Abstract
Our earlier studies of hepatitis C virus (HCV) infection rates among blood donors at the Kyiv Municipal Blood Center revealed a 3.45% HCV+ prevalence in these “healthy” hosts. In the study here, we analyzed HCV (as well as cytomegalovirus [CMV]) prevalence among Chernobyl nuclear power plant (NPP) accident sufferers—cleanup workers, local residents, NPP workers, and convalescent patients—who suffered acute radiation syndrome (ARS) as a result of the 1986 accident, and individuals who had not been exposed to ionizing radiation (IR). Serological analyses of antibodies against each pathogen (via enzyme-linked immunosorbent assay [ELISA]) revealed the highest HCV (i.e., 27.2%) and CMV (85.6%) prevalence in the convalescent hosts. Though the HCV prevalence (reflecting a current/past infection) among the cleanup workers (and other groups) was lower (i.e., 11–25%), viral presence was “associated” with a higher incidence of selected somatic diseases, for example, thyroiditis, goiter, hypertension, Type 1 diabetes, chronic hepatitis/gastritis, in the cleanup workers. A similar scenario with respect to CMV was also seen, i.e., lower prevalence rates [relative to in ARS patients] and “association” between CMV status and incidence of chronic gastritis, arthritis, and bronchitis, in the cleanup workers and IR-non-exposed controls. Further, irrespective of CMV status, there was a clear delineation between incidence rate(s) of each of the pathologies and whether or not the person was/was not exposed in 1986. We also investigated, due to a high incidence of chronic lymphocytic leukemia (CLL) among Chernobyl sufferers, if there was homology between immunoglobulins (Igs) generated by these transformed cells and known antiviral and antimicrobial Igs. Polymerase chain reaction (PCR) analyses of Ig heavy-chain variable (IgHV) genes in cells from CLL patients who were/were not exposed in 1986 revealed a significant homology of some IgHV genes with Igs directed against infectious agents. However, no differences were found between the sequences from IR-exposed and IR-non-exposed CLL patients. Based on the findings here, we conclude that a past/ongoing presence of certain viral infections (i.e., CMV and/or HCV) in a host can modify (aggravate) the clinical course of certain somatic (i.e., non-tumor) diseases and promote malignancies (i.e., CLL), and that each of these outcomes could be modulated as a result of that host’s past exposure to IR.
Introduction
Multiple studies of the effects of the 1986 Chernobyl nuclear power plant (NPP) accident paid close attention to the potential problem of increases in oncological diseases, i.e., thyroid tumors first of all, and of non-tumor pathologies of the cardiovascular, bronchopulmonary, digestive, and urogenital systems in exposed cleanup workers and non-NPP individuals living in the vicinity of the plant (Ivanov et al., Citation2000, Citation2006; Balonov, Citation2007; Sumner, Citation2007; Romanenko et al., Citation2008). As a dose–effect ratio was not strictly evident in many of the documented cases, our own investigations focused on the potential for non-radiation-based components, i.e., a presence of persisting infections, to contribute to the onset/progression of any observed changes in incidence of the various pathologies (Chumak et al., Citation2000, Citation2003, Citation2005).
There are numerous publications in the literature about virus-induced oncogenesis in which the influence of viral infection on genomic instability, which is a hallmark of most human cancers, is discussed (see Lehous et al., Citation2009; Szostek et al., Citation2009; Harde, Citation2010). Common fragile sites are preferential targets for human papilloma virus (HPV)-16 integrations in cervical tumors (Thorland et al., Citation2003). When maintained as an episome, HPV-16 DNA can disturb centrosome homeostasis and subvert genomic integrity of the host cell during early stages of the viral infection. The two HPV-encoded oncoproteins, E6 and E7, can independently induce chromosomal abnormalities (Spardy et al., Citation2009). The fragile histidine triad (FHIT) gene, which is frequently lost in many cancers, has been identified as a candidate tumor suppressor gene at chromosome 3p locus 14.2 (Pichiorri et al., Citation2008; Hassan et al., Citation2010). Alterations of FHIT were found in several kinds of viral-associated tumors including hepatitis C virus (HCV)-associated hepatocellular carcinoma (Terry et al., Citation2004; Zekri et al., Citation2005; Wang et al., Citation2006; Deng et al., Citation2007).
Viruses or their products also cause modification sites on chromosomes that are known to look like non-staining gaps. The CKR5 structural gene mapped to human chromosome 3p21 gene codes for the chemokine receptor 5 (CKR5) protein that serves as a secondary receptor on CD4+ T-lymphocytes for certain strains of human immunodeficiency virus (HIV)-1 (Sanduzzi et al., Citation2001; Castro-Volio and Valle-Bourrette, Citation2001).
The link between viral infections and some somatic diseases is also under intense examination. There are data that cytomegalovirus (CMV) may contribute to atherosclerotic events, peripheral occlusive artery disease, Type 1 diabetes, and/or rheumatoid arthritis (Ozdemir et al., Citation2007; Rabczynski et al., Citation2007; Aarnisalo et al., Citation2008; Qiu et al., Citation2008; Kurbanov and Mamedov, Citation2009). The association between CMV infection and subsequent development of allergy, specifically allergic asthma, has been known since the late 1970s (Frick et al., Citation1979; Bratke et al., Citation2007). HCV infection is associated not only with liver diseases, but also numerous hematologic, renal, dermatologic, rheumatic, and autoimmune disorders, including arthalgia, arthritis, vasculitis, Sicca syndrome, myalgia, and fibromyalgia (Buskila, Citation2009). The persistence of HCV in lymphocytes causes their oligoclonal and monoclonal proliferation, the appearance of non-organ-specific autoantibodies, and leads to autoimmune Type II mixed cryoglobulinemia and development of several types of indolent non-Hodgkin’s lymphomas (de Re et al., Citation2009; Moorman et al., Citation2009; Atta et al., Citation2010).
Our laboratories have been involved in examining the problem of persisting infections for almost 20 years. This interest was sparked by a 1993 cooperative study (under the supervision of Professor H. Yoshizawa from Hiroshima University, Japan) of HCV infection prevalence among blood donors at the Kyiv Municipal Blood Center (Chumak et al., Citation1996). Building upon those and other earlier investigations, the studies presented here reflect our most recent investigations of the potential links between somatic and non-somatic diseases and persisting infections among individuals (cleanup workers, residents of nearby radiation-contaminated territories, and power plant workers) who had suffered from the Chernobyl NPP accident in 1986 and thereafter.
Materials and methods
Patients
Patients were investigated during their hospitalization in the in-patient clinic at the Research Centre for Radiation Medicine, Academy of Medical Sciences of Ukraine (RCRM). Persons who were included in the Clinical–Epidemiological Registry of the RCRM were also investigated in the context of their biennial clinical-laboratory surveillance visit(s). The Ethics Commission of the RCRM approved all study designs used herein; patients were entered into the study only after they had provided informed consent and full medical histories of any past/ongoing tumor- and/or non-tumor-based (i.e., somatic) diseases.
The following groups of patients were recruited for these studies: convalescents of acute radiation syndrome (ARS), n = 118 (mean age 49.3 [± 0.82 {SE}] years); Chernobyl NPP cleanup workers, n = 1017 (mean age 47.6 [± 0.57] years); dwellers of radiation-contaminated territories in the Ukraine, n = 578 (mean age 46.5 [± 0.37] years); and NPP workers, n = 122 (mean age 42.2 [± 0.73] years). While the “n” values noted here represent the total size of the various populations recruited, not every subject in a group was assessed for every endpoint outlined below. For comparison purposes, a group of patients with different somatic diseases, and who had not been exposed to ionizing radiation (IR; n = 367, mean age 44.8 [± 1.17] years) and the earlier blood donors of Kyiv Municipal Blood Center (n = 41,021, mean age 39.7 [± 1.17] years) were employed as controls.
In addition, for analyses of any relationship(s) between infection (past/ongoing) status and IR exposure history, patients with chronic lymphoproliferative disorders (294 with chronic lymphocytic leukemia [CLL] and four with stable large granular lymphocytosis [LGL]) were also recruited and studied. The diagnosis of CLL and LGL was based on clinical history, lymphocyte morphology, and immunophenotypic criteria. The stage of CLL was established according to classifications of Rai (Citation1987) and the International Workshop on Chronic Lymphocytic Leukemia (Citation1989). CLL patients were divided into two groups according to IR exposure: the first group included 63 IR-exposed CLL patients and the second group (the nosologic control group) consisted of 233 IR non-exposed CLL patients.
The group of IR-exposed CLL patients included 48 cleanup workers, 10 inhabitants of nuclide-contaminated areas, and five evacuees, who were observed in the Department of Hematology of RCRM during June 2004–December 2008. Information about doses for the cleanup workers was available from the State Registry of Chernobyl Catastrophe Sufferers of Ukraine. Among the cleanup workers, estimated doses ranged from 25.0 to 120 cSv among those who were on-site the earliest; doses for those who arrived later in 1986 and then over 1987–1989 ranged from 0.14 to 9.9 cSv. Accumulated doses (since 1986 to the diagnosis of CLL; range 0.23–2.12 cSv) in the residents of contaminated areas were calculated on the basis of the data on 137Cs soil contamination density (mean age dose typical for a given level of local contamination) and information on measurements of radioactive Cs in the body at the CLL diagnosis. The control (IR-non-exposed) group included CLL patients who were observed in the Department of Hematology and Bone Marrow Transplantation of RCRM (129 patients) and the Department of Hematology of Poltava Regional Hospital (102 patients) during the same period without special selection of patients.
Blood sampling and preparation of blood plasma for antibody analyses
As part of any given patient’s routine diagnostic protocol, fasting blood (10 mL) from the cubital vein was collected (in the morning) into polypropylene tubes containing heparin (Teruno Corp., Tokyo, Japan). The blood was mixed with the heparin by gentle rocking (at least five times) and avoiding foaming. The cellular elements were then removed by centrifugation at 200 × g for 10 min. The interval between the collection and centrifugation of the blood did not exceed 30 min and all samples were held at 18–20°C during the post-collection period. The resultant plasma was then carefully recovered and aliquoted into small Eppendorf tubes (at least two tubes/each patient). The tubes were marked, logged, and then placed in a −20°C freezer (Liebherr-Hydraulik-bagger GmbH, Kirchdorf, Germany) until analysis.
Confirmation of serum anti-HCV and anti-CMV antibodies
Anti-HCV (IgG) and/or anti-CMV (IgG and IgM) antibodies in an isolated serum sample was assessed using commercially available enzyme-linked immunosorbent assay (ELISA) diagnostics kits (Sanofi Diagnostique Paster, Marnes-La-Cosuette, France; RPC Diagnostic Systems, Nizhniy Novgorod, Russia; DiaProf Ltd., Kyiv, Ukraine; Vector-Best, Novosibirsk, Russia; and, Diagnostic Systems Laboratories (DSL), Webster, TX) and following manufacturer’s instructions.
Immunophenotyping of peripheral blood mononuclear cells
Sub-populations of peripheral blood lymphocytes were quantified using two-color laser flow cytometry. The differential tagging of cells was performed with monoclonal antibodies of the Leu series obtained from Becton Dickinson (San Jose, CA) using standard protocols and following manufacturer’s instructions. All data were then analyzed using the software provided by the manufacturer.
PCR analyses of CMV DNA presence in cell samples
The presence of CMV DNA was investigated in the fraction of peripheral blood mononuclear cells by polymerase chain reaction (PCR). In brief, DNA was extracted from the cells using a sorption method. PCR of the samples was then performed using kits from DNA-Technology JSC (Moscow, Russia) and the following regimen: 5 cycles of 94°C for 90 s, 94°C for 50 s, 67°C for 45 s, and 72°C for 30 s, then followed by 40 cycles of 94°C for 20 s, 67°C for 20 s, and 72°C for 20 s. The resulting PCR products were then electrophoresed over 1.5% agarose gels and visualized using standard ethidium bromide staining.
PCR analyses of IgHV genes in CLL cell samples
The rearrangements of IgHV were studied according to the BIOMED-2 consortium rules (van Dongen et al., Citation2003) as described previously (Abramenko et al., Citation2008). In brief, total RNA was extracted from peripheral blood and/or bone marrow cells using guanidine isothiocyanate–phenol–chloroform extraction according to Chomczynski and Sacchi (Citation1987). Total cellular RNA (1–5 µg) was reverse-transcribed to cDNA using MuLV reverse transcriptase enzyme (Applied Biosystems, Branchburg, NJ) and random hexamers as primers. The cDNA was amplified in a single multiplex PCR bearing six IgHV framework 1 primers combined with an IgJH consensus primer as designed by the BIOMED-2 consortium. All reactions were carried out in a 50 µL final volume containing 10 pmol of each primer, 200 nM dNTPs, 1 U AmpliTaq Gold, and 10× PCR buffer II (Applied Biosystems). The cycling conditions were: pre-activation at 94°C for 7 min; 35 cycles of 94°C for 45 s, 60°C for 45 s, and 72°C for 45 s; and, a final 10 min cycle at 72°C.
PCR products were then analyzed on a 1.5% agarose gel and visualized with ethidium bromide staining. Products were spin column-purified using a PCR purification kit (Promega, Madison, WI) and sequenced directly using forward and reverse primers in an automated DNA sequencer ABI-310 (Applied Biosystems) using BigDye Terminator Cycle Sequencing Reaction Kit (Perkin Elmer, Foster City, CA). The closest germ line IgHV gene for each B-CLL IgHV sequence was assigned using current databases, for example, IgBlast (National Center for Biotechnology Information, Bethesda, MD), IMGT (International Immunogenetics Database; Lefranc, Citation2001), and JoinSolver (Souto-Carneiro et al., Citation2004). The length of the HCDR3 was calculated, followed, established IMGT criteria. Sequences reported in this study can be found in GenBank database under accession numbers: EF091900–EF091931, EF175384–EF175440, EF407822–EF407849, EF441744–EF441761, EF583659–EF583683. In some cases with homologous IgHV sequences, Ig variable light κ or λ chains (IgVκ and IgVλ) were amplified according to the BIOMED-2 protocol.
Statistical analysis
Data from all groups were analyzed using multivariate analysis of variance (MANOVA) to evaluate the relationship between group and presence/absence of past/ongoing infection with the viral agent(s). Post-hoc LSD multiple comparisons procedures were conducted to simultaneously examine comparisons between all possible pairs of group means. All statistical analyses were conducted using the SPSS 11.0 software package (SPSS, Chicago, IL), general linear model.
Data about ages of the investigated persons are presented as their means ± standard errors (SE). The chi-square test for categorical variables was used to compare characteristics between different subgroups of patients. All P values are two-sided, and significance was set at P ≤ 0.05.
Results
HCV infection in persons suffered due to the Chernobyl NPP accident
The presence of anti-HCV antibodies was assessed in 965 cleanup workers, 114 convalescents of ARS, 578 dwellers of radiation-contaminated territories of Ukraine, and 87 NPP workers. Samples from 41,021 blood donors at the Kyiv Municipal Blood Center were examined for comparison. As given in , a very significant (P < 0.0001) greater prevalence of HCV infection was found in all groups of individuals who suffered from the Chernobyl incident as compared with that among the Kyiv blood donors. In a separate PCR analysis of the HCV found in the blood samples from HCV+ subjects, a prognostically unfavorable 1b genotype of HCV—one associated with frequent development of cirrhosis—was the most prevalent form found (i.e., incidence of 86.92%) among all the subgroups analyzed (PCR analyses were performed at the Department of Hygiene, in the School of Medicine of Hiroshima University, Hiroshima, Japan, under the supervision of Professor H. Yoshizawa).
Table 1. Presence of anti-hepatitis C virus (HCV) serum antibodies in individuals in the indicated populations.
How and why these individuals became HCV+ were also of interest to our investigation. For this purpose, groups of 199 male cleanup workers (71 persons received blood transfusions) and 140 male IR-non-exposed persons (75 persons received blood transfusions) were studied. In both groups we found a strong association (P < 0.001) between the presence of anti-HCV antibodies and prior blood transfusion(s). Among persons who received blood transfusions, HCV seropositivity was significantly higher (P < 0.001) in the group of cleanup workers (39.4%) as compared with among the IR-non-exposed persons (14.3%). No significant differences were found among individuals who had not received blood transfusions (12.2% versus 6.7%; P > 0.05).
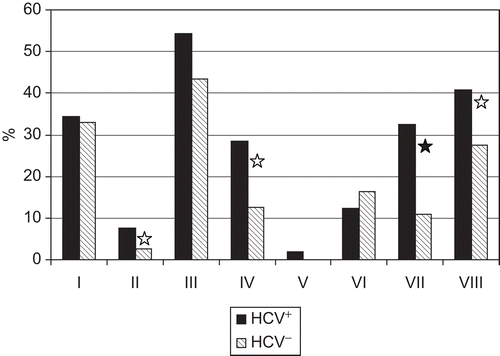
The studies here also sought to determine, in part, if there was any association between persistence of HCV in these patients and selected somatic diseases. The data here showed that in a group of cleanup workers (specifically, 368 patients without malignant diseases), HCV infection was associated with active chronic hepatitis (32.4% versus 11.0% among cleanup workers without anti-HCV serum antibodies; P < 0.001), chronic gastritis (40.9% versus 27.4%; P < 0.05), acute myocardial infarction in anamnesis (7.6% versus 2.6%; P < 0.05), thyroiditis, and nodular goiter (28.6% versus 12,5%; P < 0.01), thrombocytopenia (4.8% versus 0.7%; P < 0.05), lymphadenopathy (14.3% versus 4.9%; p < 0.05), and/or hemorrhagic vasculitis (2.8% versus 0.4%; P < 0.05) (). Thus, a significant prevalence of HCV infection among these IR-exposed persons appears to unfavorably influence their health in many manners.
Figure 1. The frequency of some somatic disease in cleanup workers as related to hepatitis C virus (HCV) seropositivity. I: coronary heart disease, II: acute myocardial infarction in anamnesis; III: essential hypertension; IV: thyroiditis/goiter; V: type 1 diabetes; VI: type 2 diabetes/impaired glucose tolerance; VII: chronic hepatitis; VIII: chronic gastritis. Differences between HCV+ and HCV− patients are significant at P < 0.05 (white star) or P < 0.001 (black star).
CMV infection in persons that suffered due to the Chernobyl NPP accident
As compared with the control group of IR-non-exposed persons, male subjects in each of the various groups of individuals who suffered from the Chernobyl incident (except for the dwellers of radiation-contaminated areas) had a significantly greater incidence of CMV infection as determined by the presence of anti-CMV IgG antibodies (i.e., values ranging from 79.4 to 85.6% versus 69.2%; P < 0.05) (). Specifically, the level of significance of these differences in titers between the male IR-non-exposed persons and the male cleanup workers, ARS convalescents, and NPP workers were P < 0.001, P < 0.001, and P < 0.05, respectively. In addition, both the cleanup workers and ARS convalescents had significantly more frequent laboratory signs of active CMV infection as determined by positive PCR measures for detection of viral DNA (i.e., 14.3 and 16.2%, respectively, versus 5.51%; P < 0.05) (only male persons were included in this analysis). As a consequence of repeated viral activation, we estimated that there has also been likely a presence of high titers of anti-CMV IgG antibodies in the serum of the vast majority of these exposed subjects. Lastly, a correlation between IR-absorbed dose and the level of CMV seropositivity was found within the ARS convalescents group (i.e., r = +0.3244; P = 0.028).
Table 2. Presence of anti-cytomegalovirus (CMV) IgG serum antibodies in individuals in the indicated populations.
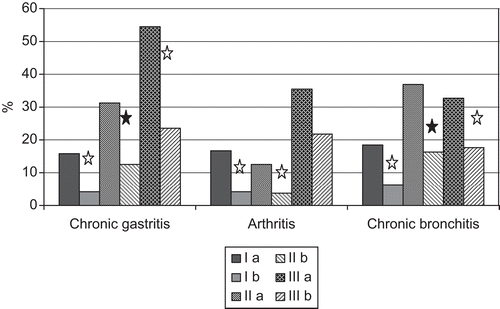
In all groups of observed subjects (a total n = 1017), the presence of anti-CMV IgG antibodies was associated with somatic diseases (76.86% versus 49.11% among persons without chronic somatic diseases; P < 0.001), especially with chronic gastritis, bronchitis, and different types of arthritis (). Furthermore, a simultaneous existence of bronchopulmonary and digestive system diseases was typical for the CMV+ patients comparing with CMV− patients. Such co-morbidity was observed in 13.19% of the CMV+ patients versus 4.16% of the CMV− patients among the IR-non-exposed subjects (P < 0.05) and in 36.84% versus 11.24% of patients in a group of cleanup workers (P < 0.01).
Figure 2. The frequency of some somatic diseases in the observed patients appears to depend on cytomegalovirus (CMV) seropositivity. Group I = IR-non-exposed persons (Ia: CMV+, Ib: CMV−); Group II = cleanup workers (IIa: CMV+, IIb: CMV−); and Group III = ARS convalescents (IIIa: CMV+, IIIb: CMV−). Differences between CMV+ and CMV− patients are significant at P < 0.05 (white star) or P < 0.001 (black star).
The repeated reactivation of CMV significantly impaired the course of HCV infection (note: this analysis was performed in group of 105 HCV+ cleanup workers). The clinical signs of active hepatitis (e.g., liver enlargement, elevated alanine and asparagine aminotransferase activities, and extrahepatic symptoms) were observed in 44.4% of HCV+CMV+ patients, but only in 21.1% of HCV+CMV− patients (P < 0.05). In a one-way layout analysis, the presence of anti-CMV antibodies (r = 0.3418; P < 0.0001), masculine gender (r = 0.3553; P < 0.0001), serological signs of viral hepatitis B in anamnesis (r = 0.2896; P = 0.003), and an increase in absolute CD8+ T-lymphocyte counts (only in CMV+ patients, r = 0.5745; P = 0.005) were the major risk factors for prediction of active HCV-associated hepatitis. It is possible to suppose that CMV may potentiate any hepatotropic action of HCV by the influence of the former on the liver parenchyma or by causing increases in the activity of cytotoxic T-lymphocytes.
Because there are some data that indicate that persistent CMV activation can trigger and maintain the clonal expansion of large granular T-lymphocytes (Zambello and Semenzato, Citation2009), we also examined a small group (n = 4) of cleanup workers with LGL. In all of these patients, substrate cells were found, which belonged to the class of mature post-thymic T-lymphocytes (i.e., CD3+, CD8+, CD4−, CD16+, and/or CD57+). A presence of CMV in blood mononuclear cells was found in two of these patients (by PCR). One of these patients who had stable LGL over the last 4 years (WBC 4.1–6.1 × 109/L, with a differential count of 56–68% lymphocytes, and 85–87% of these cells had a CD3+CD8+CD16+ phenotype); after intensive anti-CMV therapy (i.e., anti-CMV IgG, ganciclovir), the patient’s blood cell profile was completely restored and the patient displayed an improved general condition (i.e., no signs of chronic infections and episodes of febrile fever).
CLL cells display homology of their Ig receptors with antiviral or antimicrobial antigens
Over the previous decade, CLL has been the subject of increasingly intensive research. Several research groups identified so-called homologous CLL cases that expressed immunoglobulins (Igs) composed from stereotyped combinations of heavy-chain variable (HV), diversity (D), and joining (J) genes and very close heavy-chain complementarity-determining region-3 (HCDR3) (Ghiotto et al., Citation2004; Messmer et al., Citation2004; Tobin et al., Citation2004; Widhopf et al., Citation2004; Stamatopoulos et al., Citation2007). Most of the CLL clusters were also characterized by specific Ig light kappa (κ) or lambda (λ) chain gene usage (Widhopf et al., Citation2008; Hadzidimitriou et al., Citation2009). Together with a biased repertoire of Ig genes (Chiorazzi and Ferrarini, Citation2003), a similarity of CLL Ig sequences and antibodies against autoantigens and microbial/viral antigens (Lanemo Myhrinder et al., Citation2008) and some epidemiological data (Landgren et al., Citation2006, Citation2007) has led to the hypothesis that there is a possible role of viral/microbial antigens in the development of this (and other non-somatic) disease(s).
In the studies here, we analyzed Ig gene rearrangements in 294 CLL sequences from Ukrainian patients (63 of them who suffered from the Chernobyl NPP catastrophe) and identified 46 cases presenting a homology with antibody sequences of known specificity (Bilous et al., Citation2010). No differences were found between sequences from IR-exposed and IR-non-exposed CLL patients. Among the 46 CLL cases, 26 evidenced a HCDR3 homology with antibacterial or antiviral Ig clones, namely, anti-PPS antibodies against Streptococcus pneumonia polysaccharides (12 cases), Igs that recognized capsular polysaccharide of Neisseria meningitidis (three cases), anti-staphylococcal protein A Ig clones (two cases), rotavirus-specific Ig clones (four cases), anti-rabies Igs (four cases), and anti-herpes simples virus type 1 (one case). Additionally, 20 Ukrainian CLL cases demonstrated HCDR3 homology with autoreactive clones. These included with homologies to: Igs in rheumatoid arthritis patients (five cases); anti-cardiolipin antibody (six cases); Igs peculiar to systemic lupus erythematosus (anti-Sm; five cases); Ig with autoantibody activity (two cases); Ig from a bone marrow transplant recipient with graft-versus-host disease (GVHD; one case); and Ig from B-cell lymphoma secreting anti-Pr2 erythrocyte autoantibodies (1 case) (see ).
Table 3. Ukrainian chronic lymphocytic leukemia (CLL) cases showing HCDR3 homology with immunoglobulins (Igs) of known specificity.
Discussion
On the basis of our observations of several different groups of individuals who suffered during and after the Chernobyl NPP catastrophe, as well as a control group of persons without any previous direct exposure to IR, a high prevalence of HCV and CMV infections among the afflicted groups was established.
It is well-established that preceding blood transfusions are considered one major reason for HCV seropositivity in epidemiologic studies of disease (i.e., HCV) incidence. The data from the current studies confirmed this phenomenon. Nevertheless, even if transfusions were a major source of infection, it is known that after the initial infection, HCV may be completely eliminated in ≈17–25% of the affected subjects. By comparing persons who received blood transfusions relative to IR exposure, these studies revealed that there was a significantly higher (P < 0.001) frequency of anti-HCV antibodies present among cleanup workers than in IR-non-exposed individuals. Based on our previous studies that revealed considerable disturbances in T-lymphocyte-mediated immunity among Chernobyl cleanup workers—and especially among ARS convalescents (see Chumak et al., Citation2000), it is possible to suggest that this state of immunomodulation may be one reason for the high level of HCV infection among these individuals—regardless of whether or not the initial infection was due to a transfusion or not.
Our data concerning the prevalence of anti-HCV serum antibodies in the cleanup workers and ARS convalescents were in agreement with data from Japanese investigators who had earlier concluded that radiation exposure may accelerate the progress of chronic liver disease associated with HCV in atomic bomb survivors (Fujiwara et al., Citation2000). Recently, it was noted that the nonstructural protein 3 of HCV interacts with the ataxia-telangiectasia-mutated (ATM) protein and, in turn, delayed dephosphorylation of phosphorylated forms of ATM (Lai et al., Citation2008). These same investigators also described that this HCV protein also impairs the efficiency of DNA repair and renders infected cells more sensitive to DNA damage after IR exposure (Lai et al., Citation2008). This interaction may contribute to the observed HCV infection-associated pathogeneses among the IR-exposed patients here and in the Japanese studies. Specifically, among the group of HCV+ Chernobyl cleanup workers monitored here, some diseases (e.g., chronic hepatitis, chronic gastritis, thyroid diseases, thrombocytopenia, and acute heart attack in anamnesis) were found to have occurred with greater frequently in comparison with that among HCV− cleanup workers.
From the studies here and those of other investigators, some mechanisms to explain the potential for interactions between CMV and IR have been revealed as well. For example, it was demonstrated (in different experimental models) that CMV gene expression was increased by exposure to oxygen radicals or after irradiation (Vereecque et al., Citation2003; Kinoshita et al., Citation2006; Berezhnoy et al., Citation2008). This may be a major reason for the observed increases in CMV seropositivity and repeated CMV reactivation among our Chernobyl cleanup workers and ARS convalescents. The increases in CMV infectivity status (likewise HCV) were also found to be positively associated with increases in the occurrence of somatic diseases, especially chronic gastritis, bronchitis, and different types of arthritis among the CMV+ patients.
Studies here and elsewhere have also noted a significant homology between IgHV genes in the lymphoid cells of CLL patients with antiviral and antimicrobial antibodies. Recently, Lanemo Myhrinder et al. (Citation2008) revealed that several CLL Igs reacted with bacterial components (S. pneumonia polysaccharides and phosphorylcholine) as well as molecular structures exposed on apoptotic cells (e.g., vimentin, proline-rich acidic protein-1, and oxidized LDL). These findings led to the suggestion that viral and bacterial infections in synergy with self-antigens or apoptotic cells could drive CLL. Among the CLL patient examined here, similar findings of CLL Igs that react with viral and bacterial components were proof that infections—even almost a quarter of a century after the nuclear accident—may contribute to CLL pathogenesis.
In summary, it appears that persistent viral infections, especially when accompanied by exposure(s) to IR, can be quite deleterious. The worst-case scenario, where each of these factors have occurred/are ongoing, is one that appears to result in a modified (i.e., aggravated) clinical course of somatic diseases and/or to promote malignancies (i.e., CLL) among these hosts. Nevertheless, even under these seemingly “dire conditions,” the persisting infections can be transformed into “steerable/manageable constituents” with antiviral treatments, even if the patient had been exposed earlier to an irradiation. In those cases, the patients can be medically steered to an eventually vastly improved general condition.
Acknowledgement
The study was supported by KIHEV Kinderhilfe Kiew e.V. (Weil am Rhein, Germany).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Aarnisalo, J., Veijola, R., Vainionpaa, R., Simell, O., Knip, M., and Ilonen, J. 2008. Cytomegalovirus infection in early infancy: Risk of induction and progression of autoimmunity associated with Type 1 diabetes. Diabetologia 51:769–772.
- Abramenko, I., Bilous, N., Chumak, A., Davydova, E., Kryachok, I., Martina, Z., Nachaev, S., Dyagil, I., Bazyka, D., and Bebeshko, V. G. 2008. Chronic lymphocytic leukemia patients exposed to ionizing radiation due to the Chernobyl NPP accident—With focus on immunoglobulin heavy chain gene analysis. Leukemia Res. 32:535–545.
- Atta, A. M., Oliveira, I. S., Sousa, G. M., Parana, R., and Atta, M. L. 2010. Seruma cytokine profile in hepatitis C virus carriers presenting cryoglobulinaemia and non-organ-specific autoantibodies. Microb. Pathol. 48:53–56.
- Balonov, M. I. 2007. The Chernobyl Forum: Major findings and recommendations. J. Environ. Radioact. 96:6–12.
- Berezhnoy, A. E., Vaynson, A. A., Kasatkina, N. N., Khokhlova, O. A., Ostrovskaia, A. S., Volodina, E. V., Baryshnikov A. Yu., Georgiev, G. P., and Larin, S. S. 2008. High-dose gamma-irradiation enhance expression of transgene under control of immediate-early CMV promoter in stably transfected tumor cells. Mol. Biol. (Mosk.) 42:501–509.
- Bilous, N., Bomben R., Dal Bo, M., Capello, D., Forconi, F., Laurenti, L., Bertoni, F., Efremov, D. G., Marasca, R., Del Poeta, G., Martina Z., Kryachouk I., Dyagil, I., Gaidano, G., Chumak, A., Gattei, V., and Abramenko I. 2010. Molecular and clinical features of chronic lymphocytic leukemia with stereotyped B-cell receptors in an Ukrainian cohort. Leukemia Lymphoma 51:822–838.
- Bomben, R., Dal, B. M., Capello, D., Forconi, F., Maffei, R., Laurenti, L., Rossi, D., Del Principe, M. I., Zucchetto, A., Bertoni, F., Rossi, F. M., Bulian, P., Cattarossi, I., Ilariucci, F., Sozzi, E., Spina, V., Zucca, E., Degan, M., Lauria, F., Del Poeta, G., Efremov, D. G., Marasca, R., Gaidano, G., and Gattei, V. 2009. Molecular and clinical features of chronic lymphocytic leukemia with stereotyped B-cell receptors: Results from an Italian multicentre study. Br. J. Haematol. 144:492–506.
- Bratke, K., Krieghoff, L., Kuepper, M., Luttmann, W., and Virchow, J. C. 2007. CD8+ T-cell activation and differentiation in allergic asthma and the impact of cytomegalovirus serological status. Clin. Exp. Immunol. 149:311–316.
- Buskila, D. 2009. Hepatitis C-associated rheumatic disorders. Rheum. Dis. Clin. North Am. 35:111–123.
- Castro-Volio, I., and Valle-Bourrette, L. 2001. Chromosomal defects in 34 male homosexuals, half of them with HIV antibodies. Rev. Biol. Trop. 50:347–353.
- Chiorazzi, N., and Ferrarini, M. 2003. B-Cell chronic lymphocytic leukemia: Lessons learned from studies of the B-cell antigen receptor. Ann. Rev. Immunol. 21:841–894.
- Chomczynski, P., and Sacchi, N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 162:156–159.
- Chumak, A. A., Abramenko, I. V., Bazyka, D. A., Boychenko, P. K., and Pleskach, O. Y. 2005. Persisting viral infections and genome instability at the remote post irradiation period. Probl. Radiat. Med. Radibiol. 11:193–198.
- Chumak, A. A., Bazyka, D. A., Beliaeva, N. V., Azarslova, M. V., Minchenko, Zh. N., Pleskach, O. Y., and Mishenko, L. P. 2000. Immunological effects in acute radiation sickness convalescents—Results of thirteen years of follow-up. Int. J. Radiat. Med. 1:65–83.
- Chumak, A. A., Kovalenko, A. N., Abramenko, I. V., Bazyka, D. A., Boychenko, P. K., Beliy, D. A., and Pleskach, O. Y. 2003. Reactivation of cytomegalovirus infection in convalescents of acute radiation sickness and its significance in somatic pathology. Ukr. Med. J. 4:113–116.
- Chumak, A. A., Pleskach, O., Yurchenko, L., Ivaniskaya, N., and Zanevskaya, L. 1996. Blood banking in Kiev Municipal Blood Center. In: Abstract Book of ISBT ’96. Kiev, p. 32.
- de Re, V., Simula, M. P., Pavan, A., Garziera, M., Marin, D., Dolcetti, R., de Vita, S., Sansonno, D., Geremia, S., and Toffoli, G. 2009. Characterization of antibodies directed against the immunoglobulin light kappa chain variable chain region (VK) of hepatitis C virus-related Type-II mixed cryoglobulinemia and B-cell proliferation. Ann. N. Y. Acad. Sci. 1173:152–160.
- Deng, Y. F., Zhou, D. N., and Lu, Y. D. 2007. Frequent allelic loss at the FRA3B site in endemic nasopharyngeal carcinoma: Association with clinical features and Epstein-Barr virus infection. J. Laryngol. Otol. 121:1073–1078.
- Frick, O. L., German, D. F., and Mills, J. 1979. Development of allergy in children. I. Association with virus infections. J. Allergy Clin. Immunol. 63:228–241.
- Fujiwara, S., Kusumi, S., Cologne, J., Akahoshi, M., Kodama, K., and Yoshizawa, H. 2000. Prevalence of anti-hepatitis C virus antibody and chronic liver disease among atomic bomb survivors. Radiat. Res. 154:12–19.
- Ghiotto, F., Fais, F., Valetto, A., Albesiano, E., Hashimoto, S., Dono, M., Ikematsu, H., Allen, S. L., Kolitz, J., Rai, K. R., Nardini, M., Tramontano, A., Ferrarini, M., and Chiorazzi, N. 2004. Remarkably similar antigen receptors among a subset of patients with chronic lymphocytic leukemia. J. Clin. Invest. 113:1008–1016.
- Hadzidimitriou, A., Darzentas, N., Murray, F., Smilevska, T., Arvaniti, E., Tresoldi, C., Tsaftaris, A., Laoutaris, N., Anagnostopoulos, A., Davi, F., Ghia, P., Rosenquist, R., Stamatopoulos, K., and Belessi, C. 2009. Evidence for the significant role of immunoglobulin light chains in antigen recognition and selection in chronic lymphocytic leukemia. Blood 113:403–411.
- Hardie, D. R. 2010. Human gamma-herpesviruses: A review of 2 divergent paths to oncogenesis. Transfus. Apher. Sci. 42:177–183.
- Hassan, M. I., Naiyer, A., and Ahmad, F. 2010. Fragile histidine triad protein: Structure, function, and its association with tumorogenesis. J. Cancer Res. Clin. Oncol. 136:333–350.
- International Workshop on Chronic Lymphocytic Leukemia. 1989. Chronic lymphocytic leukemia: Recommendations for diagnosis, staging, and response criteria. Ann. Intern. Med. 110:236–238.
- Ivanov, V. K., Maksioutov, M. A., Chekin, S. Yu., Kruglova, Z. G., Petrov, A. V., and Tsyb, A. F. 2000. Radiation-epidemiological analysis of incidence of non-cancer diseases among the Chernobyl liquidators. Health Phys. 78:495–501.
- Ivanov, V. K., Maksioutov, M. A., Chekin, S. Yu., Petrov, A. V., Biryukov, A. P., Kruglova, Z. G., Mayash, V. A., Tsyb, A. F., Manton, K. G., and Kravchenko, J. S. 2006. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 90:199–207.
- Kinoshita, A., Kobayashi, D., Saitoh, Y., Tanabe, N., Uchino, K., Nishiquchi, K., Okumura. K., and Komada, F. 2006. Regulation of exogenous gene expression by superoxide. Pharm. Res. 23:2536–2541.
- Kurbanov, S. A., and Mamedov, M. K. 2009. Serological markers of viral infections in patients with rheumatoid arthritis. Georgian Med. News 166:65–67.
- Lai, C. K., Jeng, K. S., Machida, K., Cheng, Y. S., and Lai, M. M. 2008. Hepatitis C virus NS3/4A protein interacts with ATM, impairs DNA repair and enhances sensitivity to ionizing radiation. Virology 370:295–309.
- Landgren, O., Engels, E. A., Caporaso, N. E., Gridley, G., Mellemkjaer, L., Hemminki, K., Linet M. S., and Goldin, L. R. 2006. Patterns of autoimmunity and subsequent chronic lymphocytic leukemia in Nordic countries. Blood 108:292–296.
- Landgren, O., Rapkin, J. S., Caporaso, N. E., Mellemkjaer, L., Gridley G., Goldin, L. R., and Engels, E. A. 2007. Respiratory tract infections and subsequent risk of chronic lymphocytic leukemia. Blood 109:2198–2201.
- Lanemo Myhrinder, A., Hellqvist, E., Sidorova, E., Soderberg, A., Baxendale, H., Dahle, C., Willander, K., Tobin, G., Backman, E., Soderberg, O., Rosenquist, R., Horkko, S., and Rosen, A. 2008. A new perspective: Molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood 111:3838–3848.
- Lefranc, M. P. 2001. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 29:207–219.
- Lehous, M., D’Abramo, C. M., and Archambault, J. 2009. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public Health Genom 12:268–280.
- Messmer, B. T., Albesiano, E., Efremov, D. G., Ghiotto, F., Allen, S. L., Kolitz, J., Foa, R., Damle, R. N., Fais, F., Messmer, D., Rai, K. R., Ferrarini, M., and Chiorazzi, N. 2004. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J. Exp. Med. 200:519–525.
- Moorman, J., Dong, Z. P., Ni, L., Zhang, C., Borthwick, T., and Yao, Z. Q. 2009. Abnormal B-cell activation associated with TALL-1 over-expression and SOCS-1 suppression during chronic hepatitis C virus infection. Immunology 128:227–235.
- Murray, F., Darzentas, N., Hadzidimitriou, A., Tobin, G., Boudioora, M., Scielzo, C., Laoutaris, N., Karlsson, K., Baran-Marzsak, F., Tsaftaris, A., Moreno, C., Anagnostopoulos, A., Caligaris-Cappio, F., Vaur, D., Ouzonis, C., Belessi, C., Ghia, P., Davi, F., Rosenquist, R., and Smatatopoulos, K. 2008. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia: Implications for the role of antigen selection in leukemogenesis. Blood 111:1524–1533.
- Ozdemir, F. N., Akgul, A., Altunoglu, A., Bilgic, A., Arat, Z., and Haberal, M. 2007. The association between cytomegalovirus infection and atherosclerotic events in renal transplant recipients. Transplant Proc. 39:990–992.
- Pichiorri, F., Ishii, H., Okumura, H., Trapasso, F., Wang, Y., and Huebner, K. 2008. Molecular parameters of genome instability: Roles of fragile genes at common fragile sites. J. Cell Biochem. 104:1525–1533.
- Qiu, H., Straat, K., Rahbar, A., Wan, M., Soderberg-Naucler, C., and Haeggstrom, J. Z. 2008. Human CMV infection induced 5-lipoxygenase expression and leukotriene B4 production in vascular smooth muscle cells. J. Exp. Med. 205:19–24.
- Rabczynski, M., Jakobsche, U., and Adamiec, R. 2007. Serological markers of chronic Chlamydia pneumoniae and cytomegalovirus (CMV) infection in patients with peripheral occlusive artery disease—An initial report. Przegl. Lek. 64:416–418.
- Rai, K. R. 1987. A critical analysis of staging in CLL. In: Chronic Lymphocytic Leukemia (Gale, R. P., and Rai, K. R., Eds.), New York: Alan R. Liss, Inc, pp. 253–264.
- Romanenko, A. Y., Finch, S. C., Hatch, M., Lubin, J. H., Bebeshko, V. G., Bazyka, D. A., Gudzenko, N., Dyagil, I. S., Reiss, R. F., Bouvill, A., Chumak, V. V., Trosiuk, N. K., Babkina, N. G., Belyayev, Y., Masnyk, I., Ron, E., Howe, G. R., and Zablotska, L. B. 2008. The Ukrainian-American study of leukemia and related disorders among Chernobyl cleanup workers from Ukraine: III. Radiation risks. Radiat. Res. 170:711–720.
- Sanduzzi, A., Fraziano, M., and Mariani, F. 2001. Monocytes/macrophages in HIV infection and tuberculosis. J. Biol. Regul. Homeost. Agents 15:294–298.
- Souto-Carneiro, M. M., Longo, N. S., Russ, D. E., Sun, H. W., and Lipsky, P. E. 2004. Characterization of the human Ig heavy chain antigen binding complementary determining region 3 using a newly-developed software algorithm, JOINSOLVER. J. Immunol. 172:6790–6802.
- Spardy, N., Covella, K., Cha, E., Hoskins, E. E., Wells, S. I., Duensing, A., and Duensing, S. 2009. Human papillomavirus 16 E7 oncoprotein attenuates DNA damage checkpoint control by increasing the proteolytic turnover of claspin. Cancer Res. 69:7022–7029.
- Stamatopoulos, K., Belessi, C., Moreno, C., Boudjograh, M., Guida, G., Smilevska, T., Belhoul, L., Stella, S., Stavroyianni, N., Crespo, M., Hadzidimitriou, A., Sutton, L., Bosch, F., Laoutaris, N., Anagnostopoulos, A., Montserrat, E., Fassas, A., Dighiero, G., Caligaris-Cappio, F., Merle-Beral, H., Ghia, P., and Davi, F. 2007. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood 109:259–270.
- Sumner, D. 2007. Health effects resulting from the Chernobyl accident. Med. Confl. Surviv. 23:31–45.
- Szostek, S., Zawilinska, B., Kopec, J., and Kosz-Vnenchak, M. 2009. Herpesviruses as possible cofactors in HPV-16-related oncogenesis. Acta Biochim. Pol. 56:337–342.
- Terry, G., Ho, L., Londesborough, P., Cross, P., Lopes, A., Monaghan, J., and Cuzick, J. 2004. The role of human papillomavirus type 16 and the fragile histidine triad gene in the outcome of cervical neoplastic lesions. Br. J. Cancer 91:2056–2062.
- Thorland, E. C., Myers, S. L., Gostout, B. S., and Smith, D. I. 2003. Common fragile sites are preferential targets for HPV 16 integrations in cervical tumors. Oncogene 22:1225–1237.
- Tobin, G., Thunberg, U., Karlsson, K., Murray, F., Laurell, A., Willander, K., Enblad, G., Merup, M., Vilpo, J., Juliusson, G., Sundstrom, C., Soderberg, O., Roos, G., and Rosenquist, R. 2004. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood 104:2879–2885.
- van Dongen, J. J., Langerak, A. W., Bruggemann, M., Evans, P. A., Hummel, M., Lavender, F. L., Delabesse, E., Davi F., Schuuring E., Garcia-Sanz, R., van Krieken, J. H., Droese, J., Gonzalez, D., Bastard, C., White, H. E., Spaargaren, M., Gonzalez, M., Parreira, A., Smith, J. L., Morgan, G. J., Kneba, M., and Macintyre, E. A. 2003. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17:2257–2317.
- Vereecque, R., Saudemont, A., Wickham, T. J., Gonzalez, R., Hetuin, D., Fenaux, P., and Quesnel, B. 2003. γ-Irradiation enhances transgene expression in leukemic cells. Gene Ther. 10:227–233.
- Wang, J., Cheng, Y. W., Wu, D. W., Chen, J. T., Chen, C. Y., Chou, M. C., and Lee, H. 2006. Frequent FHIT gene loss of heterozygosity in human papillomavirus-infected non-smoking female lung cancer in Taiwan. Cancer Lett. 235:18–25.
- Widhopf, G. F., Goldberg, C. J., Toy, T. L., Rassenti, L. Z., Wierda, W. G., Byrd, J. C., Keating, M. J., Gribben, J. G., Rai, K. R., and Kipps, T. J. 2008. Non-stochastic pairing of immunoglobulin heavy and light chains expressed by chronic lymphocytic leukemia B-cells is predicated on the heavy chain CDR3. Blood 111:3137–3144.
- Widhopf, G. F., Rassenti, L. Z., Toy, T. L., Gribben, J. G., Wierda, W. G., and Kipps, T. J. 2004. Chronic lymphocytic leukemia B cells of more than 1% of patients express virtually identical immunoglobulins. Blood 104:2499–2504.
- Zambello, R., and Semenzato, G. 2009. Large granular lymphocyte disorders: New etiopathogenetic clues as a rationale for innovative therapeutic approaches. Hematologica 94:1341–1345.
- Zekri, A. R., Bahnassy, A. A., Hafez, M., El-Shehaby, A. M., Sherif, G. M., Khaled, H. M., and Zakhary, N. 2005. Alterations of the fragile histidine triad gene in hepatitis C virus-associated hepatocellular carcinoma. J. Gastroenterol. Hepatol. 20:87–94.