, and culture; however, the general trend is that the prevalence increases with age and the prevalence is generally higher in people of lower socioeconomic condition and in females. Overall, simplexvirus seroprevalence rates in North America, Europe, Asia, and Africa frequently exceed 80% and often approach 100% in certain demographic groups (McDonald et al., Citation1974; Nahmiase et al., Citation1990; Fleming et al., Citation1997; Howard et al., Citation2003; Malkin, Citation2004).
Macaque Simplexvirus infections: Macaques are frequently infected by Macacine herpesvirus 1 (McHV1) (Elmore and Eberle, Citation2008) which was first described in 1934 by Albert Sabin (Sabin and Wright, Citation1934). The virus was originally cultured from tissues obtained from a young physician, Dr. W. B., who died of encephalomyelitis approximately two weeks after being bitten on the finger while performing polio research on an apparently normal rhesus macaque. Sabin recapitulated the disease in animals inoculated with cell cultures from virus obtained from Dr. W. B. and referred to the virus as “B virus” in his honor; many people still use B virus as a common term for McHV1 (Sabin and Wright, Citation1934). Since the virus was first described, over two dozen people in the United Stated have died from B virus-induced encephalomyelitis after direct or indirect exposure to infected macaques (Whitley and Hilliard, Citation2007). Orthologous viruses are found in a wide variety of laboratory monkey species including herpes tamarinus, simian agent 8 (SA8), and Papine herpesvirus 2 (PaHV2) in tamarins, vervets, and baboons, respectively.
Like humans, macaques can have both oral and genital infections with B virus, and primary infection is usually acute and self-limiting with little morbidity. Infected macaques are usually asymptomatic; however, when lesions are seen they are typically mild and can include conjunctivitis, vaginitis, and oral vesicles or ulcerations (Weigler et al., Citation1990; Anderson et al., Citation1994). Pathogenesis of B virus infection in macaques is similar to that for HSV 1 and 2: virus infects local epithelial cells, spreads to sensory nerve fibers, and retrograde transport moves the virus capsid to the sensory nerve cell body. Transmission occurs via B virus capsid production in the sensory nerve nucleus, virus envelope formation in the cytoplasm, and anterograde transport to the distal epithelial cells where original infection occurred. B virus then infects and is released from lytically-infected mucosal epithelial cells. Sites of B virus shedding can include the buccal, gingival, conjuncitval, anal, or genital mucosal sites (Daniel et al., Citation1984; Anderson et al., Citation1994). Shedding of virus can occur from sites with or without observable B virus-associated lesions; as such, one must always exercise care when working with laboratory macaques (Weigler et al., Citation1992, Citation1993). Since B virus is a neurotropic virus, infections are typically localized within the nervous system; however, viremia has been rarely reported in B virus-infected macaques (Simon et al., Citation1993).
The rate of B virus prevalence in free-ranging and captive macaques is very high in populations of infected animals (Kessler et al., Citation1989; Weigler et al., Citation1993). Seroprevalence rates typically increase as animals age with adolescent macaques (2–3-years-old) at greatest risk of infection and with most animals infected by 4 years-of-age (Weigler et al., Citation1993). B virus-negative colonies have been established by testing and removing seropositive animals {Foster, Citation1976 #3432} and B virus is commonly included in the minimal definition of specific pathogen-free (SPF) (Lerche et al., Citation1994; Desrosiers, Citation1997; Mansfield, Citation2005; Lerche and Simmons, Citation2008).
Reactivation of B virus resulting in observable lesions or death has been reported in laboratory macaques (Chellman et al., Citation1992; Carlson et al., Citation1997). In one instance, 3 of 14 macaques given a high-dose immune-suppressive drug in a 6-month toxicology study developed B virus-associated oral lesions after 3 mo on study, which resulted in affected animals being removed from the study for personnel safety (Chellman et al., Citation1992). The other report describes the death of two macaques from fatal disseminated B virus infection and notes that the animals were infected with the immune suppressive Betaretrovirus, simian type-D retrovirus (SRV) that could have potentiated B virus recurrence from latency and fatal dissemination (Carlson et al., Citation1997). Simian type-D retrovirus is described in detail elsewhere in this issue (Lerche, 2009).
Varicellovirus
The prototypical Varicellovirus (αV) is human varicella-zoster virus (VZV) (International Committee on the Taxonomy of Viruses, Citation2006). Varicella, which occurs with primary infection by VZV, is generally an acute and self-limiting disease that is characterized by fever, malaise, and a pruritic vesicular rash that is often called “chickenpox” (Gershon and Silverstein, Citation2002; Davidovici et al., Citation2007). Varicella-zoster virus is rapidly spread via respiratory droplets and is commonly a disease of early childhood in temperate climates, throughout the world. The tissue tropic sites for VZV are T-lymphocytes, epithelial cells, and dorsal root ganglia (Cohen et al., Citation2007). As a neurotropic alpha herpesvirus, the site of VZV latency is within sensory ganglia.
Reactivation of virus from latency, known as zoster and commonly referred to as “shingles,” typically occurs in 15% of VZV-infected adults; disease incidence greatly increases in people over 50 years-of-age (Hope-Simpson, Citation1965). Zoster typically begins as a localized unilateral coalescing skin eruption involving 1–3 dermatomal segments (Gershon and Silverstein, Citation2002). Zoster typically occurs in people that are relatively immune suppressed due to age, disease, chemotherapy, or radiotherapy (Gershon and Silverstein, Citation2002). During reactivation in more severely immune-suppressed patients, VZV can disseminate to the lungs, liver, CNS, and other organs from sites of localized reactivation (Feldman et al., Citation1977; Locksley et al., Citation1985).
Varicella infection rates are very high and approach 100% in most countries throughout the world. The attack rate for VZV is also very high, with 80–90% of exposed people within a household developing clinical disease (Ross, Citation1962). Subclinical infections have also been documented, as three-quarters of adults that report no history of varicella infection have antibodies to the virus (LaRussa et al., Citation1985).
Macaque Varicellovirus infections: Simian varicella virus (SVV), also known as Cercopithecine herpesvirus 9 (CeHV-9), has been associated with outbreaks of varicella-like disease in a variety of Old World non-human primates (NHP) (Gray, Citation2004, Citation2008). The described clinical disease is typically a multifocal erythematous and vesicular dermatitis, and can include hemorrhagic necrosis of the liver, lungs, and other visceral organs. Outbreaks of SVV-associated clinical disease, resulting in deaths of 2–74% of clinically-affected animals, have been described in patas (Erythrocebus patas), African green (Chlorocebus aethiops) and macaque (Macaca spp.) monkeys (Gray, Citation2008). In addition, antibodies to SVV have been described in a colony of olive baboons (Papio cynocephalus anubis), with no reports of clinical varicella-like disease or death (Payton et al., Citation2004).
Transmission of SVV occurs primarily via inhalation of aerosolized respiratory droplets; however, direct transmission via contact with cutaneous lesions is possible (Wolf et al., Citation1974; Lehner et al., Citation1984). Based upon data from intratracheal inoculation studies, the virus initially replicates in respiratory epithelial cells; by Day 3 post-inoculation, virus can be detected in circulating T- and B-lymphocytes that disseminate the virus throughout the body (White et al., Citation2002). Viremia is transient and virus is cleared from the bloodstream by Day 11 post-inoculation. The incubation period for SVV following natural or experimental infection appears to be 7–14 days, and is similar to that of VZV infection in humans (Gray et al., Citation1998; Gray, Citation2004). The first clinical signs of disease appear around Day 10 post-inoculation as an inguinal rash and disseminate to a generalized cutaneous rash over the following two days that is particularly visible on the face, thorax and abdomen, with the palmar and plantar surfaces of the distal appendages being spared (Gray et al., Citation1998). Other clinical signs of SVV infection can include fever, malaise, inappetence, and hepatitis (Gray, Citation2004). In other intratracheal inoculation studies in African green monkeys, no clinically-observable signs of disease were noted; however, SVV DNA was found to localize to and to establish latency in the dorsal root ganglia of sensory neurons (Mahalingam et al., Citation1991).
While outbreaks of SVV-associated clinical disease have been well documented, and experimental infection studies have been performed in both macaques and African green monkeys, the epidemiology of natural SVV infection and transmission in colonies of immune competent macaques is poorly understood. This is, in part, due to the fact that SVV is not commonly included as an SPF agent and thus macaque colonies are seldom tested for antibodies to the virus (Lerche et al., Citation1994; Desrosiers Citation1997; Mansfield, Citation2005; Lerche and Simmons, Citation2008). Also complicating the understanding of macaque SVV epidemiology is the fact that reports of outbreaks of SVV in macaque colonies occurred before the description and implementation of routine testing for the immune suppressive Betaretrovirus SRV in captive macaque colonies (Marx et al., Citation1984). Thus, SVV-associated clinical disease in these outbreaks may have been exacerbated by immune suppression. Further, colony managers have not reported observing varicella-like lesions in captive bred animals, which is consistent with the report of 40% seroprevalence of SVV in immune competent infant and juvenile baboons in the absence of clinically observable disease (Payton et al., Citation2004).
There have been two recent reports of SVV-associated disease in macaques undergoing immune suppressive experimental protocols (Kolappaswamy et al., Citation2007; Schoeb et al., Citation2008). In the first report, a rhesus macaque (Macaca mulatta) contracted disseminated SVV infection 105 days after receiving 600 cGy of total-body irradiation. The macaque was seronegative for SVV before the study was initiated and the Authors speculated that the affected macaque contracted SVV by exposure to another macaque during the study that had an inapparent SVV reactivation (Kolappaswamy et al., Citation2007). The second SVV outbreak occurred in a group of 11 of 75 rhesus macaques, some of which were subjected to organ transplant and immune suppression and some that were not experimentally manipulated. The source of infection in this case is not definitively known; however, retrospective analysis indicated that two animals were seropositive for SVV before the outbreak occurred. Thus, the Authors speculated that one or both of these monkeys had a reactivation from latency, thus exposing the naïve and otherwise immune-suppressed group to SVV (Schoeb et al., Citation2008).
Betaherpesvirinae
Betaherpesviruses are characterized by restricted host range, a long reproductive cycle, and the slow progression of infection in cell cultures (Pellett and Roizman, Citation2007). In vivo and in vitro, infected cells can develop both karyomegaly (enlarged nuclei) and cytomegaly (generalized cellular enlargement). Sites of latency usually include lymphoreticular cells, secretory glands, kidneys, endothelial cells, as well as various other tissues. There are three recognized genera within the subfamily: Cytomegalovirus, Muromegalovirus, and Roseolovirus. Of these three genera, only the Cytomegaloviruses are currently known to infect both human and non-human primates (International Committee on the Taxonomy of Viruses, Citation2006).
Cytomegalovirus
The prototypical Cytomegalovirus (βC) is a human herpes virus 5 (HHV-5) that is commonly referred to as human cytomegalovirus (HCMV) (International Committee on the Taxonomy of Viruses, Citation2006). Human CMV is a ubiquitous opportunist that infects most people throughout the world by early adulthood. Primary infection is generally subclinical, indicating that the virus and host are well adapted to one another, which is supported by the 80 million year co-evolution between herpesviruses and their mammalian hosts (Griffiths and Emery, Citation2002; McDonagh et al., Citation2006; Mocarski et al., Citation2007). Like all herpesviruses, HCMV infections are life-long once infection occurs; however, the HHV-5 replication strategy for host maintenance appears to be via sites of persistent active replication or via frequent virus reactivation throughout the host’s lifespan, even in immune-competent individuals (Jarvis and Nelson, Citation2007). Cytomegaloviruses are also the only group of herpesviruses that are known to cross the placenta (McDonagh et al., Citation2006). Ante- and post-mortem biopsy specimens indicate that HCMV can be found in a wide variety of tissues, including the lung, heart, adrenal glands, salivary glands, brain, spinal cord, esophagus, colon, etc. (Griffiths and Emery, Citation2002) and that it can infect a wide variety of cell types. However, smooth muscle, myeloid, and endothelial cells appear to be critical sites of virus latency and persistence (Vossen et al., Citation1996; Jarvis and Nelson, Citation2002, Citation2007). The platelet-derived growth factor-α receptor was recently identified as a required receptor for HHV-5 infection (Soroceanu et al., Citation2008).
Cytomegaloviruses are ideal opportunists since they cause little or no clinical disease in immune competent hosts; however, if the host is immune incompetent, for example due to very young age, or immune suppressed due to old age, HIV infection status, or therapeutic immunomodulatory treatment, HCMV can reactivate and cause significant clinical disease. Ten years ago, the estimated additional cost of managing HCMV infection in heart, liver, and kidney transplant patients was $22,598–$42,111 per year (Mocarski et al., Citation2007). Further, HCMV is a significant disease risk in HIV-infected individuals (Griffiths and Emery, Citation2002; Mocarski et al., Citation2007).
Macaque Cytomegalovirus infections: The best-characterized macaque cytomegalovirus (CMV) is rhesus CMV (RhCMV), also known as Macacine herpesvirus 8 (McHV8) (Hansen et al., Citation2003; International Committee on the Taxonomy of Viruses Citation2006). Orthologous CMVs exist in a variety of other New and Old World non-human primate species, including owl monkeys, baboons, African green monkeys, mandrils, and chimpanzees (Pellett and Roizman, Citation2007). The pathogenesis of RhCMV in macaques recapitulates that of natural HCMV infection, with little or no clinically observable disease upon primary infection and with a potential for significant disease in immune suppressed NHPs (Barry et al., Citation2006; Barry and Strelow, Citation2008). Rhesus CMV also replicates in tissues and cell types that are similar to those in HCMV-infected humans (Lockridge et al., Citation1999). In one controlled infection study, incipient infection resulted in plasma viremia for a period of 1–2 weeks, followed by dissemination to a variety of organs with spleen, particularly the endothelial cells and macrophages, being the organ infected in all animals studied (Lockridge et al., Citation1999). Bone marrow, one of the suspected sites of latency for HCMV, was also found to be reservoir of infection in the macaque as well (Hahn et al., Citation1998; Lockridge et al., Citation1999).
The epidemiology of infection by, and seroconversion to, RhCMV in both wild and captive adult macaque populations was assessed to be 95–100% in three large disparate colony surveys, indicating that the seroprevalence of RhCMV in macaques mirrors that of HCMV in the general human population (Vogel et al., Citation1994; Andrade et al., Citation2003; Jones-Engel et al., Citation2006). Infection of macaques by RhCMV appears to occur fairly early in life, as 50% of juvenile macaques (≈6 months-of-age) are seropositive for anti-RhCMV antibody (Vogel et al., Citation1994). Macaques infected with RhCMV have been found to persistently shed virus in their saliva and urine, which likely explains the rapid dissemination of RhCMV within susceptible populations (Asher et al., Citation1974; Huff et al., Citation2003). Interestingly, while transplacental HCMV transmission has been rarely documented in humans, to date, transplacental RhCMV transmission has not been documented in macaques. However, detailed studies that might determine if transplacental transmission of RhCMV occurs have not been completed (Barry and Strelow, Citation2008).
Opportunistic disease associated with RhCMV has been observed in infected macaques that were immune suppressed by immune modulation with other viruses or by large molecule therapy (Baskin, Citation1987; Sequar et al., Citation2002; Pearson et al., Citation2002; Kaur et al., Citation2003; Jonker et al., Citation2004; Yanai et al., Citation2006). Simian immunodeficiency virus (SIV) is a retrovirus in the genus Lentivirus that causes a human AIDS-like disease in macaques (Desrosiers, Citation1988) that is described in detail elsewhere in this issue (Lerche, 2009). Macaques that are opportunistically infected with RhCMV and SIV by natural exposure commonly die from disseminated RhCMV-associated disease, with histopatholgical lesions being found in the brain, lung, lymph nodes, liver, spleen, small intestine, testicles, nerves, and arteries (Baskin, Citation1987). Severe disseminated RhCMV-associated vascular lesions have also been described in SIV-infected macaques, which are consistent with the endothelial tropism of cytomegaloviruses (Yanai et al., Citation2006). Co-infection of macaques with SIV and RhCMV recapitulates the human AIDS-associated opportunistic HCMV disease (Sequar et al., Citation2002; Kaur et al., Citation2003). Additionally, RhCMV-infected macaques that are immune suppressed with monoclonal antibodies directed against CD-40 or MHC Class II to study renal allograft rejection died from disseminated cytomegalovirus-associated disease (Pearson et al., Citation2002; Jonker et al., Citation2004). Thus, macaques that are adventitiously infected with RhCMV are sensitive to disruptions in immune function that can result in loss of immunologic control of RhCMV resulting in significant disease or death.
Gammaherpesvirinae
Gammaherpesviruses have an in vivo host range that is limited to members of the same order or family and typically infect T- or B-lymphocytes and establish latency in lymphoid tissues (Pellett and Roizman, Citation2007). There are currently two recognized genera within the family, Lymphocryptovirus and Rhadionvirus; however, researchers are actively discovering gammaherpesviruses at a rapid pace and it is likely that the subfamily will both grow and undergo some rearrangement as newly discovered viruses are formally classified (International Committee on the Taxonomy of Viruses, Citation2006; McGeoch et al., Citation2006).
Lymphocryptovirus
The prototypical Lymphocryptovirus (γL) is human herpesvirus 4 (HHV-4), also known as Epstein-Barr virus (EBV), the etiologic agent of human infectious mononucleosis (Beaulieu and Sullivan, Citation2002; International Committee on the Taxonomy of Viruses, Citation2006; Pellett and Roizman, Citation2007). The pathogenesis of EBV is well described and initiated when HHV-4 contacts and likely infects both the tonsillar epithelium and crypt B-lymphocytes (Beaulieu and Sullivan, Citation2002; Rickinson and Kieff, Citation2007). After a primary infection, persistently infected B-lymphocytes are transported throughout the lymphoreticular system where latency is established (Beaulieu and Sullivan, 2002; Thorley-Lawson and Gross, 2004; Rickinson and Kieff, 2007). The site of lytic virus production is still undetermined, but there is circumstantial evidence that virus is produced in the oropharynx most likely the lingual epithelium (Rickinson and Kieff, Citation2007). In one study, EBV virus DNA was detected in the saliva of 90% of HIV-seropositive and in 48% of HIV-seronegative humans tested, which is consistent with virus production in the oropharynx and virus-contaminated saliva being a primary mechanism for virus transmission (Beaulieu and Sullivan, Citation2002; Miller et al., Citation2006; Rickinson and Kieff, Citation2007).
Epstein-Barr virus is nearly ubiquitous in adult humans, with a seroprevalence of 90-100% in most of the world, yet the timing of human infection varies widely by geographic location (Beaulieu and Sullivan, Citation2002; Rickinson and Kieff, Citation2007). For example, 82% of children in Accra, Ghana have had a primary EBV infection by 18 months-of-age and in the United States the infection rate for entering college freshmen is 50–70% (Biggar et al., Citation1978; Beaulieu and Sullivan, Citation2002). As with most herpesviruses in their well-adapted hosts, there is very little clinical disease upon primary EBV infection; however, clinical disease (typified by infectious mononucleosis) is more likely when primary infection occurs later in life (Biggar et al., Citation1978; Beaulieu and Sullivan, Citation2002). The Epstein–Barr virus has been associated with a wide variety of lymphoproliferative diseases and cancers, including chronic active EBV infections, X-linked lymphoproliferative syndrome, Burkitt’s lymphoma, post-transplantation lymphoproliferative disorders (PTLD), Hodgkin’s disease, nasopharyngeal carcinomas, and HIV-associated lymphomas and smooth muscle tumors (Biggar et al., Citation1978; Beaulieu and Sullivan, Citation2002).
Macaque Lymphocryptovirus infections: Macaques are infected by a lymphocryptovirus (LCV), Macacine herpesvirus 4 (McHV4), which is commonly referred to as rhesus lymphocryptovirus (RhLCV). Rhesus LCV demonstrates extensive genetic and biological homology to EBV (Wang et al., Citation2001; Carville and Mansfield, Citation2008). Orthologous LCVs can be found in a wide variety of other apes and New and Old World monkeys, including the chimpanzee, gorilla, orangutan, baboon, African green monkey, squirrel monkey, capuchin, and marmoset (Pellett and Roizman, Citation2007). Primary infection of naïve immune competent macaques with RhLCV is typically asymptomatic. Experimental oral inoculation of naïve rhesus macaques with RhLCV resulted in 1–3% atypical lymphocytes within one week of exposure, and axillary lymphadenopathy 3–5 weeks post-exposure (Moghaddam et al., Citation1997). An EBV enzyme-immunoassay was used to assess seroconversion and both animals in the study developed a robust antibody response that peaked two weeks post-inoculation and persisted for many months thereafter (Moghaddam et al., Citation1997). Animals were persistently infected with RhLCV, virus was shed into oral secretions, and persistently infected B-lymphocytes were recovered (Moghaddam et al., Citation1997). Further, RhLCV DNA was intermittently detectable in oropharyngeal secretions for 1.5 years post-inoculation, and both animals remained healthy with normal weight gain and no evidence of neoplasia for the duration of the 23-month study (Moghaddam et al., Citation1997). Thus, RhLCV infection of naïve macaques recapitulates EBV infection in naïve humans.
The epidemiology of RhLCV in socially-housed macaques also recapitulates that of EBV in the human population. Newly born animals acquire maternally derived antibodies and are seropositive for RhLCV until approximately 6 months-of-age. As maternal antibodies wane, animals become seronegative and thus are susceptible to infection, after which they quickly begin to seroconvert. By 1 year-of-age, ≈ 80–90% of juvenile macaques have become persistently infected by RhLCV and have developed a measurable antibody response (Vogel et al., Citation1994). Like EBV, two types of RhLCV with differing genetic arrangements in their Epstein-Barr virus nuclear antigen (EBNA) genes have been isolated from colonies of socially-housed macaques. Both genetic forms of RhLCV have been found in similar prevalence in macaque colonies and they have been designated RhLCV1 and RhLCV2 (Cho et al., Citation1999).
While clinical disease is seldom seen when macaques are incipiently infected with RhLCV, an atypical lymphocytosis lasting 10-16 weeks post-infection has been observed (Moghaddam et al., Citation1997). Like many of the other herpesviruses, immune suppressed macaques that are infected with RhLCV are susceptible to a number of different clinical diseases. Macaques that are also infected with the immune suppressive virus SIV are susceptible to oral hairy leukoplakia and non-Hodgkin’s lymphoma (NHL) (Carville and Mansfield, Citation2008). Oral hairy leukoplakia is a raised plaque of ballooning degeneration of epithelial cells found primarily on the tongue, in the esophagus, and occasionally on the skin (Habis et al., Citation2000; Baskin et al., Citation2001; Fortgang et al., Citation2004). Epithelial cells in these lesions have intranuclear inclusion bodies consistent with herpes virus infection, and they are positive for lytic RhLCV infection by immunohistochemistry (Kutok et al., Citation2004). Humans with HIV-induced AIDS commonly develop NHLs; similarly, SIV-infected immune suppressed macaques also develop NHLs. In macaques, NHLs are characterized by an extra nodal B-cell lymphoma that can involve the central nervous system or a body cavity (Baskin et al., Citation2001). Interestingly, there is a differential susceptibility to NHLs between rhesus and cynomolgus macaques with cynomolgus macaques developing NHLs at a rate of 4–15% and rhesus macaques developing them approximately 40% of the time (Carville and Mansfield, Citation2008). Macaques are also commonly used as animal models for organ transplantation studies and immune-suppressive regimens are usually implemented to prevent post-transplantation organ rejection. In one study of 160 consecutive renal transplants in cynomolgus macaques, nine developed PTLDs (Schmidtko et al., Citation2002). All PTLDs reported in this study involved lymph nodes that were effaced by an atypical polymorphous lymphoid proliferation of B-lymphocytes that were positive for EBV encoded RNA (EBER) by in situ hybridization and 6 of 9 PTLDs involved extra-nodal sites, including the liver, lung, heart, and grafted and native kidney (Schmidtko et al., Citation2002). Thus, immune-suppressed RhLCV-infected macaques are susceptible to a variety of clinically significant lymphoproliferative disorders.
Rhadinovirus
The prototypic rhadinovirus (γR) is Saimirine herpesvirus 2 (SaHV2) that was isolated from the peripheral blood mononuclear cells of healthy squirrel monkeys (Falk et al., Citation1972). Saimirine HV2 endemically infects natural populations of squirrel monkeys in Central and South America and over 80% of free-ranging animals are persistently infected (Jung et al., Citation1999). The best-characterized rhadinovirus is human herpesvirus 8 (HHV-8), which was first identified as an endothelial neoplasia associated with AIDS and transplant-associated immune suppression known as Kaposi’s sarcoma (Ganem, Citation2007). A decade and a half ago, a sophisticated molecular technique known as representational difference analysis was used to demonstrate the DNA of a novel herpesvirus in tumors from affected patients, and the virus was subsequently characterized as HHV8, but it is still commonly referred to as Kaposi’s sarcoma-associated herpesvirus (KSHV) (Chang et al., Citation1994; Moore et al., Citation1996). The reservoir for KSHV latency is not well understood, but is likely the CD19+ B-lymphocyte (Ambroziak et al., Citation1995). Biopsies taken from Kaposi’s sarcomas have a heterogeneous appearance and, in general, pathologists agree that the primary cell type is a “spindle cell”. However, recent evidence suggests that KSHV likely infects lymphatic endothelial cells and drives their gene regulation toward that of a blood endothelial cell (Regezi et al., Citation1993; Hong et al., Citation2004; Wang et al., Citation2004).
The epidemiology of KSHV is very interesting as virus prevalence varies greatly by region and demographic profile (Ganem, Citation2007). Prevalence of KSHV in North America, Northern Europe, and Asia is <5% and sexually-active homosexual men are in the highest risk group, with most infections occurring after the onset of sexual activity. In the Mediterranean, Middle East, and Caribbean, the prevalence is 5–20%, and homosexual men and older adults are most likely to be infected. In Africa and parts of the Amazon basin, the prevalence exceeds 50%, with older adults of lower socioeconomic status the most frequently infected demographic group (Kedes et al., Citation1996, Citation1997; Ganem, Citation2007).
Macaque Rhadinovirus infections: Rhadinoviruses have been grouped into two categories based upon phylogenetic relationships: γR1, which includes KSHV and retroperitoneal fibromatosis-associated herpesvirus (RFHV), and γR2, which includes rhesus rhadinovirus (RhRV), a more distantly-related macaque virus with no known human ortholog (Schultz et al., Citation2000).
Retroperitoneal fibromatosis-associated herpesvirus: Retroperitoneal fibromatosis-associated herpesvirus was genetically identified in fibro-proliferative tumors taken from macaques that were severely immune suppressed due to intercurrent SRV infection (Tsai et al., Citation1985, Citation1986; Rose et al., Citation1997). Additional sequence analysis has clearly placed RFHV within the γR1 group of herpesviruses along with orthologous viruses such as KSHV and γR1 herpesviruses of chimpanzees, gorillas, and African green monkeys (Schultz et al., Citation2000; Rose et al., Citation2003; Westmoreland and Mansfield, Citation2008). Little is known about the pathogenesis of RFHV since virus has never been isolated or cultured so that detailed studies could be performed. The lack of a serologic assay has made sero-epidemiologic studies impractical and blood based molecular analysis by polymerase chain reaction lacks sensitivity because γR1 viruses or infected cells are seldom found in circulation (Hudnall et al., Citation2008). Thus, diagnosis is often limited to histopathology or PCR performed on biopsy specimens of suspicious lesions (Westmoreland and Mansfield, Citation2008). Reported cases of RFHV were isolated to a small number of breeding colonies and, since many breeding centers have eliminated the immune suppressive macaque retroviruses from their colonies, few new cases are being reported. Since RFHV was sequenced from macaque tumors, and virus has never been cultured or fully-characterized, it is not officially recognized by the International Committee on the Taxonomy of Viruses (Citation2006).
Rhesus rhadinovirus: Macaques are often infected by the γR2 virus Macacine herpesvirus 5 (McHV5), also commonly known as rhesus rhadinovirus (RhRV) (Damania and Desrosiers, Citation2001; International Committee on the Taxonomy of Viruses, Citation2006; Westmoreland and Mansfield Citation2008). While there are no known human γR2 viruses, there are orthologous primate viruses in chimpanzees and squirrel, spider, and African green monkeys (Damania and Desrosiers, Citation2001; Langermans et al., Citation2005; International Committee on the Taxonomy of Viruses, Citation2006; Pellett and Roizman, Citation2007). Rhesus RV is a well-characterized virus and two isolates from disparate primate centers in the United States have been sequenced and found to be closely related virus strains (Searles et al., Citation1999; Alexander et al., Citation2000). Like other members of the Gammaherpesvirinae, RhRV infects peripheral blood mononuclear cells and establishes latency in B-lymphocytes (Bergquam et al., Citation1999; Mansfield et al., Citation1999). Experimental inoculation studies have been performed in which naïve antibody-negative rhesus and pig tailed-macaques were inoculated intravenously with rhadinovirus isolates from either macaque species (Mansfield et al., Citation1999). Following virus inoculation in this study, naïve animals seroconverted to RhRV and viral DNA was detected in lymph nodes, oral mucosa, skin, and peripheral blood mononuclear cells by PCR. A peripheral lymphadenopathy, characterized by an effacing paracortical lymphocytic hyperplasia, was accompanied by an abundance of small arborizing vessels and a hyperplastic endothelium. The peripheral lymphadenopathy developed as early as 2 weeks post-inoculation, resolved by 12 weeks, and these animals subsequently remained disease-free (Mansfield et al., Citation1999).
Serologic surveys for RhRV in large United States’ primate centers revealed that 90–95% of juvenile macaques have a robust antibody response to RhRV (Desrosiers et al., Citation1997; Bergquam et al., Citation1999; Mansfield et al., Citation1999). The age of seroconversion suggests that a high percentage of naïve macaques are infected with RhRV as juveniles in the interval between waning of maternally-derived antibody and the onset of sexual maturity (Westmoreland and Mansfield, Citation2008). While the route of virus transmission has not been fully explored, it is most likely via virus-contaminated saliva, which would allow the virus to be rapidly transmitted among socially-housed macaques (Westmoreland and Mansfield, Citation2008).
Given the nearly ubiquitous presence of RhRV in adult socially-housed macaques and the lack of reports of associated disease, RhRV is likely of low virulence in immunologically-normal animals (Desrosiers et al., Citation1997). As previously described, RhRV-inoculated macaques develop an acute self-limiting peripheral lymphadenopathy that resolves by 12 weeks post-inoculation (Mansfield et al., Citation1999). Macaques that were experimentally co-inoculated with RhRV and SIV developed a marked B-cell lymphocytosis with hypergammaglobulinemia, angiofollicular lymphoid hyperplasia, and an aplasmacytic infiltration consistent with Castleman’s disease (CD) (Mansfield et al., Citation1999). Castleman’s disease is associated with increased interleukin (IL)-6 expression, is commonly seen in immune suppressed KSHV-infected humans, and KSHV DNA sequences have been associated with CD lesions (Yoshizaki et al., Citation1989; Brandt et al., Citation1990; Soulier et al., Citation1995; Ganem, Citation2007). Rhesus RV produces a viral homolog of IL-6, which is consistent with RhRV-associated CD in macaques (Kaleeba et al., Citation1999; Mansfield et al., Citation1999). A retrospective analysis of macaques that were naturally infected with RhRV and subsequently inoculated with SIV reported that RhRV was rarely found in simian AIDS-associated lymphomas and that its presence, when detected, was consistent with an occasional tumor cell infiltration or blood contamination of the sample (Ruff et al., Citation2003). Thus, RhRV likely has little impact on toxicology studies unless biopsies are taken during early infection when an otherwise clinically normal animal might have some virus-induced lymphadenopathy.
Conclusions
Laboratory macaques are frequently infected with a diverse herpesvirus flora that results in persistent life-long infections with intermittent periods of virus reactivation and shedding. In immune competent animals primary infection usually results in an acute, subclinical and self-limiting disease course, with a large percentage of socially-housed animals infected with multiple herpesviruses by the time they are young adults. Since macaques are infected with many herpesviruses when they are quite young, around the time maternal antibody begins to wane at approximately 4–6 months-of-age, it is impractical to create a large supply of macaques that are herpesvirus-free. However, because of the potential human health consequences of zoonotic B virus infection, B virus-negative colonies have been derived and B virus-negative sources such as Mauritian macaques are available (Foster, Citation1976; Elmore and Eberle, Citation2008). As rhesus macaques are more frequently used in academic research in the United States, their herpesvirus flora is much better characterized than is the flora of other macaques such as the cynomolgus macaque. Many of the herpesviruses that infect macaques are preceded by rhesus as a modifier; however, it is very likely that all macaques are infected by similar herpesviruses that probably only vary slightly in their genetic and serologic reactivity, and information regarding the pathogenesis of the various herpesviruses in rhesus macaques is very likely applicable to all macaques species.
The potential impact of herpesvirus infections on toxicology studies depends upon the age or infection status of the animals used and the potential immunomodulatory affects of the compound being studied. For example, young animals that are at the peak of infection and seroconversion could impact on studies by having an increased incidence of background lymphoid hyperplasia. If an immunomodulatory compound is used, it could exacerbate lesions that are seen in a primary infection or could cause a recurrence from latency in an older animal. Regardless, the herpesvirus flora of laboratory macaques closely resembles that of the general human population, and a compound that potentiates clinical herpesvirus disease in macaques would a priori likely have a similar effect in humans. Toxicologists that use macaques in pre-clinical studies need to be aware of basic herpesvirus biology and disease pathogenesis. This way, they can anticipate and mitigate the consequences of adventitious macaque herpesvirus diseases on toxicology studies.
Acknowledgements
The Author would like to thank the ILSI Health and Environmental Sciences Institute for sponsoring and facilitating the Workshop on Naturally Occurring Infections in Nonhuman Primates and Immunotoxicity Implications, and Mr. Robert Zaccardi of Charles River for his assistance with graphics.
Declaration of interest
The Author reports no conflicts of interest. The Author is alone responsible for the content and writing of the paper.
References
- Alexander, L., Denekamp, L., Knapp, A., Auerbach, M. R., Damania, B., and Desrosiers, R. C. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26–95: Sequence similarities to Kaposi’s sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388–3398.
- Ambroziak, J. A., Blackbourn, D. J., Herndier, B. G., Glogau, R. G., Gullett, J. H., McDonald, A. R., Lennette, E. T., and Levy, J. A. 1995. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science 268:582–583.
- Anderson, D. C., Swenson, R. B., Orkin, J. L., Kalter, S. S., and McClure, H. M. 1994. Primary Herpesvirus simiae (B-virus) infection in infant macaques. Lab. Anim. Sci. 44:526–530.
- Andrade, M. R., Yee, J., Barry, P., Spinner, A., Roberts, J. A., Cabello, P. H., Leite, J. P., and Lerche, N. W. 2003. Prevalence of antibodies to selected viruses in a long-term closed breeding colony of rhesus macaques (Macaca mulatta) in Brazil. Am. J. Primatol. 59:123–128.
- Asher, D. M., Gibbs, C. J., Jr., Lang, D. J., Gajdusek, D. C., and Chanock, R. M. 1974. Persistent shedding of cytomegalovirus in the urine of healthy Rhesus monkeys. Proc. Soc. Exp. Biol. Med. 145:794–801.
- Barry, P. A., Lockridge, K. M., Salamat, S., Tinling, S. P., Yue, Y., Zhou, S. S., Gospe, S. M., Jr., Britt, W. J., and Tarantal, A. F. 2006. Non-human primate models of intrauterine cytomegalovirus infection. ILAR J. 47:49–64.
- Barry, P. A., and Strelow, L. 2008. Development of breeding populations of rhesus macaques (Macaca mulatta) that are specific pathogen-free for rhesus cytomegalovirus. Comp. Med. 58:43–46.
- Baskin, G. B. 1987. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am. J. Pathol. 129:345–352.
- Baskin, G. B., Cremer, K. J., and Levy, L. S. 2001. Comparative pathobiology of HIV- and SIV-associated lymphoma. AIDS Res. Hum. Retroviruses 17:745–751.
- Beaulieu, B. L., and Sullivan, J. L. 2002. Epstein-Barr Virus. In: Clinical Virology (Richman, D. D., Whitley, R. J., and Hayden, F. G., Eds.), Washington, DC: ASM Press, pp. 479–494.
- Bergquam, E. P., Avery, N., Shiigi, S. M., Axthelm, M. K., and Wong, S. W. 1999. Rhesus rhadinovirus establishes a latent infection in B-lymphocytes in vivo. J. Virol. 73:7874–7876.
- Biggar, R. J., Henle, W., Fleisher, G., Bocker, J., Lennette, E. T., and Henle, G. 1978. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int. J. Cancer 22:239–243.
- Brandt, S. J., Bodine, D. M., Dunbar, C. E., and Nienhuis, A. W. 1990. Dysregulated interleukin-6 expression produces a syndrome resembling Castleman’s disease in mice. J. Clin. Invest. 86:592–599.
- Carlson, C. S., O’Sullivan, M. G., Jayo, M. J., Anderson, D. K., Harber, E. S., Jerome, W. G., Bullock, B. C., and Heberling, R. L. 1997. Fatal disseminated cercopithecine herpesvirus 1 (herpes B) infection in cynomolgus monkeys (Macaca fascicularis). Vet. Pathol. 34:405–414.
- Carville, A., and Mansfield, K. G. 2008. Comparative pathology of macaque lymphocrypto-viruses. Comp. Med. 58:57–67.
- Chang, Y., Cesarman, E., Pessin, M. S., Lee, F., Culpepper, J., Knowles, D. M., and Moore, P. S. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266:1865–1869.
- Chellman, G. J., Lukas, V. S., Eugui, E. M., Altera, K. P., Almquist, S. J., and Hilliard, J. K. 1992. Activation of B virus (Herpesvirus simiae) in chronically immunosuppressed cynomolgus monkeys. Lab. Anim. Sci. 42:146–151.
- Cho, Y. G., Gordadze, A. V., Ling, P. D., and Wang, F. 1999. Evolution of two types of rhesus lymphocryptovirus similar to type 1 and type 2 Epstein-Barr virus. J. Virol. 73:9206–9612.
- Cohen, J. I., Straus, S. E., and Arvin, A. M. 2007. Varicella-zoster virus replication, pathogenesis, and management. In: Fields Virology (Knipe, D. M., and Howley, P. M., Eds.), Philadelphia, PA: Lippincott Williams & Wilkens, pp. 2773–2818.
- Damania, B., and Desrosiers, R. C. 2001. Simian homologues of human herpesvirus 8. Phil. Trans. Royal Soc. London. Series B: Biol. Sci. 356:535–543.
- Daniel, M. D., King, N. W., Letvin, N. L., Hunt, R. D., Sehgal, P. K., and Desrosiers, R. C. 1984. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science 223:602–605.
- Davidovici, B. B., Balicer, R. D., Klement, E., Green, M. S., Mendelson, E., Smetana, Z., and Cohen, D. I. 2007. Comparison of the dynamics and correlates of transmission of Herpes Simplex Virus-1 (HSV-1) and Varicella-Zoster Virus (VZV) in a sample of the Israeli population. Eur. J. Epidemiol. 22:641–646.
- Davison, A. J., Eberle, R., Ehlers, B., Hayward, G. S., McGeoch, D. J., Minson, A. C., Pellett, P. E., Roizman, B., Studdert, M. J., and Thiry, E. 2009. The order Herpesvirales. Arch. Virol. 154:171–177.
- Desrosiers, R. C. 1988. Simian immunodeficiency viruses. Annu. Rev. Microbiol. 42:607–625.
- Desrosiers, R. C. 1997. The value of specific pathogen-free rhesus monkey breeding colonies for AIDS research. AIDS Res. Hum. Retroviruses 13:5–6.
- Desrosiers, R. C., Sasseville, V. G., Czajak, S. C., Zhang, X., Mansfield, K. G., Kaur, A., Johnson, R. P., Lackner, A. A., and Jung, J. U. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi’s sarcoma-associated herpesvirus. J. Virol. 71:9764–9769.
- Ehlers, B., Borchers, K., Grund, C., Frolich, K., Ludwig, H., and Buhk, H. J. 1999. Detection of new DNA polymerase genes of known and potentially novel herpesviruses by PCR with degenerate and deoxyinosine-substituted primers. Virus Genes 18:211–220.
- Elmore, D. B., and Eberle, R. 2008. Monkey B Virus (Cercopithecine herpesvirus 1) Comp. Med. 58:11–21.
- Falk, L. A., Wolfe, L. G., and Deinhardt, F. 1972. Isolation of Herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus). J. Natl.Cancer Inst. 48:1499–1505.
- Feldman, S., Chaudary, S., Ossi, M., and Epp, E. 1977. A viremic phase for herpes zoster in children with cancer. J. Pediat. 91:597–600.
- Fleming, D. T., McQuillan, G. M., Johnson, R. E., Nahmias, A. J., Aral, S. O., Lee, F. K., and St Louis, M. E. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. New Engl. J. Med. 337:1105–1111.
- Fortgang, I. S., Srivastav, S. K., Baskin, G. B., Schumacher, P. M., and Levy, L. S. 2004. Retrospective analysis of clinical and laboratory factors associated with lymphoma in simian AIDS. Leuk. Lymph. 45:161–169.
- Foster, H. L. 1976. Progress report on the Charles River Breeding Laboratories’ free-ranging rhesus monkey breeding colony on Key Lois, FL. Lab. Anim. Sci. 26:374–382.
- Ganem, D. 2007. Kaposi’s sarcoma-associated herpesvirus. In: Fields Virology (Knipe, D. M., and Howley, P. M., Eds.), Philadelphia, PA: Lippincott Williams & Wilkens, pp. 2847–2888.
- Gershon Anne, A., and Saul J., Silverstein. . 2002. Varicella-zoster virus. In: Clinical Virology (Richman, D. D., Whitley, R. J., and Hayden, F. G., Eds.), Washington, DC: ASM Press, pp. 413–432.
- Gray, W. L. 2004. Simian varicella: A model for human varicella-zoster virus infections. Rev. Med. Virol. 14:363–381.
- Gray, W. L., Williams, R. J., Chang, R., and Soike, K. F. 1998. Experimental simian varicella virus infection of St. Kitts vervet monkeys. J. Med. Primatol. 27:177–183.
- Gray, W. L. 2008. Simian varicella in Old World monkeys. Comp. Med. 58:22–30.
- Griffiths, P. D., and Emery, V. C. 2002. Cytomegalovirus. In: Clinical Virology (Richman, D. D., Whitley, R. J., and Hayden, F. G., Eds.), Washington, DC: ASM Press, pp. 433–461.
- Habis, A. G., Baskin, G., Simpson, L., Fortgang, I., Murphey-Corb, M.,and Levy, L. S. 2000. Rhesus lymphocryptovirus infection during the progression of SAIDS and SAIDS-associated lymphoma in the rhesus macaque. AIDS Res. Hum. Retroviruses 16:163–171.
- Hahn, G., Jores, R., and Mocarski, E. S. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937–3942.
- Hansen, S. G., Strelow, L. I., Franchi, D. C., Anders, D. G., and Wong, S. W. 2003. Complete sequence and genomic analysis of rhesus cytomegalovirus. J. Virol. 77:6620–6636.
- Hong, Y. K., Foreman, K., Shin, J. W., Hirakawa, S., Curry, C. L., Sage, D. R., Libermann, T., Dezube, B. J., Fingeroth, J. D., and Detmar, M. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36:683–685.
- Hope-Simpson, R. E. 1965. The nature of herpes zoster: A long-term study and a new hypothesis. Proc. Royal Soc. Med. 58:9–20.
- Howard, M., Sellors, J. W., Jang, D., Robinson, N. J., Fearon, M., Kaczorowski, J., and Chernesky, M. 2003. Regional distribution of antibodies to herpes simplex virus type 1 (HSV-1) and HSV-2 in men and women in Ontario, Canada. J. Clin. Microbiol. 41:84–89.
- Hudnall, S. D., Chen, T., Allison, P., Tyring, S. K., and Heath, A. 2008. Herpesvirus prevalence and viral load in healthy blood donors by quantitative real-time polymerase chain reaction. Transfusion 48:1180–1187.
- Huff, J. L., Eberle, R., Capitanio, J., Zhou, S. S., and Barry, P. A. 2003. Differential detection of B virus and rhesus cytomegalovirus in rhesus macaques. J. Gen. Virol. 84:83–92.
- International Committee on the Taxonomy of Viruses. 2006. Index of Viruses—Herpesviridae Columbia University 2006 [cited February 2009. Available from http://www.ncbi.nlm.nih.gov/ICTVdb/Ictv/fs_index.htm.
- Jarvis, M. A., and Nelson, J. A. 2002. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr. Opin. Microbiol. 5:403–407.
- Jarvis, M. A., and Nelson, J. A. 2007. Human cytomegalovirus tropism for endothelial cells: Not all endothelial cells are created equal. J. Virol. 81:2095–2101.
- Jones-Engel, L., Engel, G. A., Heidrich, J., Chalise, M., Poudel, N., Viscidi, R., Barry, P. A., Allan, J. S., Grant, R., and Kyes, R. 2006. Temple monkeys and health implications of commensalism, Kathmandu, Nepal. Emerg. Infect. Dis. 12:900–906.
- Jonker, M., Ringers, J., Kuhn, E. M., Hart, B. T., and Foulkes, R. 2004. Treatment with anti-MHC-Class-II antibody postpones kidney allograft rejection in primates but increases the risk of CMV activation. Am. J. Transplant. 4:1756–1761.
- Jung, J. U., Choi, J. K., Ensser, A., and Biesinger, B. 1999. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin. Cancer Biol. 9:231–239.
- Kaleeba, J. A., Bergquam, E. P., and Wong, S. W. 1999. A rhesus macaque rhadinovirus related to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 encodes a functional homologue of interleukin-6. J. Virol. 73:6177–6181.
- Kaur, A., Kassis, N., Hale, C. L., Simon, M., Elliott, M., Gomez-Yafal, A., Lifson, J. D., Desrosiers, R. C., Wang, F., Barry, P., Mach, M., and Johnson, R. P. 2003. Direct relationship between suppression of virus-specific immunity and emergence of cytomegalovirus disease in simian AIDS. J. Virol. 77:5749–5758.
- Kedes, D. H., Ganem, D., Ameli, N., Bacchetti, P., and Greenblatt, R. 1997. The prevalence of serum antibody to human herpesvirus 8 (Kaposi sarcoma-associated herpesvirus) among HIV-seropositive and high-risk HIV-seronegative women. JAMA 277:478–481.
- Kedes, D. H. E., Operskalski, E., Busch, M., Kohn, R., Flood, J., and Ganem, D. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): Distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918–924.
- Kessler, M. J., London, W. T., Madden, D. L., Dambrosia, J. M., Hilliard, J. K., Soike, K. F., and Rawlins, R. G. 1989. Serological survey for viral diseases in the Cayo Santiago rhesus macaque population. P. R. Health Sci. J. 8:95–97.
- Kolappaswamy, K., Mahalingam, R., Traina-Dorge, V., Shipley, S. T., Gilden, D. H., Kleinschmidt-Demasters, B. K., McLeod, C. G., Jr., Hungerford, L. L., and DeTolla, L. J. 2007. Disseminated simian varicella virus infection in an irradiated rhesus macaque (Macaca mulatta). J. Virol. 81:411–415.
- Kutok, J. L., Klumpp, S., Simon, M., MacKey, J. J., Nguyen, V., Middeldorp, J. M., Aster, J. C., and Wang, F. 2004. Molecular evidence for rhesus lymphocryptovirus infection of epithelial cells in immunosuppressed rhesus macaques. J. Virol. 78:3455–3461.
- Langermans, J. A., Doherty, T. M., Vervenne, R. A., van der Laan T., Lyashchenko, K., Greenwald, R., Agger, E. M., Aagaard, C., Weiler, H., van Soolingen D., Dalemans, W., Thomas, A. W., and Andersen, P. 2005. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 23:2740–2750.
- LaRussa, P., Steinberg, S. P., Seeman, M. D., and Gershon, A. A. 1985. Determination of immunity to varicella-zoster virus by means of an intradermal skin test. J. Infect. Dis. 152:869–875.
- Lehner, N. D., Bullock, B. C., and Jones, N. D. 1984. Simian varicella infection in the African green monkey (Cercopithecus aethiops). Lab. Anim. Sci. 34:281–285.
- Lerche, N. W., Yee, J. L., and Jennings, M. B. 1994. Establishing specific retrovirus-free breeding colonies of macaques: An approach to primary screening and surveillance. Lab. Anim. Sci. 44:217–221.
- Lerche, N. W., and Simmons, J. H. 2008. Beyond specific pathogen-free: Biology and effect of common viruses in macaques. Comp. Med. 58:8–10.
- Lockridge, K. M., Sequar, G., Zhou, S. S., Yue, Y., Mandell, C. P., and Barry, P. A. 1999. Pathogenesis of experimental rhesus cytomegalovirus infection. J. Virol. 73:9576–9583.
- Locksley, R. M., Flournoy, N., Sullivan, K. M., and Meyers, J. D. 1985. Infection with varicella-zoster virus after marrow transplantation. J. Infect. Dis. 152:1172–1181.
- Mahalingam, R., Smith, D., Wellish, M., Wolf, W., Dueland, A. N., Cohrs, R., Soike, K., and Gilden, D. 1991. Simian varicella virus DNA in dorsal root ganglia. Proc. Natl. Acad. Sci. USA 88:2750–2752.
- Malkin, J. E. 2004. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes 11(S1):2A–23A.
- Mansfield, K. G., Westmoreland, S. V., DeBakker, C. D., Czajak, S., Lackner, A. A., and Desrosiers, R. C. 1999. Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses. J. Virol. 73:10320–10328.
- Mansfield, K. 2005. Development of specific pathogen-free non-human primate colonies. In: The Laboratory Primate (Wolfe-Coote, S., Ed.), London: Elsevier Academic Press, pp. 229–239.
- Marx, P. A., Maul, D. H., Osborn, K. G., Lerche, N. W, Moody, P., Lowenstine, L. J., Henrickson, R. V., Arthur, L. O., Gilden, R. V., Gravell, M., London, W. T., Sever, J. L., Levy, J. A., Munn, R. J., and Gardner, M. B. 1984. Simian AIDS: Isolation of a type D retrovirus and transmission of the disease. Science 223:1083–1086.
- McDonagh, S., E., ?Maidji, E., Chang, H. T., and Pereira, L. 2006. Patterns of human cytomegalovirus infection in term placentas: A preliminary analysis. J. Clin. Virol. 35:210–215.
- McDonald, A. D., Williams, M. C., West, R., and Stewart, J. 1974. Neutralizing antibodies to herpesvirus types 1 and 2 in Montreal women. Am. J. Epidemiol. 100:124–129.
- McGeoch, D. J., Dolan, A., and Ralph, A. C. 2000. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J. Virol. 74:10401–10406.
- McGeoch, D. J., Rixon, F. J., and Davison, A. J. 2006. Topics in herpesvirus genomics and evolution. Virus Res. 117:90–104.
- Miller, C. S., Berger, J. R., Mootoor, Y., Avdiushko, S. A., Zhu, H., and Kryscio, R. J. 2006. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active anti-retroviral therapy. J. Clin. Microbiol. 44:2409–2415.
- Mocarski, E. S., Jr., Shenk, T., and Pass, R. F. 2007. Cytomegaloviruses. In: Fields Virology (Knipe, D. M., and Howley, P. M., Eds.), Philadelphia, PA: Lippincott Williams & Wilkens, pp. 2701–2772.
- Moghaddam, A., Rosenzweig, M., Lee-Parritz, D., Annis, B., Johnson, R. P., and Wang, F. 1997. An animal model for acute and persistent Epstein–Barr virus infection. Science 276:2030–2033.
- Moore, P. S., Gao, S. J., Dominguez, G., Cesarman, E., Lungu, O., Knowles, D. M., Garber, R., Pellett, P. E., McGeoch, D. J., and Chang, Y. 1996. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcoma. J. Virol. 70:549–558.
- Nahmias, A. J., Lee, F. K., and Beckman-Nahmias, S. 1990. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 69:19–36.
- Payton, M. E., d’Offay, J. M., Prado, M. E., Black, D. H., Damania, B., White, G. L., and Eberle, R. 2004. Comparative transmission of multiple herpesviruses and simian virus 40 in a baboon breeding colony. Comp. Med. 54:695–704.
- Pearson, T. C., Trambley, J., Odom, K., Anderson, D. C., Cowan, S., Bray, R., Lin, A., Hollenbaugh, D., Aruffo, A., Siadak, A. W., Strobert, E., Hennigar, R., and Larsen, C. P. 2002. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation 74:933–940.
- Pellett, P. E., and Roizman, B. 2007. The family Herpesviridae: A brief introduction. In: Fields Virology (Knipe, D. M., and Howley, P. M., Eds.), Philadelphia, PA: Lippincott Williams & Wilkens, pp. 2479–2499.
- Regezi, J. A., MacPhail, L. A., Daniels, T. E., DeSouza, Y. G., Greenspan, J. S., and Greenspan, D. 1993. Human immunodeficiency virus-associated oral Kaposi’s sarcoma. A heterogeneous cell population dominated by spindle-shaped endothelial cells. Am. J. Pathol. 143:240–249.
- Rickinson, A. B., and Kieff, E. 2007. Epstein–Barr virus. In: Fields Virology (Knipe, D. M., and Howley, P. M., Eds.), Philadelphia, PA: Lippincott Williams & Wilkens, pp. 2655–2700.
- Roizman, B., Carmichael, L. E., Deinhardt, F., de-The, G., Nahmias, A. J., Plowright, W., Rapp, F., Sheldrick, P., Takahashi, M., and Wolf, K. 1981. Herpesviridae. Definition, provisional nomenclature, and taxonomy. The Herpesvirus Study Group, the International Committee on Taxonomy of Viruses. Intervirology 16:201–217.
- Roizman, B., Knipe, D. M., and Whitley, R. J. 2007. Herpes simplex viruses. In: Fields Virology (Knipe, D. M., and Howley, P. M., Eds.), Philadelphia, PA: Lippincott Williams & Wilkens, pp. 2501–2601.
- Rose, T. M., Ryan, J. T., Schultz, E. R., Raden, B. W., and Tsai, C. C. 2003. Analysis of 4.3 kilobases of divergent locus B of macaque retroperitoneal fibromatosis-associated herpesvirus reveals a close similarity in gene sequence and genome organization to Kaposi’s sarcoma-associated herpesvirus. J. Virol. 77:5084–5097.
- Rose, T. M., Strand, K. B., Schultz, E. R., Schaefer, G., Rankin, G. W., Jr., Thouless, M. E., Tsai, C. C., and Bosch, M. L. 1997. Identification of two homologs of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J. Virol. 71:4138–4144.
- Ross, A. H. 1962. Modification of chicken pox in family contacts by administration of gamma globulin. New Engl. J. Med. 267:369–376.
- Ruff, K., Baskin, G. B., Simpson, L., Murphey-Corb, M., and Levy, L. S. 2003. Rhesus rhadinovirus infection in healthy and SIV-infected macaques at Tulane National Primate Research Center. J. Med. Primatol. 32:1–6.
- Sabin, A. B., and Wright, A. M. 1934. Acute ascending myelitis following a monkey bite, with the isolation of a virus capable of reproducing the disease. J. Exp. Med. 59:115–136.
- Saitou, N., and Nei, M. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evolution 4:406–425.
- Schmidtko, J., R., ?Wang, R., Wu, C. L., Mauiyyedi, S., Harris, N. L., Della Pelle, P., Brousaides, N., Zagachin, L., Ferry, J. A., Wang, F., Kawai, T., Sachs, D. H., Cosimi, B. A., and Colvin, R. B.. 2002. Post-transplant lymphoproliferative disorder associated with an Epstein-Barr-related virus in cynomolgus monkeys. Transplantation 73:1431–1439.
- Schoeb, T. R., Eberle, R., Black, D. H., Parker, R. F., and Cartner, S. C. 2008. Diagnostic exercise: Papulovesicular dermatitis in rhesus macaques (Macaca mulatta). Vet. Pathol. 45:592–594.
- Schultz, E. R., Rankin, G. W., Jr., Blanc, M. P., Raden, B. W., Tsai, C. C., and Rose, T. M. 2000. Characterization of two divergent lineages of macaque rhadinoviruses related to Kaposi’s sarcoma-associated herpesvirus. J. Virol. 74:4919–4928.
- Searles, R. P., Bergquam, E. P., Axthelm, M. K., and Wong, S. W. 1999. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040–3053.
- Sequar, G., Britt, W. J., Lakeman, F. D., Lockridge, K. M., Tarara, R. P., Canfield, D. R., Zhou, S. S., Gardner, M. B., and Barry, P. A. 2002. Experimental co-infection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: Pathogenesis. J. Virol. 76:7661–7671.
- Simon, M. A., Daniel, M. D., Lee-Parritz, D., King, N. W., and Ringler, D. J. 1993. Disseminated B virus infection in a cynomolgus monkey. Lab. Anim. Sci. 43:545–550.
- Soroceanu, L., Akhavan, A., and Cobbs, C. S. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455:391–395.
- Soulier, J., Grollet, L., Oksenhendler, E., Cacoub, P., Cazals-Hatem, D., Babinet, P., d’Agay, M. F., Clauvel, J. P., Raphael, M., Degos, L., and Sigaux, F. 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86:1276–1280.
- Springer, M. S., Murphy, W. J., Eizirik, E., and O’Brien, S. J. 2003. Placental mammal diversification and the Cretaceous–Tertiary boundary. Proc. Natl. Acad. Sci. USA 100:1056–1061.
- Tamura, K., Dudley, J., Nei, M., and Kumar, S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evolution 24:1596–1599.
- Thorley-Lawson, D. A., and Gross, A. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. New Engl. J. Med. 350:1328–1337.
- Tsai, C. C., Giddens, W. E., Jr., Morton, W. R., Rosenkranz, S. L., Ochs, H. D., and Benveniste, R. E. 1985. Retroperitoneal fibromatosis and acquired immunodeficiency syndrome in macaques: Epidemiologic studies. Lab. Anim. Sci. 35:460–464.
- Tsai, C. C., Giddens, W. E., Jr., Ochs, H. D., Morton, W. R., Knitter, G. H., Blakley, G. A., and Benveniste, R. E. 1986. Retroperitoneal fibromatosis and acquired immunodeficiency syndrome in macaques: Clinical and immunologic studies. Lab. Anim. Sci. 36:119–125.
- VanDevanter, D. R., Warrener, P., Bennett, L., Schultz, E. R., Coulter, S., Garber, R. L., and Rose, T. M. 1996. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 34:1666–16671.
- Vogel, P., Weigler, B. J., Kerr, H., Hendrickx, A. G., and Barry, P. A.. 1994. Seroepidemiologic studies of cytomegalovirus infection in a breeding population of rhesus macaques. Lab. Anim. Sci.44:25–30.
- Vossen, R. C., van Dam-Mieras, M. C., and Bruggeman, C. A.. 1996. Cytomegalovirus infection and vessel wall pathology. Intervirology 39:213–221.
- Wang, F., Rivailler, P., Rao, P., and Cho, Y. 2001. Simian homologues of Epstein-Barr virus. Phil. Trans. Royal Soc. London. Series B: Biol. Sci. 356:489–497.
- Wang, H. W., Trotter, M. W., Lagos, D., Bourboulia, D., Henderson, S., Makinen, T., Elliman, S., Flanagan, A. M., Alitalo, K., and Boshoff, C. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36:687–693.
- Weigler, B. J. 1992. Biology of B virus in macaque and human hosts: A review. Clin. Infect. Dis. 14:555–567.
- Weigler, B. J., Hird, D. W., Hilliard, J. K., Lerche, N. W., Roberts, J. A., and Scott, L. M. 1993. Epidemiology of cercopithecine herpesvirus 1 (B virus) infection and shedding in a large breeding cohort of rhesus macaques. J. Infect. Dis. 167:257–263.
- Weigler, B. J., Roberts, J. A., Hird, D. W., Lerche, N. W., and Hilliard, J. K. 1990. A cross-sectional survey for B virus antibody in a colony of group housed rhesus macaques. Lab. Anim. Sci. 40:257–261.
- Westmoreland, S. V., and Mansfield, K. G. 2008. Comparative pathobiology of Kaposi sarcoma-associated herpesvirus and related primate rhadinoviruses. Comp. Med. 58:31–42.
- White, T. M., Mahalingam, R., Traina-Dorge, V., and Gilden, D. H. 2002. Persistence of simian varicella virus DNA in CD4+ and CD8+ blood mononuclear cells for years after intratracheal inoculation of African green monkeys. Virology 303:192–198.
- Whitley, R. J., and Hilliard, J. 2007. Cercopithecine herpes virus 1 (B virus). In: Fields Virology (Knipe, D. M., and Howley, P. M., Eds.), Philadelphia, PA: Lippincott Williams & Wilkens, pp. 2889–2903.
- Wolf, R. H., Smetana, H. F., Allen, W. P., and Felsenfeld, A. D. 1974. Pathology and clinical history of Delta herpesvirus infection in patas monkeys. Lab. Anim. Sci. 24:218–221.
- Yanai, T., Lackner, A. A., Sakai, H., Masegi, T., and Simon, M. A. 2006. Systemic arteriopathy in SIV-infected rhesus macaques (Macaca mulatta). J. Med. Primatol. 35:106–112.
- Yoshizaki, K., Matsuda, T., Nishimoto, N., Kuritani, T., Taeho, L., Aozasa, K., Nakahata, T., Kawai, H., Tagoh, H., and Komori, T. 1989. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood 74:1360–1367.
- Zuckerkandl, E., and Pauling, L. 1965. Evolutionary divergence and convergence in proteins. In: Evolving Genes and Proteins (Bryson, V.and Vogel, H. J., Eds.), New York: Academic Press, pp. 97–166.