Abstract
Teucrium ramosissimum (Lamiaceae), a native and endemic plant from South Tunisia, has traditionally been used as a treatment for inflammation and for ulcers. Though the plant and its products are widely used, very few studies have analyzed the pharmacological/toxicological properties of this plant. Thus, the aim of these studies was to evaluate the anti-inflammatory/anti-ulcerogenic activities of various extracts (i.e., methanolic, aqueous, and total oligomer flavonoid [TOF]-enriched) from leaves of T. ramosissimum. In vitro, the effects from each extract on lysosomal enzyme activity and proliferation of, respectively, freshly isolated peritoneal macrophages and splenic lymphocytes were assessed. The extracts alone clearly affected macrophage function, as evidenced by a significant modulation of cell lysosomal enzyme activity and ability to form and/or release nitric oxide. These extracts were also found to be able to significantly modify the proliferation of splenocytes, even when lipopolysaccharide or lectin mitogens were absent. With respect to the anti-ulcerogenic activity of the extracts, these studies found that the leaf extracts were able to exert significant protective effects against ethanol-induced ulcers in a rat model; at some doses, the extract effects were even greater than that obtained using a cytoprotective histamine H2-antagonist, cimetidine. Based on these studies, we conclude that the extracts from T. ramosissimum appear to be potentially potent modulators of innate immunity and that their efficacy against ulcer formation may be due, in part, to a cytoprotective effect. Further, these results fortify the ethnopharmacological importance of the use of T. ramosissimum products as anti-inflammatory and anti-ulcer agents. Nevertheless, ongoing/further studies are needed to clarify more precisely mechanisms underlying effects against ulcers and on lymphocyte and macrophage functionality, as well as the causative agents.
Introduction
Inflammation or phlogosis is a pathophysiological response of mammalian tissues to a variety of hostile agents including infectious organisms, toxic chemical substances, physical injury or tumor growth leading to local accumulation of plasmic fluid and blood cells (Sobota et al., Citation2000). Although inflammation is a defense mechanism, the complex events and mediators involved in the inflammatory reaction can induce, maintain and aggravate many disorders. Hence, the employment of anti-inflammatory agents may be helpful in the therapeutic treatment of those pathologies associated with inflammatory reactions (Sosa et al., Citation2002).
The clinical treatment of inflammatory diseases is dependent on drugs that belong either to the nonsteroidal or steroidal chemical therapeutics. The nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin, indomethacin, and ibuprofen inhibit early steps in the biosynthesis pathway of prostaglandins by inhibition of cyclo-oxygenase (COX) enzymes and are the main drugs used to reduce the untoward consequences of inflammation (Albert et al., Citation2002). However, the side effects of the currently available anti-inflammatory drugs pose a major problem in their clinical use. For instance, NSAIDs cause several serious adverse effects like gastric injury and ulceration, renal damage, and bronchospasm due to their nonselective inhibition of both isoforms of the COX enzyme (Tapiero et al., Citation2002). The use of steroidal drugs as anti-inflammatory agents is also becoming highly controversial due to their multiple side effects (van den Worm et al., Citation2001). Therefore, a need arises for the development of newer anti-inflammatory agents from natural sources with more powerful activity and with lesser side effects as substitutes for chemical therapeutics.
A recent review of references indicates that the anti-ulcerogenic effects of many taxa of medicinal plants have been assessed worldwide (Yesilada and Gürbüz, Citation2003). Although in most cases the etiology of ulcer is unknown, it is generally accepted that it results from an imbalance between aggressive factors such as acid and pepsin and the maintenance of mucosal integrity through endogenous defense mechanisms (Wallace and Granger, Citation1996). There are many products used for the treatment of gastric ulcers, such as antacids, proton pump inhibitors, or anti-histaminic agents, but most of these drugs produce several adverse reactions (Brunton, Citation1996). Thus, there is a need for more effective and less toxic anti-ulcer agents. Different therapeutic agents, including plant extracts, are used to inhibit gastric acid secretion or to boost mucosal defense mechanisms by increasing mucus production, stabilizing surface epithelial cells, or interfering with prostaglandin (PG) synthesis (Lewis and Hanson, Citation1991). Plants are some of the most attractive sources of new drugs, and have been shown to produce promising results for the treatment of gastric ulcer (Alkofahi and Atta, Citation1999). The literature reveals that plants contain a large diversity of natural products that have demonstrated anti-ulcerogenic properties (Lewis and Hanson, Citation1991).
Teucrium species have been used medicinally since ancient Greek times as analgesic, hypoglycemic, hypolipidemic, anti-pyretic, anti-inflammatory, and anti-ulcer agents (Tariq et al., Citation1989; Barrachina et al., Citation1995; Saracoglu et al., Citation1997; Bellomaria et al., Citation1998; Abdollahi et al., Citation2003; Panovska et al., Citation2007). From the nonpharmacologic perspective, Maccioni et al. (Citation2007) has noted the importance of Teucrium species as edible plants, as some of them are currently used in the preparation of flavored wines, herbal teas, bitters, and liqueurs; infusions of the leaves and flowers are used for flavoring beers in some areas. The importance of this genus in the food industries lies also in the fact that many species display anti-microbial, anti-oxidant, and anti-fungal activities, rendering them useful as natural preservative ingredients (Ulubelen et al., Citation2000; Özkan et al., Citation2007; Saroglou et al., Citation2007).
The aim of this work was to investigate the in vitro immunomodulatory activities of plant leaf extracts from Teucrium ramosissimum on mouse lymphocyte proliferation and peritoneal macrophage inflammatory functions. We also investigated the same extracts for their anti-ulcerogenic property.
Materials and methods
Plant material
The aerial part of Teucrium ramosissimum was collected in January 2005 from the mountainous region of Gafsa in Southeast Tunisia. The plant was identified by Professor. Mohamed Chaieb (Department of Botany, Faculty of Sciences, University of Sfax, Sfax, Tunisia) according to the Flora of Tunisia (Pottier-Alapetite, Citation1979). A voucher specimen (Tr-02-05) was deposited at the Herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, University of Monastir in Tunisia, for future reference. The leaves were shade dried, powdered, and stored in a tightly closed container at ambient temperature (in darkness) for future use.
Preparation of extracts
Methanol (MeOH) extract was obtained by Soxhlet extraction (4 h) using 100 g of the powdered leaves and 1 L of solvent (Normapur, VWR International Ltd., Leighton Buzzard, UK). This extract was then concentrated to dryness and the residue was kept at 4°C. In order to obtain an extract enriched in total oligomer flavonoids (TOF), the powdered leaves were macerated in water-acetone mixture (1:2) for 24 h, with continuous stirring. The extract was then filtered and the acetone was evaporated under low pressure in order to obtain an aqueous phase. Tannins were partially removed by precipitation with an excess of NaCl during 24 h at 5°C, and the supernatant was recovered. The latter was extracted with ethyl acetate, concentrated, and precipitated with an excess of chloroform. The precipitate was separated and yielded the TOF extract, which was then dissolved in water (Ghedira et al., Citation1991).
The fresh leaves of T. ramosissimum were dried at room temperature and reduced to coarse powder. One hundred grams of the powdered leaves were extracted with boiling water (1 L) for 15–20 min. After filtration, the crude extract obtained was frozen and lyophilized, leading to the aqueous extract that was dissolved in water.
Animals
Specific pathogen-free BALB/c mice (6–8-week-old males, 18–22 g) were obtained from the Pasteur Institute (Tunis, Tunisia). The animals were housed in polypropylene cages in an air-conditioned area at 25 (± 2)°C with 12-h light:12-h dark cycle, and provided a standard pellet diet and clean drinking water ad libitum. All animal experiments (including the later-described ones using rats) were performed in accordance with the guidelines for the care and use of laboratory animals published by the United States National Institutes of Health.
Cell preparation from mice
Splenic lymphocytes were obtained as previously reported (Harizi et al., Citation2001). Briefly, the mice were euthanized by cervical dislocation and their spleens aseptically removed and homogenized by mincing with sterile forceps. Splenocytes were then isolated by centrifugation (1500 rpm, 10 min) and red blood cells present in the pellet lysed by incubation in lysing buffer (144 mM NH4Cl, 1.7 mM Tris base, [pH 7.4]) for 5 min at 4°C. The remaining cells were then washed twice with phosphate-buffered saline (PBS) and then resuspended in complete RPMI medium (RPMI 1640 medium bearing 10% fetal bovine serum and 100 µg gentamycin/ml; all materials from Gibco-BRL, Paisley, UK).
Some mice were used to provide peritoneal macrophages by methods we have previously reported (Harizi et al., Citation2001; Limem et al., Citation2011). Immediately after sacrifice, peritoneal cells were obtained by exposing and then lifting of the peritoneal wall, injection of 4 ml sterile PBS, massaging of the peritoneum, and slowly withdrawing of the fluid (≈ 4 ml) back into the syringe. Cells were washed twice by centrifugation (1500 rpm, 10 min, 4°C) and then resuspended in complete RPMI 1640 medium. The cell number was determined by counting in a hemocytometer and cell viability was evaluated/confirmed using the trypan-blue dye exclusion technique.
Nitrite assay
Nitrite levels were measured using the diazotization method based on the Griess reaction, which is an indirect assay for nitric oxide (NO) production as previously described by Green et al. (Citation1982). Cells were incubated with 5 µg lipopolysaccharide/ml (LPS, rough strain, Escherichia coli EH100, Sigma Aldrich, Hamburg, Germany; positive control) or in the presence of increasing concentrations of leaf extracts. Nitrite was measured by adding 100 µl Griess reagent (1% sulphanilamide and 0.1% naphthylenediamine in 5% phosphoric acid) to 100 µl culture supernatant. The optical density at 540 nm (OD540) was then measured with a microplate reader (Multiskan FC, Thermo Scientific, Braunschweig, Germany). The concentration of nitrite in each sample was calculated by comparison with the OD540 of standard solutions of sodium nitrite that were prepared in parallel in culture medium.
Assessment of lysosomal enzyme activity
Measurements of acid phosphatase (AP) were used to assess cellular lysosomal enzyme activity in macrophages, as previously described (Manosroi et al., Citation2005) with minor modification. Briefly, macrophage suspensions (100 µl, at 6 × 106 cells/ml) were seeded into a flat-bottom 96-well plate, and 100 µl of plant extracts (dissolved in complete RPMI medium) were added to each well to obtain final concentrations of 1, 25, 50, or 100 µg/ml. Control cells consisted of macrophages treated with RPMI alone. The cultures were then incubated at 37°C in a 5% CO2 humidified atmosphere for 24 h. The medium was then removed by aspiration and 20 µl of 0.1% Triton-X100 (Sigma Chemical, St. Louis, MO), 100 µl p-nitrophenyl phosphate solution (Sigma; 100 mM), and 50 µl citrate buffer (pH 5.0, 0.1 M) were added to each well. The plate was incubated a further 30 min at 37°C; 150 µl borate buffer (0.2 M, pH 9.8) was then added and the absorbance was measured at 405 nm. The percentage of lysosomal enzyme activity was calculated as previously reported (Manosroi et al., Citation2003) by the following equation:
In vitro proliferation assay
Lymphocyte proliferation was assessed using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Mossmann, Citation1983). Aliquots (100 µl) of splenocytes (3 × 106 cells/ml) were placed into wells of a 96-well plate and preincubated in RPMI 1640 medium for 24 h before the addition of the mitogens LPS or lectin each at 5 µg/ml (FITC conjugate, Bandeiraea simplicifolia, Sigma) alone or with increasing concentrations of extract (e.g., 1, 10, or 100 µg/ml) solubilized in RPMI. Controls for the LPS- or lectin-only groups were cells incubated in RPMI 1640 medium only. Controls for the various extracts were the LPS- or lectin-only groups. All cells were then incubated at 37°C in humidified 5% CO2 atmosphere for an additional 24 h. After centrifugation at 1500 rpm for 10 min, each cell pellet was re-suspended in 50 µl of MTT (5 mg/ml in RPMI media) and incubated a further 4 h. The medium was then removed after centrifugation and 100 µl dimethyl sulfoxide (neat) was added. Absorbance in each well was measured at 570 nm and percentage proliferation calculated using the following equation (Green et al., Citation1982):
Anti-ulcerogenic activity
Gastric lesions were produced in albino rats of both sexes (120–180 g; n = 6/treatment group) according to the method of Robert et al. (Citation1979). Twenty-four hours before the procedure, food was withdrawn but free access to water was still allowed. For these studies, the test samples were delivered at 200 and 300 mg/kg body weight (BW) as a suspension in physiological water; control rats were given vehicle and received the same experimental handling as those in the test groups. The cytoprotective drug cimetidine (at 100 mg/kg) was used as a reference compound. Each dose of test sample was administrated by oral gavage 30 min before an oral application of a 96% ethanol solution (5 ml/kg corporal weight) to each group of six rats. Sixty minutes later, all animals were euthanized via cervical dislocation. The stomach of each rat was removed and inflated with 2 ml of a 5% formalin solution; after 30 min, the organ was opened along the greater curvature, rinsed with physiological water to remove gastric contents and blood clots, and examined under a dissecting microscope to assess the ulcer formation. The sum of length (mm) of all lesions for each stomach was used as the ulcer index (UI). The percentage inhibition of ulceration was calculated by the following formula:
Statistical analysis
All experiments were performed in triplicate and the results were expressed as mean ± SD. A Student’s t-test was used to analyze statistical significance of the differences between the control and the treated values. p values less than 0.05 were considered significant.
Results
Effects of the extracts on NO production
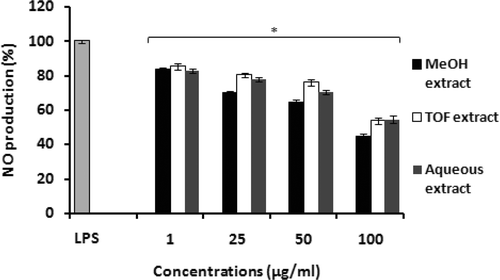
The anti-inflammatory activity of leaf extracts from Teucrium ramosissimum was studied in vitro by analyzing their inhibitory effects on the release of chemical mediators like NO from macrophages. Treatment of peritoneal macrophages with LPS (5 µg/ml) for 24-h induced NO production (assessed by measuring accumulation of nitrite, a stable metabolite of NO, using the Griess reaction) in the media. The beneficial anti-inflammatory effect of extracts from T. ramosissimun on the inhibition of production of NO by macrophages is shown in . Incubation of peritoneal macrophages with T. ramosissimum extracts induced a significant inhibitory effect on LPS-induced NO production. The extract that most effectively inhibited NO production was the methanol extract, with a value that reached only 44.27% of that from LPS alone (standardized to 100%) at the highest concentration tested (100 µg/ml). The inhibitory potency of TOF and the aqueous extracts were lower; production values of 53.52 and 54.50%, respectively, were noted with these extracts at a concentration of 100 µg/ml (). These extracts – even up to a concentrations of 100 µg/ml – were not cytotoxic to the macrophages; viabilities of >85% were routinely noted for each extract.
Figure 1. Production of nitrite by mouse peritoneal macrophages in response to tested extracts from T. ramosissimum. Macrophages (6 × 106 cells/well) were incubated in microtiter plates in RPMI 1640 medium in the presence of increasing concentrations of extracts for 24 h. Cells treated with lipopolysaccharide (5 µg LPS/ml) alone were used as a positive control. Data plotted represent the mean (± SD) percentage of NO production of three independent experiments. Results are expressed as percentage of NO production. Cells incubated in RPMI 1640 medium alone did not produce NO. Each value represents the mean (± SD) of triplicate samples compared to the control; *p < 0.05.
Assessment of cellular lysosomal enzyme activity
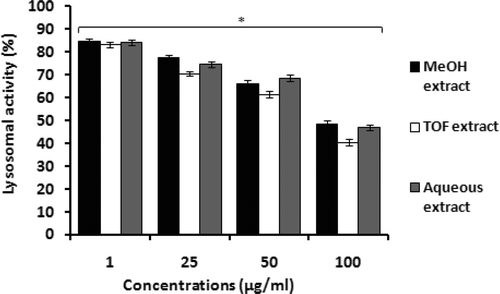
As macrophages play a critical role in immune defense against various infectious agents because of their secretory and phagocytic functions, mouse peritoneal macrophages were used to analyze effects of T. ramosissimum leaf extracts on the secretory endpoint. As shown in , all tested extracts markedly affected macrophage lysosomal enzyme activity in a somewhat dose-related manner. Use of 1 µg/ml of the methanol, aqueous, or TOF extracts stimulated lysosomal enzyme activity by 84.74%, 84.18%, and 83.19%, respectively. At concentrations ≥25 µg/ml, the aqueous extract appeared to be the least effective extracts for stimulating lysosomal enzyme activity. In fact, at higher concentrations, all extracts appeared to have increasingly less and less capacity to induce macrophage lysosomal function.
Figure 2. Stimulation of mouse peritoneal macrophage lysosomal enzyme activity by tested extracts from T. ramosissimum. Macrophages (6 × 106 cells/well) were incubated in microtiter plates in the presence of increasing concentrations of extracts for 24 h. Controls included cells incubated with RPMI 1640 medium only. Lysosomal enzyme activity was assessed as indicated in the “Materials and methods.” Data plotted represent the mean (± SD) percentage of lysosomal enzyme activity of three independent experiments. Each value represents the mean (± SD) of triplicates samples compared to the control; *p < 0.05.
Mitogen-induced splenocyte proliferation
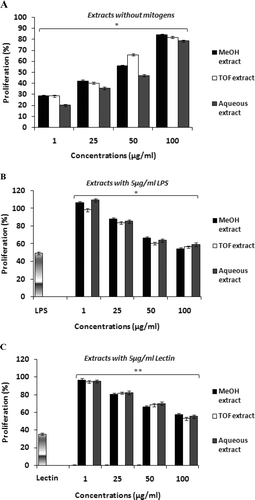
Lymphocyte-mediated immunity plays an important role in cellular and humoral immune responses. As the capacity to elicit an effective T- and B-lymphocyte response is ultimately dependent of the proliferative capacity of these cells, the effects of T. ramosissmum extracts on lymphocyte proliferation (in absence and presence of mitogen) was evaluated using splenocytes from naïve hosts (–). Each extract alone demonstrated an ability to induce proliferation; these effects appeared to be dose related (. Mitogen-induced splenocyte proliferation seems to be enhanced by extracts in an inversely dose-depending manner ( and ). At 1 µg/ml, the aqueous, TOF-enriched, and methanol extracts caused the maximum proliferative enhancement in the presence of LPS, with proliferation percentages of 109.51, 98.30, and 106.23%, respectively. In contrast, in the presence of lectin, 1 µg/ml of each extract enhanced cell proliferation to 94.95, 94.62 and 96.72%.
Figure 3. In vitro effects of tested extracts from T. ramosissimum on proliferation response of splenocytes: (A) cells incubated with increasing concentrations of extracts without mitogens for 24 h, (B) cells incubated with lipopolysaccharide (5 µg LPS/ml) in the absence and presence of extracts for 24 h, and (C) cells treated with lectin (5 µg/ml) in the absence and the presence of extracts for 24 h. Controls for the LPS- or lectin-only groups were cells incubated in RPMI 1640 medium only; controls for the extract groups were the LPS- or lectin-only groups. Cell proliferation was assessed by an MTT test. Data plotted represent the mean (± SD) percentage of proliferation (calculated using equation presented in section on “In vitro proliferation assay” above) of three independent experiments. **p < 0.01 and *p < 0.05; percentage of proliferation that is significantly different compared with mitogen (LPS or lectin)-only-treated cells.
Ethanol-induced gastric ulceration
All tested extracts significantly protected rats from ethanol-induced ulcers (). There were significant (p < 0.001) dose-related reductions in the ulcer indices relative to those seen in control animals. The aqueous extract of T. ramosissimum appeared to possess the highest protective effect, that is, 100% inhibition against ethanol-induced ulcerogenesis, at a dose of 300 mg extract/kg. At a comparable dose, the next most potent anti-ulcerogenic effect was noted for the methanol extract (88.80%), followed by the TOF extract (81.94%). While use of either dose of each extract (i.e., 200 or 300 mg/kg) led to significant reductions in the Ulcer Index Lesion values, there was no significant difference between the numbers of mucosal lesions found in animals treated with extracts in comparison to that in those treated with cimetidine.
Table 1. Effects of leaf extracts from T. ramosissimum on ethanol-induced gastric ulcer in rats.
Discussion
This study focused on how extracts from a family of medicinal plants can modulate immune cell functions, such as macrophage secretory function and lymphocyte proliferation, to bring about reported anti-inflammatory and anti-ulcerogenic effects. During inflammatory responses, both as part of normal anti-pathogen (Manosroi et al., Citation2005) and in some cases aberrant responses (i.e., allergy; Benjamini et al., Citation2000), macrophages release a slew of toxic products, including free radicals and NO, and release several lysosomal enzymes, including AP (alkaline phosphatase). Compared with LPS, which is a potent inducer of macrophage NO release (MacMicking et al., Citation1997), it was shown here that the methanol, aqueous, and TOF extracts from T. ramosissimum inhibited macrophage release of NO. While such results could suggest interference with the ability of the macrophages to interact with/respond to LPS, it is equally plausible that there might be bioactive molecules in the extract that lead to reductions in NO formation directly (i.e., scavenging effects) or by impacting on the nitric oxide synthase system (NOS); further studies are ongoing to help clarify this matter.
The inhibitory effects on NO release by the methanol, aqueous and TOF extracts from T. ramosissimum appeared to be more important when compared to other Teucrium species. For example, the essential oil from T. flavum induced >92% inhibition of NO production at a dose of 200 µg/ml (Menichini et al., Citation2009). The ethanolic extract (at a dose of 500 mg/kg BW) of T. polium, produced significant inhibition of carrageenan-induced inflammation and cotton-pellet granuloma (Tariq et al., Citation1989). The essential oil of the same species was found to be responsible for the analgesic properties of the plant when studied in a pain model in mice (Abdollahi et al., Citation2003). Though less active, the oil from T. flavum, more specifically its MeOH and methylene chloride (CH2Cl2) extracts, still caused significant anti-inflammatory effects against carrageenan-induced edema in rats (Barrachina et al., Citation1995). A survey of the literature reveals that other Teucrium spp. have potent anti-inflammatory activity against both the acute and delayed phases of inflammation (Fernández Puntero et al., Citation1997).
The capacity to elicit an effective T- and B-lymphocyte response is ultimately dependent of the proliferative capacity of each of these cells. These cells, especially T-lymphocytes, are also key to triggering inflammatory responses, in part, by their abilities to release factors that ultimately activate macrophages. Mitogens have routinely been used to determine the effects of agents like the extracts to impact on lymphocytic functions (Nakamura et al., Citation1986). LPS is used for T-independent B-lymphocyte proliferation whereas lectin is used for T-lymphocyte proliferation. In the studies here, the extracts – with and without LPS or lectin – produced different effects on splenocyte proliferation. As each extract alone enhanced cell proliferation activity, as well as when the cells were in the co-presence of LPS or lectin, we conclude that both B-lymphocytes and T-lymphocytes were sensitive to the extracts.
The results with the lowest extract dose in the LPS studies, along with the data derived from the mitogen-free studies, suggest the leaf extracts may contain some mitogenic substrates capable of stimulating cell proliferation. A presence of LPS/other mitogens in these plant extracts argues in favor of this explanation. Such mitogens have been reported by other investigators to possess a wide range of anti-tumor, immunomodulatory, and anti-oxidation properties (Benjamini et al., Citation2000; Ben Ammar et al., Citation2007; Jiménez-Medina et al., Citation2008). Nevertheless, the data with the extracts alone (see still seem incongruous with the other findings reported here; apart from a potential “mitogen presence” in the plant extracts themselves, it remains uncertain as to how this “stimulation” is occurring.
These ex vivo/in vitro studies clearly showed that the extracts had the potential to act as anti-inflammatory agents in situ. To test this hypothesis, we examined the anti-ulcerogenic effects of the extracts. There is a basis for expecting such effects; Galati et al., (Citation1997, Citation2000) and Fernandez Puntero et al. (1997) each have described the utility of Teucrium genus products to treat gastric disorder. In fact, the results of the studies of protection against ulcer formation in situ here were found to be consistent with those observed by Aguwa (Citation1985), Ezaki et al. (Citation1985), Aguwa and Lawal (Citation1988), Vilegas et al. (Citation1999), Leite et al. (Citation2001), Gonzalez and Di Stasi (Citation2002) and Khennouf et al. (Citation2003) who each examined the anti-ulcerogenic properties of several types of natural products/by-products (e.g., Calliandra portoricensis, Rhigiocarya racemifera, Linderae umbellatae, Wilbrandia ebracteata, Maytenus ilicifolia, M. aquifolium, M. aquifolium, Quercus suber, and Q. coccifera). In each case, those Authors suggested that tannins, flavonoids, and polyphenols found in the natural products they examined may have been the agent(s) that imparted the observed anti-ulcerogenic effects, albeit by (as-yet) undefined mechanisms.
In the studies here, ethanol (EtOH) was used to induce ulcers in the stomachs of rats. EtOH-induced ulcers are due to a direct necrotizing effect on the gastric mucosa (Miller and Henagan, Citation1984). Specifically, EtOH causes necrosis of superficial epithelial cells in the mucosa (Oates and Hakkinen, Citation1988) and, consequently, erosion. It is accepted that EtOH-induced ulcers are not only inhibited by anti-secretory agents such as cimetidine, but also by agents that enhance mucosal defensive factors (Wolfe and Sachs, Citation2000). Based on our findings here, it is plausible to suggest there might be some similar “cytoprotective” agents in T. ramosissimum extracts. Such findings would be consistent with those by Souza-Formigoni et al. (Citation1991) and by Antônio and Souza-Brito (Citation1998) who each compared the anti-ulcerogenic activity of lyophilized infusions of, respectively, M. ilicilifolia and M. qualifolium and of Turnera ulmifolia to that of cimetidine, In each case, the precise mechanisms underlying the observed protective effects of those natural products remains unresolved. It has been suggested by others that agents (potentially tannins, polyphenols, and/or flavonoids) impart a measure of gastric mucosa protection through effects on prostaglandin formation (Rainsford, Citation1989), inhibition of mast cell degranulation (Cho et al., Citation1976) or proton pumps (Bossolani, Citation2000), and/or stabilization of lysosomal membranes (Pfeiffer et al., Citation1987). Ongoing studies in our laboratories seek to more precisely define the mechanisms – as well as the causative agent(s) – through which our T. ramosissimum extracts imparted their anti-ulcerogenic effects in situ.
There is an additional potential means by which the T. ramosissimum extracts helped to protect against ulcer formation. Gastric mucus is believed to play an important role in the defense against ulceration (Martin et al., Citation1991). The protective effect of mucus as an active barrier may be attributed to the presence of glycoproteins that have the property of holding water in the interstices, thereby obstructing hydrogen ion diffusion. Thus, any increase in the synthesis of mucus, as suggested by studies of Jorge et al. (Citation2004), would be consistent with those found by some investigators (Alarcón de la Lastra et al., Citation1996; Sairam et al., Citation2002) using other drugs (i.e., cisapride) or plant (i.e., Emblica officinallis) extracts. Once again, ongoing studies in our laboratory seek to ascertain if the T. ramosissimum extracts do in fact cause changes in gastric mucus content/formation.
In these studies, we have described both anti-inflammatory and anti-ulcerogenic effects observed in vitro, ex vivo, and in situ. We believe that these activities of the extracts tested here may be ascribed to polyphenols and flavonoids that are present in the samples (Virgili et al., Citation1998; Ho et al., Citation2007; Terra et al., Citation2007; Ben Sghaier et al., Citation2011a, Citation2011b). Flavonoids and polyphenols are a class of naturally occurring agents found in plants and commonly consumed in the human diet (Middleton et al., Citation2000; López-Posadas et al., Citation2008). Many types of phenolic compounds have been previously shown either to stimulate or suppress the immune system (Labadie, Citation1993). A presence of polyphenols, tannins, and flavonoids was clearly seen in phytochemical evaluations of the three T. ramosissimum extracts employed here (Ben Sghaier et al., Citation2011a, Citation2011b). Specifically, those studies showed that total polyphenols were present in 1 mg of the methanol and in 1 mg of the aqueous extracts (originally derived from 100 g of T. ramosissimum leaves) at 120.0 (± 5.5) and 121.7 (± 4.8) gallic acid (i.e., in µg) equivalents; in 1 mg of TOF extract (originally derived from 200 g of T. ramosissimum leaves), the levels were 315.0 (± 9.0) equivalents. With respect to flavonoids, levels of 565.0 (±10.5) and 320.0 (± 8.5) quercetin equivalents (i.e., in µg) were found, respectively, in these methanol and aqueous extracts, while the TOF fraction contained 662.5 (± 13.0) equivalents. Last, for the tannins, the methanol and aqueous extracts contained, respectively, 164.0 (± 5.8) and 99.4 (± 2.8) tannic acid equivalents (i.e., in µg), while the TOF had near-undetectable levels of these agents. As noted above, ongoing studies in our laboratories seek to more precisely define which is\are the causative agent(s) through which the T. ramosissimum extracts imparted their anti-inflammatory/ulcerogenic effects.
Taking the results of these studies in total, we conclude that the tested extracts from T. ramosissimum appear to be potent modulators of innate immunity and their efficacy in ulcer models mainly may potentially be due to a direct cytoprotective effect or possibly via modulation of mucus formation in the stomach. Ongoing/further studies will help us to clarify more precisely mechanisms underlying the effects against ulcers as well as those on lymphocyte and macrophage functionality. Even in the absence of specific defined mechanisms or identification of the bioactive agent(s) responsible, our results fortify the ethnopharmacological importance of T. ramosissimum extracts as anti-inflammatory and anti-ulcer agents.
Acknowledgments
The authors thank Prof. Boukattaya Samir, Faculty of Dental Medicine, Monastir, Tunisia, for English editing and Prof. Chouchene Lotfi (Laboratory of immune-oncology, Faculty of Medicine, Monastir) for cell culture manipulations.
Declaration of interest
The authors report no conflicts of interest. The authors are alone responsible for the content and writing of the paper.
References
- Abdollahi, M., Karimpour, H. and Monsef-Esfehani, H. R. 2003. Antinociceptive effects of Teucrium polium L total extract and essential oil in mouse writhing test. Pharmacol. Res. 48:31–35.
- Aguwa, C. N. 1985. Gastrointestinal effects of the extracts of Rhigiocarya racemifera (Menispermaceae). Gen. Pharmacol. 16:387–390.
- Aguwa, C. N. and Lawal, A. M. 1988. Pharmacologic studies on the active principles of Calliandra portoticensis leaf extracts. J. Ethnopharmacol. 22:63–71.
- Alarcón de la Lastra, C., Martin, M. J., La Casa, M. C., López, A., and Motilva, V. 1996. Effects of cisapride on ulcer formation and gastric secretion in rats: Comparison with ranitidine and omeprazol. Gen. Pharmacol. 27:1415–1420.
- Albert, D., Zündorf, I., Dingermann, T., Müller, W. E., Steinhilber, D. and Werz, O. 2002. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem. Pharmacol. 64:1767–1775.
- Alkofahi, A. and Atta, A. H. 1999. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J. Ethnopharmacol. 67:341–345.
- Antônio, M. A. and Souza Brito, A. R. 1998. Oral anti-inflammatory and anti-ulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae). J. Ethnopharmacol. 61:215–228.
- Barrachina, M. D., Bello, R., Martinez-Cuesta, M. A., Esplugues, J., and Primo-Yufera, E. 1995. Anti-inflammatory activity and effects on isolated smooth muscle of extracts from different Teucrium species. Phytother. Res. 9:368–371.
- Ammar, R. B., Bouhlel, I., Valenti, K., Sghaier, M. B., Kilani, S., Mariotte, A. M., Dijoux-Franca, M. G., Laporte, F., Ghedira, K. and Chekir-Ghedira, L. 2007. Transcriptional response of genes involved in cell defense system in human cells stressed by H2O2 and pre-treated with (Tunisian) Rhamnus alaternus extracts: Combination with polyphenolic compounds and classic in vitro assays. Chem. Biol. Interact. 168:171–183.
- Benjamini, E., Coico, R., and Sunshine, G. (Eds). 2000. Immunology: A short course. 4th Edition. New York: Wiley.
- Bellomaria, B., Arnold, N., and Valentini, G. 1998. Essential oil of Teucrium flavum subsp. Hellenicum from Greece. J. Essent. Oil Res. 10:131–133.
- Ben Sghaier, M., Bhouri, W., Neffati, A., Boubaker, J., Skandrani, I., Bouhlel, I., Kilani, S., Chekir-Ghedira, L. and Ghedira, K. 2011a. Chemical investigation of different crude extracts from Teucrium ramosissimum leaves. Correlation with their anti-genotoxic and antioxidant properties. Food Chem. Toxicol. 49:191–201.
- Ben Sghaier, M., Boubaker, J., Skandrani, I., Bouhlel, I., Limem, I., Ghedira, K., and Chekir-Ghedira, L. 2011b. Anti-mutagenic, anti-genotoxic, and antioxidant activities of phenolic-enriched extracts from Teucrium ramosissimum: Combination with their phytochemical composition. Environ. Toxicol. Pharmacol. 31:220–232.
- Bossolani, M. P. 2000. Mecanismo molecular da ação anti-secretora ácida gástrica de extratos e frações isoladas de Maytenus ilicifolia Mart e Maytenus aquifolium Mart, Tese de mestrado, Universidade Federal de São Paulo, São Paulo, p. 108.
- Brunton, L. L. 1996. Agents for control of gastric acidity and treatment of peptic ulcers. In: The Pharmacological Basis of Therapeutics 9th Edition (Hardman, J. G., Limbird, L. E., Molinnoff, P. B., and Ruddon, R. W., Eds)., New York: Macmillan, pp. 901–915.
- Cho, C. H., Ogle, C. W. and Dai, S. 1976. Effects of zinc chloride on gastric secretion and ulcer formation in pylorus-occluded rats. Eur. J. Pharmacol. 38:337–341.
- Ezaki, N., Kato, M., Takizawa, N., Morimoto, S., Nonaka, G. and Nishioka, I. 1985. Pharmacological studies on Linderae umbellatae Ramus, IV. Effects of condensed tannin related compound on peptic activity and stress-induced gastric lesions in mice. Planta Med. 34–38.
- Fernández Puntero, B., Iglesias Peinado, I. and Villar del Fresno, A. M. 1997. Anti-inflammatory and anti-ulcer activity of Teucrium buxifolium. J. Ethnopharmacol. 55:93–98.
- Galati, E. M., Mondello, M. R., D’Aquino, A., Miceli, N., Sanogo, R., Tzakou, O. and Monforte, M. T. 2000. Effects of Teucrium divaricatum Heldr. ssp. divaricatum decoction on experimental ulcer in rats. J. Ethnopharmacol. 72:337–342.
- Galati, E. M., Sanogo, R., Tzakou, O., Rossitto, A., and Germano, M. P. 1997. Anti-ulcerogenic activity of Teucrium divaricatum helder.ssp. divaricatum. Phytother. Res. 11:572–575.
- Ghedira, K., Chemli, R., Richard, B., Zeches, M., Le,and Men-Olivier, L. 1991. Contribution to the study of traditional medicines in Tunisia: Study of the aerial parts of Ajuga iva (L.) Schreb. Plantes Med. Phytother. 25:100–111.
- Gonzalez, F. G. and Di Stasi, L. C. 2002. Anti-ulcerogenic and analgesic activities of the leaves of Wilbrandia ebracteata in mice. Phytomedicine 9:125–134.
- Green, L. C., Wagner, D. A., Glogowski, J., Skipper, P. L., Wishnok, J. S. and Tannenbaum, S. R. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131–138.
- Harizi, H., Juzan, M., Grosset, C., Rashedi, M. and Gualde, N. 2001. Dendritic cells issued in vitro from bone marrow produce PGE2 that contributes to the immunomodulation induced by antigen-presenting cells. Cell. Immunol. 209:19–28.
- Ho, S. C., Hwang, L. S., Shen, Y. J. and Lin, C. C. 2007. Suppressive effect of a proanthocyanidin-rich extract from longan (Dimocarpus longan Lour.) flowers on nitric oxide production in LPS-stimulated macrophage cells. J. Agric. Food Chem. 55:10664–10670.
- Jiménez-Medina, E., Berruguilla, E., Romero, I., Algarra, I., Collado, A., Garrido, F. and Garcia-Lora, A. 2008. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer 8:78.
- Jorge, R. M., Leite, J. P., Oliveira, A. B. and Tagliati, C. A. 2004. Evaluation of anti-nociceptive, anti-inflammatory and anti-ulcerogenic activities of Maytenus ilicifolia. J. Ethnopharmacol. 94:93–100.
- Khennouf, S., Benabdallah, H., Gharzouli, K., Amira, S., Ito, H., Kim, T. H., Yoshida, T. and Gharzouli, A. 2003. Effect of tannins from Quercus suber and Quercus coccifera leaves on ethanol-induced gastric lesions in mice. J. Agric. Food Chem. 51:1469–1473.
- Labadie, R. P. 1993. Immunomodulatory compounds. In: Bioactive Natural Products: Detection, Isolation, and Structure Determination (Steven, M., and Russel, J. M., Eds)., London: CRC Press, pp. 279–317.
- Leite, J. P., Rastrelli, L., Romussi, G., Oliveira, A. B., Vilegas, J. H., Vilegas, W. and Pizza, C. 2001. Isolation and HPLC quantitative analysis of flavonoid glycosides from Brazilian beverages (Maytenus ilicifolia and M. aquifolium). J. Agric. Food Chem. 49:3796–3801.
- Lewis, D. A. and Hanson, P. J. 1991. Anti-ulcer drugs of plant origin. Prog. Med. Chem. 28:201–231.
- Limem, I., Harizi, H., Ghedira, K. and Chekir-Ghedira, L. 2011. Leaf extracts from Phlomis crinita Cav. subs. mauritanica Munby affect immune cell functions in vitro. Immunopharmacol. Immunotoxicol. 33:309–314.
- López-Posadas, R., Ballester, I., Abadía-Molina, A. C., Suárez, M. D., Zarzuelo, A., Martínez-Augustin, O. and Sánchez de Medina, F. 2008. Effect of flavonoids on rat splenocytes, a structure-activity relationship study. Biochem. Pharmacol. 76:495–506.
- Maccioni, S., Baldini, R., Tebano, M., Cioni, P. L., and Flamini, G. 2007. Essential oil of Teucrium scorodonia L. ssp. scorodonia from Italy. Food Chem. 104:1393–1395.
- MacMicking, J., Xie, Q. W. and Nathan, C. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323–350.
- Manosroi, A., Saraphanchotiwitthaya, A. and Manosroi, J. 2003. Immunomodulatory activities of Clausena excavata Burm. f. wood extracts. J. Ethnopharmacol. 89:155–160.
- Manosroi, A., Saraphanchotiwitthaya, A. and Manosroi, J. 2005. In vitro immunomodulatory effect of Pouteria cambodiana (Pierre ex Dubard) Baehni extract. J. Ethnopharmacol. 101:90–94.
- Martin, M. J., Marhuenda, E. and Alarcon de la Lastra, C. 1991. Esculine, ranitidine and carbenoxolone: Different modes of action on gastric mucosa. Gen. Pharmacol. 22:1001–1004.
- Menichini, F., Conforti, F., Rigano, D., Formisano, C., Piozzi, F., and Senatore, F. 2009. Phytochemical composition, anti-inflammatory and anti-tumor activities of four Teucrium essential oils from Greece. Food Chem. 115:679–686.
- Middleton E., Jr Kandaswami, C. and Theoharides T. C. 2000. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52:673–751.
- Miller, T. A. and Henagan, J. M. 1984. Indomethacin decreases resistance of gastric barrier to disruption by alcohol. Dig. Dis. Sci. 29:141–149.
- Mossmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 65:55–63.
- Nakamura, A., Nagai, K., Suzuki, S., Ando, K. and Tamura, G. 1986. A novel method of screening for immunomodulating substances, establishment of an assay system and its application to culture broths of microorganisms. J. Antibiot. 39:1148–1154.
- Oates, P. J. and Hakkinen, J. P. 1988. Studies on the mechanism of ethanol-induced gastric damage in rats. Gastroenterology 94:10–21.
- Özkan, G., Kuleasan, H., Çelik, S., Göktürk, R. S., and Ünal, O. 2007. Screening of Turkish endemic Teucrium montbretii subsp. pamphylicum extracts for antioxidant and antibacterial activities. Food Contr. 18:509–512.
- Panovska, T. K., Kulevanova, S., Gjorgoski, I., Bogdanova, M. and Petrushevska, G. 2007. Hepatoprotective effect of the ethyl acetate extract of Teucrium polium L. against carbon tetrachloride-induced hepatic injury in rats. Acta Pharm. 57:241–248.
- Pfeiffer, C. J., Bulbena, O., Esplugues, J. V., Escolar, G., Navarro, C. and Esplugues, J. 1987. Anti-ulcer and membrane stabilizing actions of zinc acexamate. Arch. Int. Pharmacodyn. Ther. 285:148–157.
- Pottier-Alapetite, G., 1979. Flowers of Tunisia: Angiosperms, dicotyledons, apetals, dialypetals. Ministère de L’enseignement Supérieur et de la Recherche Scientifique et Ministère de l’Agriculture, Tunis, Tunisia, 210.
- Rainsford, K. D., 1989. Mechanisms of gastrointestinal damage by non-steroidal anti-inflammatory drugs. In: Ulcer Disease: New Aspects of Pathogenesis and Pharmacology (Szabo, S., and Pfeiffer, C. J., Eds)., Boca Raton, CRC Press, FL, pp. 3–13.
- Robert, A., Nezamis, J. E., Lancaster, C. and Hanchar, A. J. 1979. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 77:433–443.
- Sairam, K., Rao, C. h. V., Babu, M. D., Kumar, K. V., Agrawal, V. K. and K Goel, R. K. 2002. Anti-ulcerogenic effect of methanolic extract of Emblica officinalis: an experimental study. J. Ethnopharmacol. 82:1–9.
- Saracoglu, I., Calis, I., Inoue, M., and Ogihara, Y. 1997. Selective cytotoxic and cytostatic activity of some phenylpropanoid glycosides. Fitoterapia 68:434–438.
- Saroglou, V., Arfan, M., Shabir, A., Hadjipavlou-Litina, D., and Skaltsa, H. 2007. Composition and anti-oxidant activity of the essential oil of Teucrium royleanum Wall. Ex Benth growing in Pakistan. Flavor Frag. J. 22:154–157.
- Sobota, R., Szwed, M., Kasza, A., Bugno, M. and Kordula, T. 2000. Parthenolide inhibits activation of signal transducers and activators of transcription (STATs) induced by cytokines of the IL-6 family. Biochem. Biophys. Res. Commun. 267:329–333.
- Sosa, S., Balick, M. J., Arvigo, R., Esposito, R. G., Pizza, C., Altinier, G. and Tubaro, A. 2002. Screening of the topical anti-inflammatory activity of some Central American plants. J. Ethnopharmacol. 81:211–215.
- Souza-Formigoni, M. L., Oliveira, M. G., Monteiro, M. G., da Silveira-Filho, N. G., Braz, S. and Carlini, E. A. 1991. Anti-ulcerogenic effects of two Maytenus species in laboratory animals. J. Ethnopharmacol. 34:21–27.
- Tapiero, H., Ba, G. N., Couvreur, P. and Tew, K. D. 2002. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 56:215–222.
- Tariq, M., Ageel, A. M., al-Yahya, M. A., Mossa, J. S. and al-Said, M. S. 1989. Anti-inflammatory activity of Teucrium polium. Int. J. Tissue React. 11:185–188.
- Terra, X., Valls, J., Vitrac, X., Mérrillon, J. M., Arola, L., Ardèvol, A., Bladé, C., Fernandez-Larrea, J., Pujadas, G., Salvadó, J. and Blay, M. 2007. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW264.7 macrophages by inhibiting NFkB signaling pathway. J. Agric. Food Chem. 55:4357–4365.
- Ulubelen, A., Topcu, G., and Sonmez, U. 2000. Chemical and biological evaluation of genus Teucrium. Studies in Natural Products Chemistry. 23(Bioactive Natural Products, Part D):591–648.
- Van den Worm, E., Beukelman, C. J., Van den Berg, A. J., Kroes, B. H., Labadie, R. P. and van Dijk, H. 2001. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur. J. Pharmacol. 433:225–230.
- Vilegas, W., Sanommiya, M., Rastrelli, L. and Pizza, C. 1999. Isolation and structure elucidation of two new flavonoid glycosides from the infusion of maytenus aquifolium leaves. Evaluation of the anti-ulcer activity of the infusion. J. Agric. Food Chem. 47:403–406.
- Virgili, F., Kobuchi, H. and Packer, L. 1998. Procyanidins extracted from Pinus maritima (Pycnogenol): Scavengers of free radical species and modulators of nitrogen monoxide metabolism in activated murine RAW 264.7 macrophages. Free Radic. Biol. Med. 24:1120–1129.
- Wallace, J. L. and Granger, D. N. 1996. The cellular and molecular basis of gastric mucosal defense. FASEB J. 10:731–740.
- Wolfe, M. M. and Sachs, G. 2000. Acid suppression: Optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology. 118:S9–31.
- Yesilada, E., and Gürbüz, I. 2003. A compilation of the studies on the anti-ulcerogenic effects of medicinal plants. In Recent Progress in Medicinal Plants, Vol. II: Phytochemistry and Pharmacology (Singh, S., Singh, V.K., and Govil, J. N., Eds.), Houston, TX: SCI Tech Publishing LLC, pp. 111–174.