Abstract
1,4-Phenylenediamine (PPD) and the structurally-related 1,4-toluenediamine (PTD) are frequently used oxidative hair dye precursors that can induce a delayed-type hypersensitivity reaction known as contact allergy. Very rare cases of Type 1 (IgE-mediated) allergic responses associated with PPD or PTD have been reported among hair dye users. As part of an effort to determine if repeated dermal exposure to the dyes could induce a T-helper-2 (TH2) response, we used a dermal exposure regimen in mice reported to identify a TH2 response. Ear swelling was evident at post-final exposure to PPD and PTD, indicating that an immune response was observed. However, cytokine mRNA after repeated topical exposure to these two chemicals showed no shift in the expression toward the typical TH2 cytokines interleukin (IL)-4 and IL-10 compared to the TH1 cytokine interferon (IFN)-γ. Consistent with these cytokine profiles, no concomitant increase in total serum IgE antibody titer or in B220+IgE+ lymphocytes in lymph nodes and skin application site skin was detected. In contrast, using an identical exposure regimen, animals topically exposed to the known respiratory (Type 1) allergen toluene 2,4-diisocyanate (TDI) showed significant expression of IL-4 and IL-10 mRNA compared to IFNγ as well as an increase in total serum IgE and in B220+IgE+ cells in lymph nodes and skin application site. The data generated are consistent with the pattern of adverse reactions to hair dyes seen clinically, which overwhelmingly is of delayed rather than immediate-type hypersensitivity. Although current animal models have a limited ability to detect rare TH2 responses to contact allergens, the present study results support the view that exposure to hair dyes is not associated with relevant TH2 induction.
Introduction
1,4-Phenylenediamine (PPD) is an arylamine used widely in the rubber, dye, and photographic industries. Furthermore, PPD and the chemically-related 1,4-toluenediamine (PTD) are important oxidative hair dye precursors that chemically represent primary intermediates. The dyeing process involves primary intermediates and couplers permeating the hair shaft, where—after activation with hydrogen peroxide—the primary intermediate is oxidized and thus activated to react with couplers. The resultant larger molecule is trapped in the hair shaft and is responsible for the shade and permanence of the dye effect (Brown, Citation1997).
Clinically, two forms of allergy are common, delayed-type hypersensitivity that presents as allergic contact dermatitis and is most frequently caused by chemical exposure and immediate-type hypersensitivity that presents as urticaria, conjunctivitis, rhinitis, asthma, and anaphylaxis, and is most frequently associated with protein exposure. Delayed-type hypersensitivity reactions (contact allergy) from hair dye precursors occurs in susceptible users, with PPD being the most frequent contact sensitizer in hair dye formulas (Krasteva et al., Citation2009). Globally, the rate of positive reactions to PPD varies generally between 2% and 8% in consecutive eczema patients, whereas the frequency of positive elicitation reactions in patch tests to PPD in the general population was typically between 0 and 1.5% (Krasteva et al., Citation2009). In very rare cases, PPD was suspected not only to induce a delayed-type allergy but also symptoms similar to an immediate-type allergy (Edwards and Edwards, Citation1984). Over the past 25 years, only 14 cases of immediate-type allergic-like reactions among oxidative hair dye users with reported positive skin prick tests, scratch tests or short-term patch tests to PPD and/or PTD were reported in the global literature (summarized in ). Compared with the billions of PPD- and PTD-containing hair colorants used during the same period, these are considered to represent extremely rare events.
Table 1. Review of the literature on Type 1 IgE-mediated hypersensitivity to hair dyes among consumers.
For hypersensitivity, T-helper-1 (TH1) cells typically mediate delayed-type hypersensitivity reactions, whereas T-helper-2 (TH2) cells typically mediate immediate-type reactions (Mosmann and Coffman, Citation1989). As far as investigated today, chemical allergens that induce TH1- or TH2-driven immune responses are positive in local lymph node assays (LLNAs) and can be distinguished by their different cytokine profile (Dearman et al., Citation2002). One prevailing approach to assess chemicals for their ability to induce TH1 or TH2 immune responses is to evaluate cytokine profiles of lymph node cells collected from animals (usually mice) exposed to the chemical. TH1-dominant immune responses are identified by high production of interferon (IFN)-γ in comparison to interleukin (IL)-4. TH2-dominant immune responses are identified by high production of IL-4 in comparison to IFNγ. In addition, TH2-dominant responses are often associated with an increase in total serum IgE. IL-10 is also described as a TH2-promoting cytokine as well as one important to regulation of the TH1/TH2 balance (Fiorentino et al., Citation1989; Akdis et al., Citation2004).
Little is known about a possible connection between immediate-type allergy and exposure to contact allergens in humans. There are only very rare cases in the literature describing an immediate-type and delayed-type allergy to the same chemical in the same patient (e.g., Edwards and Edwards, Citation1984). We are not aware of any cases where there is a shift within an individual from a delayed-type allergy to an immediate-type allergy to a chemical sensitizer. There are some very rare individuals in whom hair dye allergens such as PPD and PTD can induce a preferential TH2 immune response instead of a TH1 response (). It is interesting to note that well-known human chemical respiratory sensitizers, such as the isocyanates, seem occasionally to cause a TH1 allergic contact dermatitis, which may represent a related, but converse, phenomenon (Liippo and Lammintausta, Citation2008).
In this article, we asked whether, with the benefit of hindsight, it would have been possible to predict these very rare TH2-driven responses to hair dyes. However, no validated (or even standardized) predictive model exists for the assessment of immediate-type chemical allergens and with current knowledge, neither is it known if potency determined in a LLNA is a good predictor for immediate-type allergens (Arts and Kuper, Citation2007; Boverhof et al., Citation2008). Therefore, we chose to test PPD and PTD in an exposure regimen in BALB/c mice where the antigen-specific hypersensitivity response to the contact allergens 2,4-dinitrofluorobenzene (DNFB) and toluene 2,4-diisocyanate (TDI) was TH2-like upon five repeated topical applications within 12 or 28 days (Dearman et al., Citation1996; Nagai et al., Citation1997; Warbrick et al., Citation1998). The respiratory sensitizer TDI was only used as a positive control since both TH1 and TH2 responses can be generated to this chemical in animal models (Matheson et al., Citation2005; Ban et al., Citation2006) and in man (Liippo and Lammintausta, Citation2008). The study was not designed to compare potencies of PPD or PTD to TDI or to each other.
Materials and methods
Test substances and preparation of dosing solutions
PPD (CAS No. 106-50-3) was obtained from Sigma-Aldrich (Taufkirchen, Germany; Catalogue No. P6001). PTD (CAS No. 615-50-9; Batch 2346 [R99053665]) was obtained from Wella AG (Darmstadt, Germany). PPD and PTD were stored at room temperature and protected from light and humidity. For the formulation of PPD, an aqueous solution of PPD was made and subsequently diluted in acetone, to which olive oil was added. The final acetone and olive oil content in the formulation was 18.75% (v/v) and 25% (v/v), respectively. PTD was formulated as described for PPD except that the aqueous PTD stock solution contained 0.25% NaOH. For the vehicle control, an aqueous acetone (18.75% v/v), olive oil (25% v/v) solution was prepared. This vehicle system was chosen since it was used to generate an EC3 value for PPD and PTD in the LLNA. The positive control TDI (CAS No. 584-84-9; EC No. 2095445) was obtained from Sigma-Aldrich (Cat. No. T6889). TDI was formulated in acetone:olive oil (4:1 v/v). The concentrations of chemicals were chosen to optimize our ability to detect a TH1 and/or TH2 response. This study exaggerated the reported induction threshold for contact allergy in the mouse by a factor of 6 for PPD (EC3 equal to or very near to 0.1% [Warbrick et al., Citation1997]) and a factor of 3 for PTD (Aeby et al., Citation2004). The concentration of TDI was chosen based on doses used in other dermal exposure mouse models and shown to induce TH2 responses (van Och et al., Citation2000). These solutions were equivalent to 34.0 mM TDI, 55.5 mM PPD, and 27.2 mM PTD. All final dilutions were freshly prepared.
Animals and animal husbandry
Specific pathogen-free 6–8-week-old female BALB/c mice were obtained from Charles River Wiga GmbH (Sulzfeld, Germany) and acclimatized for 2 weeks in groups of 10 mice per Makrolon Type III cage maintained at 22 (± 3)°C, 30–70% relative humidity, and with a 12-h artificial light cycle. Treated animals were kept individually in Type I Makrolon cages. Mice were maintained on pellet standard diet (Harlan Winkelmann, Borchen, Germany) and water ad libitum. Granulated soft wood bedding from Harlan Winkelmann was used throughout. All animals were kept in accordance with the “Commission Regulation on guidelines for the accommodation and care of animals used for experimental and other specific purposes” and in alignment with the German Home Office recommendations and requirements.
Treatment of the animals and measurement of ear thickness
Animals were treated with PPD, PTD, TDI or the vehicle alone once daily on Day 0, 5, 10, 11, and 12. Treatment was performed by topical application of 25 µl of the formulated test substances or vehicle to both ears of the mice. The thickness of both ears of each mouse was recorded using a caliper on day 0 and on Days 5, 6, 7, 10, 11, and 12. On Day 14, blood samples were collected by retro-orbital puncture, centrifuged (800g), and the serum collected and stored at −80°C until analysis. The animals were sacrificed and their ears immediately excised, placed in vials containing RNA-later (Ambion Inc., Austin, TX), refrigerated for 24 h, and then frozen at −80°C for RNA extraction and cytokine analyses.
The auricular lymph nodes of the individual mice were also isolated. One lymph node per mouse was used for analysis of cell number and lymphocyte subpopulations. The lymph node was passed through a nylon cell strainer (40 µm, Falcon, BD Biosciences, Heidelberg, Germany), washed once in HBSS (Invitrogen, Karlsruhe, Germany), and re-suspended in 1 ml HBSS. The lymph node cells were then automatically counted using a TT-CASY-1 cell counter (Schärfe Systems, Reutlingen, Germany). The contra-lateral lymph node was treated with RNA-later (as described for the ears) and frozen at −80°C for later RNA extraction and cytokine analysis.
Serum IgE measurement
The sera of the treated animals were thawed immediately prior to analysis. Total IgE content was measured as described in the manufacturer’s instructions using a mouse IgE BD OptEIA™ ELISA Kit (BD Biosciences) and an ELISA-reader (Molecular Devices, Sunnyvale, CA). The detection limit in this assay was 1.6 ng/ml.
Lymphocyte population analysis of auricular lymph nodes
Antibodies were obtained from BD Biosciences. Aliquots (each 100 µl) of the lymph node samples, isolated as described above, were double-stained with a combination of α-CD3 (FITC-labeled, clone 145-2C11; 1:20 diluted) and α-CD4 (PE labeled, clone H129.19; 1:10 diluted), or α-CD8 (PE labeled, clone 53-6.7; 1:10 diluted). Another aliquot of each lymph node sample was double-stained with α-CD45R/B220 antibody (PE labeled, clone RA3-6B2, 1:10 diluted) and α-IgE antibody (FITC-labeled, clone R3572; 1:10 diluted). Samples were washed twice using CellWash and fixed with Cellfix (BD Biosciences). Population analysis was performed using a FACSCalibur flow cytometer and CellQuest software (BD Biosciences). Lymphocytes were gated according to their forward and sideward scatter characteristics. Approximately 5000 gated cells were evaluated for their surface expression of the respective markers.
mRNA expression
To perform mRNA analyses, frozen tissues (i.e., draining lymph nodes and ears) were homogenized with Tissue Lyser (Qiagen, Hilden, Germany) followed by treatment with DNAse. Total RNA (2–4 µg/sample) was translated into cDNA using a High Capacity Archive Kit (Applied Biosystems, Foster City, CA) and stored at −20°C. For mRNA quantification, 80 ng cDNA per reaction were used. All reactions were run in triplicate in a volume of 50 µl each using TaqMan Assays-on-Demand (Applied Biosystems). Additionally, for each target, three negative controls were run to detect contamination of genomic DNA. The housekeeping gene β-actin was used as endogenous control and the resulting data showed the relative mRNA expression according to the β-actin values and normalized to one random probe of the control group.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA). Significant differences from the control group were determined with the Mann–Whitney Test.
Results
Changes in ear thickness in mice treated with TDI, PPD, or PTD
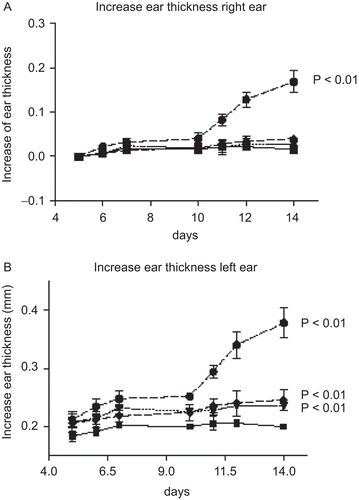
Changes in ear thickness compared to day 5 are shown in . There was a significant increase in ear thickness of both ears in animals treated with TDI starting at Day 6 to day 14 (p < 0.01). Animals treated with 0.6% PPD or PTD showed a smaller, but statistically significant, increase in ear thickness for left ears (p < 0.01). This increase in ear thickness was observed starting between days 7 and 10 to day 14.
Figure 1. The increase in ear thickness during the experimental period. ▪ vehicle (negative) control; ▾ 0.6% PPD; ♦ 0.6 % PTD; • 0.6% TDI. (A) Increase in right ear thickness; and, (B) increase in left ear thickness. Ear thickness at Day 0 was measured by a different technician than on the other days.
Auricular lymph node cell numbers from mice treated with TDI, PPD, or PTD
Control lymph nodes contained 2.87 (± 0.85) × 106 cells. In comparison, the data indicate that there was a modest, but not statistically significant, increase in the total number of cells recovered from the auricular lymph nodes of mice treated with 0.6% PPD and PTD (with 4.27 [± 1.91] × 106 cells and 4.38 [± 1.40] × 106 cells, respectively). However, there was a significant (ninefold) enhancement in total cell numbers recovered from the auricular lymph nodes of mice treated with TDI (26.5 [± 6.67] × 106 cells; p < 0.01).
Changes in lymphocyte subpopulations in auricular lymph nodes from TDI-, PPD-, and PTD-treated mice
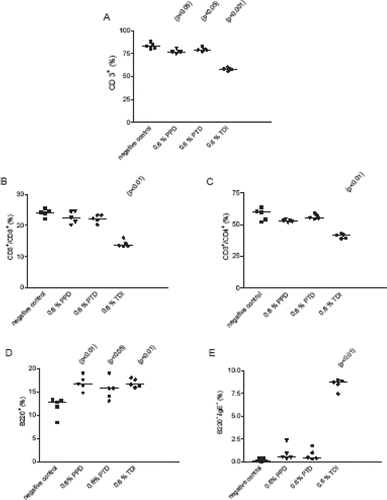
shows changes in lymphocyte subpopulations in auricular lymph nodes from mice treated with TDI, PPD, or PTD. Significantly fewer numbers of CD3+ T-cells (p < 0.001), CD8+ (p < 0.01), and CD4+ T-cells (p < 0.01) were observed in lymph nodes from TDI-treated mice compared to vehicle controls (, , and 2C). In contrast, there were statistically higher numbers of B220+ lymphocytes and B220+IgE+ cells (p < 0.01) in lymph nodes from the TDI mice compared to the controls ( and ). Lymph nodes from animals treated with 0.6% PPD or 0.6% PTD had significantly fewer CD3+ T-cells (p < 0.05) and significantly higher numbers of B220+ lymphocytes (p < 0.01) compared to the vehicle controls. In contrast to TDI, there were no differences in the CD8+, CD4+, or B220+IgE+ cell populations.
Figure 2. Flow cytometric analysis of auricular lymph node cells. Panel (A) CD3+ cells; panel (B) CD3+/CD4+ cells; panel (C) CD3+/CD8+ cells; panel (D) B220+ cells; and panel (E) B220+/IgE. • 0.6% TDI; ▾ 0.6% PPD; ♦ 0.6% PTD; ▪ vehicle control. The values are given for individual animals; the bars represent the corresponding median value. Where a result is statistically significantly different from the control, the p value is indicated.
Serum IgE levels in TDI-, PPD-, and PTD-treated mice
The total serum IgE levels from mice exposed either to PPD (94.8 ± 16.4 ng/ml) or to PTD (89.6 ± 20.4 ng/ml) were not different from the vehicle control animals (95.8 ± 3.0 ng/ml). In contrast, the total serum IgE level was significantly elevated in TDI-treated mice (2432 ± 737: p < 0.05) compared to concurrent vehicle-treated control animals.
Cytokine mRNA expression in the lymph nodes and ears from TDI-, PPD-, and PTD-treated mice
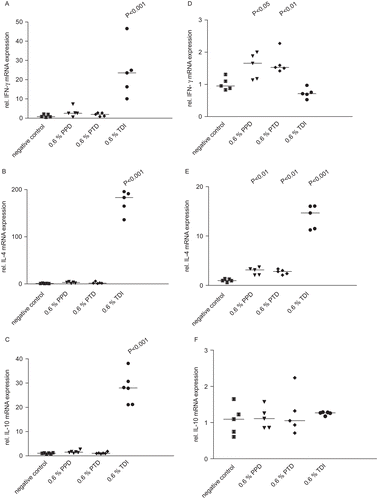
shows the relative mRNA expression for IFNγ, IL-4, and IL-10 in lymph nodes and ears from mice treated with TDI, PPD, or PTD. A significant elevation of mRNA expression for IFNγ, IL-4, and IL-10 was noted in ears taken from TDI-treated mice (p < 0.01), whereas only mRNA for IL-4 was elevated in lymph nodes. No change in IFNγ, IL-4, or IL-10 mRNA expression (compared to control) was noted in ear tissue taken from PPD- or PTD-treated mice. A small but significant increase in IFNγ and IL-4 mRNA expression was observed in lymph nodes from PPD- and PTD-treated mice. There was no change in IL-10 mRNA expression in the lymph nodes from these mice.
Figure 3. Relative IFNγ, IL-10, and IL-4 mRNA expression in lymph nodes (A, B, C) and ears (D, E, F). The values are given for individual cytokines IFNγ (A, D), IL-4 (B, E); and IL-10 (C, F). The results presented show the individual animal data and the median value. Where a result is statistically significantly different from the control, the P value is indicated. ▪ vehicle (negative) control; ▾ 0.6% PPD; ♦ 0.6 % PTD; • 0.6% TDI.
Discussion
We were not able to detect a TH2 response to PPD or PTD using the experimental conditions described in this article. Although the immune response to these dyes was not very robust, it was indicative of a TH1 response. In contrast, the immune response to TDI was clearly TH2 dominant, demonstrating that these experimental conditions were sufficient to detect such a response to a well-known allergen.
It is recognized that some of the chemicals in hair dyes can cause allergy in man, and although the extent to which this occurs has been the subject of some debate, it is evident that, overwhelmingly, such adverse reactions are a manifestation of cell-mediated (TH1) delayed-type hypersensitivity (Thyssen et al., Citation2009; Krasteva et al., Citation2009, 2010; Basketter et al., Citation2010). Only rare cases of Type 1 allergy responses following hair dye exposure were reported over a period of more than 20+ years (). One of these cases may have had a contact sensitization as well (Wong and King, Citation2003). Therefore, the likelihood that these cases were an evolution from a delayed-type to an immediate-type allergy is poor. Under experimental conditions, repeated topical application of the typical contact sensitizer dinitrochlorobenzene (DNCB) resulted in a TH2-like response in mice (Kitagaki et al., 1997). Furthermore, this phenomenon is not easily demonstrated in humans, although some reports in animal models describe IgE production after contact sensitization (e.g., Dearman et al., Citation1992, 1996; Kitagaki et al., Citation1995; Zhang et al., Citation1997; Hayashi et al., Citation2001; Plitnick et al., Citation2003). Once induced, the TH2 activity is known to promote immediate-type hypersensitivity reactions by the expression of IL-4, which promotes Ig class switching to and maintenance of an IgE antibody response (Finkelman et al., Citation1988).
Others demonstrated that five topical applications with the classic contact sensitizer DNFB in BALB/c mice induced hapten-specific IgE (Nagai et al., Citation1997). Among hair dye molecules, the most commonly suspected allergenic species are oxidation products of aromatic amines, such as PPD and PTD (Aeby et al., Citation2009; Basketter et al., Citation2010). These are readily identified in predictive assays as having the potential to give rise to cell-mediated (TH1) allergy (Warbrick et al., Citation1997; Aeby et al., Citation2004, Citation2009; Gerberick et al., Citation2005; Kern et al., Citation2010). However, there have been very occasional reports of an immediate-type hypersensitivity typically mediated by IgE antibodies (summarized in ). Consequently, in this present work, we have addressed whether it is possible to uncover any potential for PPD and PTD also to behave as immediate allergens, such that this rare type of adverse reaction could be anticipated. The study was not designed to assess potency but rather to detect evidence of a TH2 response.
As there is no standard predictive toxicology assay, a research-based approach shown previously to detect immediate hypersensitivity was adapted for the purpose (Dearman et al., Citation1996; Nagai et al., Citation1997). The BALB/c mouse was chosen since it can be used in the LLNA and it is generally used as the most sensitive (murine) system for the evaluation of TH2 responses (Mossman and Coffman, 1989; Woolhiser et al., Citation2000). The concentrations of TDI, PPD, and PTD chosen were based on information obtained from other studies: (1) a TH2 response to 2,4,6-trinitro-1-chlorobenzene was induced following repeated dermal exposure to concentrations of chemical in excess of the induction threshold for contact sensitization in the LLNA (Nagai et al., Citation1997); (2) the EC3 values for PPD and PTD in the LLNA are reported to be ~0.1%, so using the 0.6% dose is in excess of the EC3 (Warbrick et al., Citation1997, 2000; Aeby et al., Citation2004); and, (3) TDI and other isocyanates have been show to induce positive LLNA responses at doses between 0.1 and 0.3% (Dearman et al., Citation1992, 1996). Accordingly, we elected to use 0.6% for each of these allergens, considering these concentrations sufficient for induction of an immune response. These doses were not chosen for potency comparisons. In addition, we used the same vehicle as used in the EC3 study for PTD. This vehicle allowed for greater solubility of PPD and PTD and acetone is known to increase skin permeability (Tsai et al., Citation2001). Furthermore, it represented a vehicle system a little more akin to the matrix in which real-life exposure(s) would occur.
For TDI, animals treated with a concentration of 0.6% showed an increased ear thickness following repeated dermal exposure. The increase in ear thickness was evident over 5 days following challenge. There was an increase in the number of total lymph node cells and B220+/IgE+ B-cells, as well as elevated expression of the TH2 cytokine IL-4 vs. IFNγ mRNA in both lymph nodes and in ear skin, along with an increase in total serum IgE. Taken together, TDI induced a TH2-dominant immune response in BALB/c mice following repeated dermal exposures, consistent with published findings (de Vries et al., Citation1999; Dearman et al., Citation2003). In contrast, animals treated with PPD or PTD became weakly sensitized to these chemicals based on the ear swelling response upon challenge, the elevation of B220+ cells (Gerberick et al., Citation2002), and expression of IFNγ, IL-4, and IL-10 mRNA. We did note asymmetry in ear thickness increase which may simply reflect potential technical issues with measuring ear thickness or the modest extent of the immune response to PPD and PTD. Ear swelling due to irritation by the chemicals can be excluded due to the elevated B220+ cell number that will only be observed to this degree after sensitization and not after irritation (Betts et al., Citation2007). Furthermore, the test/vehicle ratio for B220+ cells for all the PPD- and PTD- as well as the TDI-treated animals was above the cutoff threshold of 1.25 for contact sensitizers (Gerberick et al., Citation2002; Betts et al., Citation2007), although a significant swelling was not observed in both ears. However, there was no evidence of a TH2-dominant immune response to PPD or PTD. In lymph nodes, no relevant increase in the number of IgE+B220+ cells was found but would have been expected for respiratory allergens (Manetz and Meade, Citation1999). Additionally, there was no elevation of total serum IgE antibody in PPD- and PTD-treated animals and the ratio of IL-4/IFNγ mRNA was not shifted toward IL-4. Taken together, our data indicate that five repeated topical applications of PPD or PTD concentrations exceeding the induction threshold for contact allergy did not induce a TH2 immune response but did induce a weak TH1 response. TDI performed as expected and clearly induced a TH2 response.
The regulatory cytokine IL-10 may also contribute to the differentiation between TH1 and TH2 immune responses. IL-10 is secreted by regulatory T-cells of two existing subgroups characterized by secretion of high levels of IL-10 or transforming growth factor (TGF)-β (Hori et al., Citation2003; Akdis et al., Citation2004). IL-10 may also facilitate development of TH2 responses, since it is known to inhibit TH1 cytokine production (Fiorentino et al., Citation1989). Additionally, IL-10 is involved in modulating cutaneous immune responses, including contact hypersensitivity (Ohmen et al., Citation1995; Wang et al., Citation1999). More specifically, it may affect the antigen-presenting function of Langerhans cells and dendritic cells, since IL-10 pre-treatment of antigen-presenting cells was found to promote TH2 cell activation (De Smedt et al., Citation1997; Morel et al., Citation1997). Cumberbatch et al. (Citation2005) demonstrated that chemical contact allergens and respiratory allergens exert differential effects on the kinetics of Langerhans cell activation and migration as a result of a differential cutaneous induction of IL-10. They concluded that immediate-type allergens may induce a cytokine environment that promotes the activation of dendritic cells with the ability to drive a TH2-type immune response that may lead to an immediate-type allergy.
In this study, TDI significantly shifted the ratio of IL-10/IFNγ gene expression toward IL-10. This was consistent with the induction of a TH2 immune response. No significant induction of the expression of IL-10 mRNA was observed in the ear tissue from PPD- and PTD-treated mice indicating that a cutaneous induction of IL-10 mRNA expression was not detectable at the timepoints selected for analysis. Neither PPD nor PTD had any effect on the IL-10 mRNA expression in the lymph nodes, again indicating that they do not promote a TH2 response.
In conclusion, we demonstrated that repeated topical applications of the oxidative hair dye precursors PPD and PTD could weakly sensitize mice under the experimental conditions described. There was no evidence that these chemicals could induce any tendency toward a TH2-dominant immune response in a mouse strain that is prone to develop TH2 responses. These data are consistent with the findings that hair dye precursors may induce delayed-type hypersensitivity (contact allergy) in susceptible consumers. The rare cases with immediate allergy symptoms among hair dye users could not be predicted from BALB/c mice under the described conditions and they are likely linked to idiosyncratic, but as yet unidentified, host factors.
Acknowledgements
The authors like to thank Naveed Honarvar for conducting the studies and technical discussions. We thank Julie Skare and Frank Gerberick for critical review of the manuscript. We thank David Basketter for his review and assistance in the preparation of the manuscript.
Declaration of Interest
The authors report no conflicts of interest. The authors are employees of The Procter and Gamble Company.
References
- Aeby, P., Wyss, C., Beck, H., Griem, P., Scheffler, H., and Goebel, C. 2004. Characterization of the sensitizing potential of chemicals by in vitro analysis of dendritic cell activation and skin penetration. J. Invest. Dermatol. 122:1154–1164.
- Aeby, P., Sieber, T., Beck, H., Gerberick, G. F., and Goebel, C. 2009. Skin sensitization to p-phenylenediamine: the diverging roles of oxidation and N-acetylation for dendritic cell activation and the immune response. J. Invest. Dermatol. 129:99–109.
- Akdis, M., Verhagen, J., Taylor, A., Karamloo, F., Karagiannidis, C., Crameri, R., Thunberg, S., Deniz, G., Valenta, R., Fiebig, H., Kegel, C., Disch, R., Schmidt-Weber, C. B., Blaser, K., and Akdis, C. A. 2004. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T-regulatory 1 and T-helper 2 cells. J. Exp. Med. 199:1567–1575.
- Arts, J. H., and Kuper, C. F. 2007. Animal models to test respiratory allergy of low molecular weight chemicals: A guidance. Methods 41:61–71.
- Ban, M., Morel, G., Langonné, I., Huguet, N., Pépin, E., and Binet, S. 2006. TDI can induce respiratory allergy with TH2-dominated response in mice. Toxicology 218:39–47.
- Basketter, D. A., Johansen, J. D., McFadden, J. P., and Sosted, H. 2010. Allergens of special interest: hair dyes. In: Contact Dermatitis, 5th Edition. (Johansen, J.D., Frosch, P., and Lepoittevin, J.P. Eds.) Berlin: Springer (In press).
- Betts, C. J., Dearman, R. J., Kimber, I., Ryan, C. A., Gerberick, G. F., Lalko, J., and Api, A. M. 2007. B220 analysis with the local lymph node assay: Proposal for a more flexible prediction model. J. Appl. Toxicol. 27:506–510.
- Boverhof, D. R., Billington, R., Gollapudi, B. B., Hotchkiss, J. A., Krieger, S. M., Poole, A., Wiescinski, C. M., and Woolhiser, M. R. 2008. Respiratory sensitization and allergy: Current research approaches and needs. Toxicol. Appl. Pharmacol. 226:1–13.
- Brown, K. C. 1997. Hair coloring. In: Hair and Hair Care (Johnson, D., Ed.), New York: Marcel Dekker, pp. 191–215.
- Cumberbatch, M., Clelland, K., Dearman, R. J., and Kimber, I. 2005. Impact of cutaneous IL-10 on resident epidermal Langerhans’ cells and the development of polarized immune responses. J. Immunol. 175:43–50.
- De Smedt, T., Van Mechelen, M., De Becker, G., Urbain, J., Leo, O., and Moser, M. 1997. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 27:1229–1235.
- de Vries, J. E., Carballido, J. M., and Aversa, G. 1999. Receptors and cytokines involved in allergic TH2 cell responses. J. Allergy Clin. Immunol. 103:S492–S496.
- Dearman, R. J., Basketter, D. A., and Kimber, I. 1996. Characterization of chemical allergens as a function of divergent cytokine secretion profiles induced in mice. Toxicol. Appl. Pharmacol. 138:308–316.
- Dearman, R. J., Warbrick, E. V., Skinner, R., and Kimber, I. 2002. Cytokine fingerprinting of chemical allergens: species comparisons and statistical analyses. Food Chem. Toxicol. 40:1881–1892.
- Dearman, R. J., Betts, C. J., Humphreys, N., Flanagan, B. F., Gilmour, N. J., Basketter, D. A., and Kimber, I. 2003. Chemical allergy: Considerations for the practical application of cytokine profiling. Toxicol. Sci. 71:137–145.
- Dearman, R. J., Mitchell, J. A., Basketter, D. A., and Kimber, I. 1992. Differential ability of occupational chemical contact and respiratory allergens to cause immediate and delayed dermal hypersensitivity reactions in mice. Int. Arch. Allergy Immunol. 97:315–321.
- Edwards, E. K., Jr, and Edwards, E.K. 1984. Contact urticaria and allergic contact dermatitis caused by paraphenylenediamine. Cutis 34:87–88.
- Finkelman, F. D., Katona, I. M., Urban, J. F., Jr, Holmes, J., Ohara, J., Tung, A. S., Sample, J. V., and Paul, W. E. 1988. IL-4 is required to generate and sustain in vivo IgE responses. J. Immunol. 141:2335–2341.
- Fiorentino, D. F., Bond, M. W., and Mosmann, T. R. 1989. Two types of mouse T-helper cell. IV. TH2 clones secrete a factor that inhibits cytokine production by TH1 clones. J. Exp. Med. 170:2081–2095.
- Fukunaga, T., Kawagoe, R., Hozumi, H., and Kanzaki, T. 1996. Contact anaphylaxis due to para-phenylenediamine. Contact Derm. 35:185–186.
- Gerberick, G. F., Cruse, L. W., Ryan, C. A., Hulette, B. C., Chaney, J. G., Skinner, R. A., Dearman, R. J., and Kimber, I. 2002. Use of a B-cell marker (B220) to discriminate between allergens and irritants in the local lymph node assay. Toxicol. Sci. 68:420–428.
- Gerberick, G. F., Ryan, C. A., Kern, P. S., Schlatter, H., Dearman, R. J., Kimber, I., Patlewicz, G. Y., and Basketter, D. A. 2005. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis 16:157–202.
- Goldberg, B. J., Herman, F. F., and Hirata, I. 1987. Systemic anaphylaxis due to an oxidation product of p-phenylenediamine in a hair dye. Ann. Allergy. 58:205–208.
- Hayashi, M., Higashi, K., Kato, H., and Kaneko, H. 2001. Assessment of preferential Th1 or Th2 induction by low-molecular-weight compounds using a reverse transcription-polymerase chain reaction method: Comparison of two mouse strains, C57BL/6 and BALB/c. Toxicol. Appl. Pharmacol. 177:38–45.
- Hori, S., Nomura, T., and Sakaguchi, S. 2003. Control of regulatory T-cell development by the transcription factor Foxp3. Science 299:1057–1061.
- Kawai, K., Kawai, K., Yasuno, H., and Shibazaki, K. 1990. Case report of anaphylactic shock from a hair dye. Rinsyou Hifuka 44:803–807.
- Kern, P. S., Gerberick, G. F., Ryan, C. A., Kimber, I., Aptula, A., and Basketter, D. A. 2010. Local lymph node data for the evaluation of skin sensitization alternatives: A second compilation. Dermatitis 21:8–32.
- Kitagaki, H., Fujisawa, S., Watanabe, K., Hayakawa, K., and Shiohara, T. 1995. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J. Invest. Dermatol. 105:749–755.
- Krasteva, M., Bons, B., Ryan, C., and Gerberick, G. F. 2009. Consumer allergy to oxidative hair coloring products: Epidemiologic data in the literature. Dermatitis 20:123–141.
- Krasteva, M., Bons, B., Tozer, S., Rich, K., Hoting, E., Hollenberg, D., Fuchs, A., and Fautz, R. 2010. Contact allergy to hair colouring products. The cosmetovigilance experience of 4 companies (2003-2006). Eur. J. Dermatol. 20:85–95.
- Kuck-Koot, N., and Lahey-de Boer, A. M. 2005. Anaphylactic reaction as a consequence of skin contact with dyestuffs in hair dyes. LN Dermatol Venereol. 15:355–358.
- Liippo, J., and Lammintausta, K. 2008. Contact sensitization to 4,4′-diaminodiphenylmethane and to isocyanates among general dermatology patients. Contact Derm. 59:109–114.
- Manetz, T. S., and Meade, B. J. 1999. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T cell-mediated hypersensitivity responses. Toxicol. Sci. 48:206–217.
- Matheson, J. M., Johnson, V. J., and Luster, M. I. 2005. Immune mediators in a murine model for occupational asthma: studies with toluene diisocyanate. Toxicol. Sci. 84:99–109.
- Morel, A. S., Quaratino, S., Douek, D. C., and Londei, M. 1997. Split activity of interleukin-10 on antigen capture and antigen presentation by human dendritic cells: Definition of a maturative step. Eur. J. Immunol. 27:26–34.
- Mosmann, T. R., and Coffman, R. L. 1989. Heterogeneity of cytokine secretion patterns and functions of helper T-cells. Adv. Immunol. 46:111–147.
- Nagai, H., Matsuo, A., Hiyama, H., Inagaki, N., and Kawada, K. 1997. Immunoglobulin E production in mice by means of contact sensitization with a simple chemical, hapten. J. Allergy Clin. Immunol. 100:S39–S44.
- Nishioka, K., Takahata, H., and Yasuno, H. 2001. Two cases of contact urticaria syndrome due to oxidative hair dyes. Environ. Dermatol. 8:88–93.
- Ohmen, J. D., Hanifin, J. M., Nickoloff, B. J., Rea, T. H., Wyzykowski, R., Kim, J., Jullien, D., McHugh, T., Nassif, A. S., and Chan, S. C. 1995. Over-expression of IL-10 in atopic dermatitis. Contrasting cytokine patterns with delayed-type hypersensitivity reactions. J. Immunol. 154:1956–1963.
- Pasche-Koo, F., French, L., Piletta-Zanin, P. A., and Hauser, C. 1998. Contact urticaria and shock to hair dye. Allergy 53:904–905.
- Plitnick, L. M., Loveless, S. E., Ladics, G. S., Holsapple, M. P., Smialowicz, R. J., Woolhiser, M. R., Anderson, P. K., Smith, C., and Selgrade, M. J. 2003. Identifying airway sensitizers: Cytokine mRNA profiles induced by various anhydrides. Toxicology 193:191–201.
- Taniguchi, T., Higashi, N., Kume, A., Miyamoto, T., Ogiwara, S., and Higami, K. 2000. A case of anaphylaxis due to p-toluenediamine in a hair dye. Jpn. J. Dermatoallergol. 8:7–11.
- Temesvari, E. 1984. Contact urticaria from paraphenylenediamine. Contact Derm. 11:125.
- Thyssen, J. P., Andersen, K. E., Bruze, M., Diepgen, T., Giménez-Arnau, A. M., Gonçalo, M., Goossens, A., Le Coz, C., McFadden, J., Rustemeyer, T., White, I. R., White, J. M., and Johansen, J. D. 2009. p-Phenylenediamine sensitization is more prevalent in central and southern European patch test centres than in Scandinavian: results from a multicentre study. Contact Derm. 60:314–319.
- Tsai, J. C., Sheu, H. M., Hung, P. L., and Cheng, C. L. 2001. Effect of barrier disruption by acetone treatment on the permeability of compounds with various lipophilicities: Implications for the permeability of compromised skin. J. Pharm. Sci. 90:1242–1254.
- Tsunoda, T., Horiuchi, N., and Sato, M. 1993. Two cases of contact urticaria syndrome by hair dye. Hifu 35:178–183.
- Tung, R. C., Taylor, J. S., and Wattanakarai, P. 1999. Immediate hypersensitivity to permanent hair color. J. Allerg. Clin. Immunol. 103:S155.
- van Och, F. M., Slob, W., de Jong, W. H., Vandebriel, R. J., and van Loveren, H. 2000. A quantitative method for assessing the sensitizing potency of low molecular weight chemicals using a local lymph node assay: Employment of a regression method that includes determination of the uncertainty margins. Toxicology. 146:49–59.
- Wang, B., Zhuang, L., Fujisawa, H., Shinder, G. A., Feliciani, C., Shivji, G. M., Suzuki, H., Amerio, P., Toto, P., and Sauder, D. N. 1999. Enhanced epidermal Langerhans cell migration in IL-10 knockout mice. J. Immunol. 162:277–283.
- Warbrick, E. V., Dearman, R. J., Lea, L. J., Basketter, D. A., and Kimber, I. 1997. Local lymph node assay responses to paraphenylenediamine: Intra- and inter-laboratory evaluations. J. Appl. Toxicol. 19:255–260.
- Warbrick, E. V., Dearman, R. J., Basketter, D. A., Gerberick, G. F., Ryan, C. A., and Kimber, I. 1998. Analysis of cytokine mRNA expression following repeated exposure of mice to chemical contact and respiratory allergens. J. Appl. Toxicol. 18:205–213.
- Wong, G. A., and King, C. M. 2003. Immediate-type hypersensitivity and allergic contact dermatitis due to para-phenylenediamine in hair dye. Contact Derm. 48:166.
- Woolhiser, M. R., Munson, A. E., and Meade, B. J. 2000. Comparison of mouse strains using the local lymph node assay. Toxicology 146:221–227.
- Zhang, Y., Lamm, W. J., Albert, R. K., Chi, E. Y., Henderson, W. R., Jr, Lamm, W. J., Albert, R. K., Chi, E. Y., Henderson, W. R., and Lewis, D. B. 1997. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am. J. Respir. Crit. Care Med. 155:661–669.