Abstract
The potential for immunotoxicological effects of ethyl tertiary butyl ether (ETBE, CAS RN 637-92-3) was studied in young adult female Crl:CD(SD) rats following subchronic oral exposures. Rats were exposed by gavage once daily for 28 consecutive days to 0, 250, 500, or 1000 mg ETBE/kg body weight (BW)/day; a concurrent positive control group received four intraperitoneal injections of at 50 mg cyclophosphamide monohydrate (CPS)/kg/day on study Days 24–27. Immunotoxicity was evaluated using a splenic antibody-forming cell (AFC) assay to assess T-cell-dependent antibody responses in rats sensitized with sheep red blood cells (SRBC). All rats survived to the scheduled necropsy. There were no effects on clinical observations, body weights, feed or water consumption, or macroscopic pathology findings in the ETBE-treated rats. No ETBE-related effects were observed on absolute or relative (to final body weight) spleen or thymus weights, spleen cellularity, or on the specific (AFC/106 spleen cells) or total activity (AFC/spleen) of splenic IgM AFC to the T-cell-dependent antigen SRBC. CPS produced expected effects consistent with its known immunosuppressive properties and validated the appropriateness of the AFC assay. Based on the results of this study, ETBE did not suppress the humoral component of the immune system in female rats. The no-observed-effect level for immunotoxicity was the highest dosage tested at 1000 mg/kg/day.
Introduction
Ethyl tertiary butyl ether (2-ethyoxy-2-methyl propane, ETBE, CAS RN 637-92-3) is an oxygenated hydrocarbon used almost entirely as a fuel oxygenate in gasoline. It is manufactured commercially from isobutylene and ethanol which, when derived from agricultural products, results in ETBE biofuel (EFOA, Citation2011). ETBE is presently the most commonly used fuel ether in Europe with consumption of 2.5 million tones in 2009 (EFOA, Citation2011). ETBE has a low molecular weight and is a highly flammable liquid of moderate volatility. It boils around 73.1°C and, at ambient temperature, appears as a pale yellow liquid with a terpene-like odor (EFOA, Citation2011).
The toxicological properties of ETBE are generally well-characterized (McGregor, Citation2007; de Peyster, Citation2010); however, data on potential immunotoxicological effects of repeated exposures are limited. Available data from repeated-exposure studies have not reported effects on hematology or organ weight or structure indicative of adverse effects to the immune system. Recently, studies have been conducted on gasoline/ETBE vapor condensate (G/ETBE), including an immunotoxicity study (White et al., Citation2004). This testing on gasoline and gasoline blended with a variety of oxygenates was mandated by EPA under Section 211(b) of the Clean Air Act (CAA). The immunotoxicity study examined target concentrations of 0, 2000, 10,000, and 20,000 mg G/ETBE/m3 (≈17% ETBE) administered by whole-body exposures for 6 h/day, 5 days/week for 4 weeks to female Sprague-Dawley rats (10/dose group). G/ETBE had no effect on the terminal body weight, absolute or relative weights of the spleen and thymus, or the total number of splenocytes/animal. Exposure to 10,000 and 20,000 mg G/ETBE/m3 did result in AFC response suppression. When evaluated as activity per 106 splenocytes, reductions of 76 and 72% were observed in the AFC for the 10,000 and 20,000 mg/m3 groups, respectively. When evaluated as total activity per spleen, reductions of 74 and 70% were observed in the AFC for the two groups, respectively. The exposure concentration associated with these effects in rats is very high (at least three orders of magnitude) relative to actual occupational or environmental exposures that might be experienced in humans exposed to gasoline evaporative emissions. Gasoline vapor condensate alone without oxygenates was also tested in the plaque assay and was negative for immunotoxicity effects. The immunosuppression finding in the G/ETBE study suggested a need for further characterization of the immunotoxic potential effects of ETBE.
The study reported here evaluated the immunotoxicity potential of exposure to neat ETBE, using a study design based upon the U.S. EPA Office of Prevention, Pesticides, and Toxic Substances (OPPTS) guideline for immunotoxicity (OPPTS 870.7800) testing (U.S. EPA, 1998). In this test, SRBC are a T-cell-dependent antigen and, thus, T-cells, B-cells, and antigen-presenting cells are required to function properly in order to obtain an appropriate AFC response. If the experimental treatment of rats affects any or a combination of these cell types, an altered antibody response to sensitization with SRBC may be observed. As a result, the T-cell-dependent IgM response to SRBC is one of the most sensitive immunotoxicological assays currently in use.
A significant modulation in the IgM AFC response, when appropriately compared to vehicle controls, indicates that a test substance is capable of modifying the humoral immune response in a host and, thus, has the potential for immunotoxicity. The AFC assay is one of the primary methods accepted by the U.S. EPA for determination of effects on the humoral response to SRBC, and is one of the Tier I assays used by the National Toxicology Program (NTP) (Luster et al., Citation1988). Although not a primary route of ETBE exposure to humans, the oral route was deemed appropriate for conduct of this screening study as: ETBE toxicity is demonstrated generally similar following oral and inhalation exposure (Medinsky et al., Citation1999; Chemicals Evaluation and Research Institute, Citation2008; Mitsubishi Chemical Safety Institute Ltd., Citation2008a; Japan Bioassay Research Center, Citation2010a, Citation2010b); the same metabolites are produced by both exposure routes (Dekant et al., Citation2001; Mitsubishi Chemical Safety Institute Ltd, Citation2008b); and the oral exposure limit dose (1000 mg/kg body weight[BW]) is estimated to have achieved a significantly higher internal dose of ETBE than for G/ETBE inhalation. The predominate human exposure to ETBE is via inhalation during fueling; however, there is also the potential for exposure indirectly via the environment including from water sources, hence oral testing was considered relevant for a screening test.
Materials and methods
Animals
Female Sprague-Dawley rats (strain Crl:CD[SD]) were obtained from Charles River Laboratories, Inc. (Raleigh, NC). Rats were ≈38 days of age upon receipt and, following a 13-day acclimation period, were randomly assigned to one of four ETBE exposure groups (0, 250, 500, or 1000 mg/kg BW/day) and one positive control group (50 mg cyclophosphamide/kg intraperitoneal [IP] injection). The SD rat was selected based on the ETBE toxicology database that provides extensive characterization of toxicity effects in rats and demonstrates generally similar toxicity in limited testing in mice (McGregor, Citation2007). No significant differences between male and female SD rats were noted with respect to general toxicity following repeated oral exposures to ETBE (McGregor, Citation2007). In addition, female rodents routinely yield higher AFC responses than male rodents for the evaluation of the humoral immune response using the antibody-forming cell (AFC) assay (Luster et al., Citation1988). Therefore, the female SD rat was the test system of choice for this study.
This species, strain, and gender are recognized as appropriate for immunotoxicity studies. The number of rats assigned to this study was the minimum required to obtain statistically and scientifically meaningful data for this rat strain (out-bred). Due to the higher variability of the immune response in out-bred rats, a slightly higher minimum number of rats/group was needed (i.e., n = 10/group) as compared to had, in-bred strains, been employed (i.e., n = 8/group). An n = 10 rats/group also helped ensure at least n = 8 rats/group (minimum number required by regulatory guidelines) were available for immunotoxicological assessment in the unexpected event of mortality during treatment/loss of samples during collection, shipment, and/or processing. The historical database for this strain of rat at ImmunoTox® Inc. (Richmond, VA) was based on an n ≥ 10 rats/group.
All study rats were maintained in accordance with the “Guide for the Care and Use of Laboratory Animals” (NRC, Citation1996) in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). Rats were housed individually in clean, stainless steel wire-mesh cages suspended above cage-board in a facility maintained at 22 ± 3°C with a 50 ± 20% relative humidity and 12-h interval light/dark cycle. Feed (PMI Nutrition International, LLC, Certified Rodent LabDiet® 5002; meal) and reverse osmosis-treated water were provided ad libitum.
Test material and exposure
Ethyl Tertiary Butyl Ether (ETBE; 96.994% purity) was received from SGS Nederland B.V., Spijkenisse, the Netherlands. The vehicle used to prepare test substance formulations and for administration to the controls was corn oil. ETBE formulations were prepared approximately weekly as single formulations for each dosage level, divided into aliquots for daily dispensation, and stored at room temperature protected from light. The formulations were stirred continuously throughout the preparation, sampling, and dose administration procedures. Due to the high volatility of ETBE, the formulation container lids were wrapped with parafilm while being stirred and were only opened when sampled and divided into aliquots, which were performed as quickly as possible to avoid test substance evaporation. Homogeneity and stability of ETBE in vehicle were established prior to initiation of dosing for the range of concentrations (62.5–250 mg/mL) that encompass the dosing concentrations used in this study. Analyzed formulations were found to contain 91–102% of the target concentrations of ETBE.
The positive control substance, cyclophosphamide monohydrate (CPS), was received from Sigma-Aldrich Inc. (St. Louis, MO). The vehicle used to prepare the CPS formulations was phosphate-buffered saline. The CPS formulation was prepared and the solution was divided into five glass septum vials within a laminar flow hood, using sterile containers and utensils, and stored at −20°C. On each day of dosing, an aliquot was thawed, kept on ice, and shaken vigorously prior to dosing. The formulation was used within ≈3 h of thawing.
The vehicle and ETBE formulations were administered orally by gavage once daily for 28 consecutive days, through the day prior to the scheduled necropsy. The dose volume for the ETBE groups was 4 mL/kg. CPS positive control was administered to a separate group of rats via IP injection once daily on study Days 24–27. The dose volume for the positive controls was 10 mL/kg. Individual doses were based on the most recently recorded body weights to provide the correct mg/kg BW/day dosage. The first day of dose administration was designated as study Day 0.
Dosage levels were selected such that the highest corresponded with the Guideline-specified limit dosage of 1000 mg/kg BW/day, and the lower dosage levels (250 and 500 mg/kg/day) were selected to span this dose/response range. Additionally, these levels have been previously tested in female SD rats in repeated-exposure oral gavage studies (McGregor, Citation2007).
General clinical evaluations
Viability and clinical observations
Rats were observed for viability twice each day of the study. Clinical examinations were performed at the time of dose administration and ≈1–2 h after dosing. On non-dosing days, positive control group animals were observed once daily. Detailed physical examinations were conducted on all animals weekly and on day of scheduled necropsy.
Body weight and feed and water consumption
Individual body weights and feed and water consumption were recorded twice and once weekly, respectively, throughout the duration of the immunotoxicity study.
Anatomic pathology
Complete gross necropsy was conducted on all animals. Animals were euthanized by CO2 inhalation. The necropsies included, but were not limited to, examination of the external surface, all orifices, and the cranial, thoracic, abdominal and pelvic cavities, including viscera. The thymus was collected and placed in 10% neutral-buffered formalin for microscopic evaluation (if needed).
Organ weights
The spleen and thymus were weighed from all animals at the scheduled necropsy.
Immunotoxicity evaluations
Four days prior to scheduled necropsy, all rats, including positive controls, received a single intravenous (IV) immunization injection of sheep red blood cells (SRBC, Lampire Biological Laboratories, Pipersville, PA: 2 × 108 cells in 0.5 mL Earle’s Balanced Salt Solution (EBSS) with HEPES [prepared by ImmunoTox® Inc.]) via the lateral tail vein. Positive control rats were treated with single daily 50 mg/kg BW/day IP injections of CPS on each of the 4 days immediately prior to the scheduled necropsy. After euthanasia, spleens from all SRBC-immunized rats were collected and harvested in EBSS with 15 mM HEPES supplemented with gentamicin.
For splenocyte preparation, spleens were made into single cell suspensions by mashing using a Stomacher® blender (Seward Inc., Bohemia, NY). Cell suspensions were then centrifuged and re-suspended in EBSS with HEPES (6 mL volume). The viability of splenocytes was determined using propidium iodide and an EPICS® XL-MCL flow cytometer (Beckman Coulter, Brea, CA). A minimum of 5000 events/sample was analyzed.
The spleen IgM AFC response to the T-cell-dependent antigen SRBC was measured using a modification of the original hemolytic plaque assay of Jerne et al. (Citation1974) and as described by White et al. (Citation2010). Isolated spleen cells (as described above) were prepared to 1:50 and 1:150 dilutions. A 0.1 mL aliquot of cells from each suspension (total number of cells here ultimately derived from Coulter counts [see below]) was placed in separate test tubes, each already containing 25 µL of guinea pig complement (Cedarlane Laboratories, Ontario, Canada), 25 µL of SRBC, and 0.5 mL of warm agar (0.5%). After thoroughly mixing, each test tube mixture was plated onto a separate petri dish, cover-slipped, and incubated at 36–38°C for 3 h. Spleen cell counts were performed on the 6 mL samples using a Model Z1 Coulter Counter (Beckman Coulter); from this data, the cells/spleen, AFC/106 spleen cells (specific activity), and AFC/spleen (total spleen activity) were calculated. Plaques were counted using a Bellco plaque viewer (Vineland, NJ). A plaque, occurring from the lysis of SRBC, is elicited as a result of the interaction of complement and antibodies (produced in response to the IV immunization of the rats) directed against SRBC. Each plaque is generated from a single IgM antibody-producing B-cell (plasma cell), permitting the number of AFC present in a whole spleen to be calculated.
Statistics
Body weight, body weight change, feed and water consumption, and organ weights were analyzed by one-way analysis of variance (ANOVA) with Dunnett’s test. The AFC data were expressed as specific activity (IgM AFC/106 spleen cells) and total spleen activity (IgM AFC/spleen), examined for homogeneity by Bartlett’s χ2 test (Bartlett, Citation1937) and homogeneous results evaluated by one-way ANOVA and subsequent Dunnett’s test. Nonhomogeneous data were evaluated using a nonparametric ANOVA. When significant differences occurred, the treatment groups were compared to the vehicle control group using the Gehan-Wilcoxon test (Gross and Clark, Citation1975) when appropriate. The Jonckheere’s test (Hollander and Wolfe, Citation1973) was used to test for dose-related trends across the vehicle and treatment groups. The positive control data were evaluated using the Student’s t-test (Sokal and Rohlf, Citation1981) and were compared to the vehicle control. The criterion for accepting the results of the positive control group in the assay was a statistically significant (p ≤ 0.05) decrease in the AFC response as compared to the vehicle control group.
Results
All rats in this experiment survived to scheduled necropsy, and no treatment-related clinical findings were observed. Body weights were also not affected by ETBE exposure, whereas the CPS group exhibited statistically significant body weight losses during the 4-day (study Days 24–27) dosing period when compared to the vehicle control group. This effect was consistent with known effects of CPS and, therefore, considered related to CPS administration (). Feed and water consumption were not altered by ETBE or CPS exposure.
Table 1. Body weight (g) and organ weights (mg) in female Crl:CD(SD) rats exposed to ethyl tertiary butyl ether (ETBE) for 28 days.
In the ETBE-treated rats, there were no test substance-related macroscopic findings at necropsy. Small thymuses were noted in 4 of 10 rats in the 50 mg CPS/kg/day group. This finding was consistent with known effects of CPS and so considered related to CPS administration. There were no ETBE-related effects on spleen weights (absolute or relative to final body weight) as compared to the vehicle control group. As expected, CPS produced statistically significant decreases in both absolute and relative spleen weights (57 and 55%, respectively), when compared to values among the vehicle animals (). There were also no ETBE-related effects on thymus weights (absolute or relative to final body weight) as compared to the vehicle controls. CPS produced statistically significant expected decreases in both absolute and relative thymus weights (76 and 71%, respectively) when compared to the vehicle animals ().
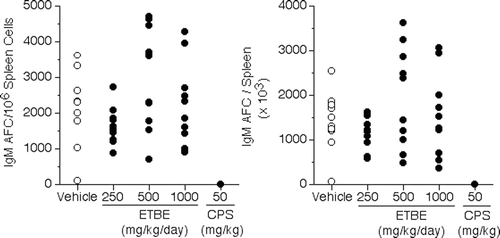
The functional results of the spleen IgM AFC response, i.e., the Plaque Assay, are shown in . There was no effect on spleen cell number for the rats treated with any dose level of ETBE as compared to vehicle rats. As expected, the CPS positive control produced a statistically significant decrease in spleen cell number (86%) when compared to that of vehicle rats. There were no significant differences between vehicle rats and any of the ETBE treatment rats in the AFC response (presented as mean ± standard error values for specific activity [AFC/106 spleen cells] and total spleen activity [AFC/spleen]). As anticipated, CPS produced statistically significant decreases in specific activity (100%) and total spleen activity (100%) in the female SD rats.
Table 2. Spleen antibody-forming cell response to T-dependent antigen sheep erythrocytes (SRBC) in female Crl:CD(SD) rats exposed to ethyl tertiary butyl ether (ETBE) for 28 days.
The results of the plaque assay where the AFC response is expressed as individual animal values for specific activity (AFC/106 spleen cells) or as total spleen activity (AFC/spleen) are shown in . also shows that the known immunosuppressive agent, CPS, produced a decreased response in rats which was statistically different from that of the vehicle hosts. This demonstrated that if an immunosuppressive effect had occurred due to ETBE, it could have been detected in this assay.
Figure 1. Individual IgM antibody-forming cell responses to sheep erythrocytes in female Crl:CD (SD) rats exposed to ETBE via oral gavage for 28 days—Day 4 response. Results of AFC response expressed as individual animal values for specific activity (AFC/106 spleen cells; left panel) or as total spleen activity (AFC/spleen; right panel). N = 10 rats/exposure level for 0, 250, 500, and 1000 mg ETBE/kg/day and 50 mg CPS/kg/day (study Days 24–27).
Discussion
This study provides new data on the potential immunotoxicological effects of subchronic exposure to high doses of ETBE. An earlier study of a vapor condensate of gasoline and ETBE (G/ETBE) found a dose-dependent decrease in the AFC response when evaluated as either AFC/106 spleen cells or AFC/spleen. Reductions of 76 and 72% in the activity expressed per 106 splenocytes and reductions of 74 and 70% activity expressed as total activity per spleen were observed in the PFC for rats whole body exposed to 10,000 and 20,000 mg G/ETBE/m3, respectively. Gasoline vapor condensate alone without ETBE tested in a plaque assay was negative for immunotoxic effects, suggesting that ETBE may have contributed to the positive finding in the mixture vapor condensate.
The present study of neat ETBE, however, did not find any treatment-related effects on the functional ability of the humoral component of the immune system of female rats following 28 days of oral exposure to 1000 mg ETBE/kg BW. Parameters assessed included spleen and thymus weights and AFC response to the T-cell-dependent antigen, SRBC. These same parameters were tested using identical methods in the G/ETBE study.
The circumstances of ETBE exposure were different in the two studies, with 6 h of inhalation exposure to ETBE in the vapor condensate vs. single oral gavage doses with neat ETBE, both over the course of 4 weeks. The estimated internal dose of ETBE to the rats exposed to G/ETBE concentrations of 20,000 mg/m3 can be calculated for comparison with the oral dose of neat ETBE tested here. Analysis of the chamber vapor indicated ≈17% ETBE or 3400 mg/m3 (810 ppm) at the 20,000 mg G/ETBE/m3 concentration; this equates to ≈500 mg ETBE/kg BW/day (assuming a 6-h rat respiratory volume of 0.29 m3/kg BW and 50% absorption via inhalation; ECHA, 2008). These assumptions are conservative, as the respiratory volume/h that has been used (45 L/h/kg) is very high in comparison to another default commonly used (i.e., assumes 6 L/h for a 500 g-male rat; Gold et al., Citation1984). Secondly, pulmonary retention of 50% is likely close to 2-fold higher than the actual value for rats. Human data indicate 26% pulmonary retention for ETBE (Nihlèn et al., 1998). These alternative parameters would reduce the retained inhalation doses even further; hence, the oral gavage dose of ETBE achieved greater than 2-fold higher doses of ETBE than did the G/ETBE exposures.
With oral gavage dosing, the first-pass effect would impact the ETBE levels post-hepatic metabolism as compared to after an inhalation exposure; however, the acetaldehyde metabolite of ETBE produced in the liver would be higher with the gavage exposure. There is a suggestion that acetaldehyde may have contributed to the immunotoxicity findings in the G/ETBE study, as a test conducted for a gasoline vapor condensate with ethanol (G/EtOH, which also metabolizes to acetaldehyde) also produced immunosuppression (White et al., Citation2004). Therefore, the present study dose level of 1000 mg ETBE/kg/day appears to have exceeded the ETBE (and metabolite) concentrations experienced by rats in the G/ETBE study. Hence, this dosage appears adequate for testing the individual contribution of ETBE to this study’s findings.
The lack of immunotoxic findings in the present ETBE study is supported by the absence of adverse effects to immune system tissues in repeated-exposure toxicity studies. ETBE has been well studied for systemic toxicity effects in a number of subchronic and chronic oral and inhalation toxicity studies conducted in rodents. Two repeated oral toxicity studies have been performed in male and female F344 rats that received ETBE either by oral gavage (at doses up to 400 mg/kg/day for 6 months; Chemicals Evaluation and Research Institute, Citation2008) or via drinking water at received doses of ≈540–560 mg/kg/day for up to 2 years (Japan Bioassay Research Centre, 2010a). The studies were GLP-compliant and comparable in design to OECD Guideline 452 and OECD 453, respectively. There were no identified adverse structural effects to spleen, bone marrow, thymus, and lymph nodes related to ETBE treatment in these studies.
Repeated dose inhalation toxicity data are available for the rat, covering durations of exposure of 28 days (IIT Research Institute, Citation1991), 90 days (Medinsky et al. Citation1999; Mitsubishi Chemical Safety Institute Ltd, Citation2008a) and 2 years (Japan Bioassay Research Center, Citation2010b). Subchronic inhalation toxicity data are available also for the mouse (Medinsky et al. Citation1999). There was no evidence of ETBE-related effects on immune system organs observed in the rat 28-day repeated inhalation study (OECD Guideline 12). In subchronic exposure studies with rats and mice according to EPA OTS 798.2450 (90-day inhalation toxicity) Guideline or OECD 413, the only reported change to an immune system organ was bone marrow congestion (female rats, 1750 and 5000 ppm; Medinsky et al., Citation1999). Although this congestion was associated with treatment in that study, hematopoietic cell populations appeared unaffected. This change itself is not associated with immunotoxicity, and the finding was not observed in other repeated-exposure studies. A second 90-day inhalation toxicity study conducted in rats evaluated ETBE exposures up to 5000 ppm, but did not find changes to immune system organs, including the bone marrow (Mitsubishi Chemical Safety Institute Ltd., 2008a). A chronic ETBE inhalation toxicity study is also available in the rat (Japan Bioassay Research Centre, 2010b), following OECD Guideline 453 and performed under GLP. This study also did not find any evidence of ETBE treatment-related effects to immune system organs.
The present study demonstrated that ETBE is not immunotoxic to the female SD rat at a dosage level of 1000 mg/kg/day (limit dose). Furthermore, the findings of the G/ETBE study cannot be explained by ETBE alone as the contributor immunotoxicant.
Conclusions
In conclusion, treatment of female Crl:CD(SD) rats with ETBE via oral gavage for 28 days did not produce biologically relevant effects on the humoral immune component of the immune response when evaluated using the antibody-plaque forming cell assay. Based on the results here, the no-observed-effect level for immune system suppression in female SD rats administered ETBE via oral gavage for 28 days was 1000 mg/kg/day, the highest dose evaluated.
Declaration of interest
The study was funded by The European Fuel Oxygenates Association of CEFIC. Author M.I.B is employed by a company that manufactures ETBE. The Authors report no conflicts of interest. The Authors are alone responsible for the content and writing of the paper.
References
- Bartlett, M. S. 1937. Sub-sampling for attributes. J. R. Stat. Soc. Suppl. 4:131–135.
- Chemicals Evaluation and Research Institute. 2008. A 180-Day Repeated Dose Oral Toxicity Study of ETBE in Rats. Testing laboratory: Hita Laboratory, Chemicals Evaluation and Research Institute, 3-822, Ishii-cho, Hita-shi Oita 877-0061, Japan. Report no. D19-0002.
- Dekant, W., Bernauer, U., Rosner, E., and Amberg, A. 2001. Biotransformation of MTBE, ETBE and TAME after inhalation or ingestion in rats and humans. Health Effects Institute Research Report. Report no.: 102.
- de Peyster, A. 2010. Ethyl t-butyl ether: Review of reproductive and developmental toxicity. Birth Defects Res. B. Dev. Reprod. Toxicol. 89:239–263.
- ECHA (European Chemicals Agency). 2008. Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.8: Characterisation of Dose [concentration]-Response for Human Health. (Helsinki, Finland).
- EFOA (European Fuel Oxygenates Association). 2011. Fuel Ethers. Available at: http://www.efoa.eu/en/fuel-ethers.aspx. Accessed on April 21, 2011.
- Gold, L. S., Sawyer, C. B., Magaw, R., Backman, G. M., de Veciana, M., Levinson, R., Hooper, N. K., Havender, W. R., Bernstein, L., and Peto, R. 1984. A carcinogenic potency database of the standardized results of animal bioassays. Environ. Health Perspect. 58:9–319.
- Gross, A. J., and Clark, V. A. 1975. Gehan-Wilcoxon Test. In: Survival Distribution: Reliability Applications in Biomedical Sciences. (Gross, A. J., and Clark, V. A. Eds.), New York: John Wiley and Sons, pp. 120–123.
- IIT Research Institute. 1991. Four-week Inhalation Toxicity Study of Ethyl-tert-butyl Ether (ETBE) in Rats. Testing laboratory: IIT Research Institute. Report no. 1544.
- Japan Bioassay Research Center. 2010a. Carcinogenicity Test of 2-ethoxy-2-methylpropane in Rats (Drinking Water Study). Testing laboratory: Japan Bioassay Research Center, Japan Industrial Safety and Health Association, 2445 Hirasawa, Hadano, Kanagawa, Japan. Report no. 0691.
- Japan Bioassay Research Center. 2010b. Carcinogenicity Test of 2-Ethoxy-2-methylpropane in Rats (Inhalation Study). Testing laboratory: Japan Bioassay Research Center, Japan Industrial Safety and Health Association, 2445 Hirasawa, Hadano, Kanagawa, Japan. Report no. 0686.
- Hollander, M., and Wolfe, D. A. 1973. Jonckheere’s Test. In: Non-parametric Statistical Methods, 1st Ed. (Hollander, M., and Wolfe, D. A., Eds)), New York: John Wiley and Sons, pp. 120–123.
- Jerne, N. K., Henry, C., Nordin, A. A., Fuji, H., Koros, A. M., and Lefkovits, I. 1974. Plaque forming cells: Methodology and theory. Transplant. Rev. 18:130–191.
- Luster, M. I., Munson, A. E., Thomas, P. T., Holsapple, M. P., Fenters, J. D., White, K. L., Jr, Lauer, L. D., Germolec, D. R., Rosenthal, G. J., and Dean, J. H. 1988. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program’s guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 10:2–19.
- McGregor, D. 2007. Ethyl tertiary-butyl ether: A toxicological review. Crit. Rev. Toxicol. 37:287–312.
- Medinsky, M. A., Wolf, D. C., Cattley, R. C., Wong, B., Janszen, D. B., Farris, G. M., Wright, G. A., and Bond, J. A. 1999. Effects of a thirteen-week inhalation exposure to ethyl tertiary butyl ether on Fisher-344 rats and CD-1 mice. Toxicol. Sci. 51:108–118.
- Mitsubishi Chemical Safety Institute Ltd. 2008a. A 90-Day Repeated Dose Toxicity Study of ETBE by Whole-body Inhalation Exposure in Rats. Testing laboratory: Mitsubishi Chemical Safety Institute Ltd, 4-2-8 Shibaura, Minato-ku, Toyko, Japan. Report no. B061829.
- Mitsubishi Chemical Safety Institute Ltd. 2008b. Pharmacokinetic study in rats treated with [14C] ETBE repeatedly for 14 days (translation). Testing laboratory: Mitsubishi Chemical Safety Institute Ltd, Kumamoto 869-0425, Japan. Report no. P070497.
- Nihlén, A., Löf, A., and Johanson, G. 1998. Controlled ethyl tert-butyl ether (ETBE) exposure of male volunteers. I. Toxicokinetics. Toxicol. Sci. 46:1–10.
- NRC (National Research Council). 1996. Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources, Commission on Life Sciences. Washington, DC: National Academy Press.
- Sokal, R. R., and Rohlf, F. J. ( Eds.) 1981. Biometry. San Francisco: Freeman, pp. 222–229.
- U.S. EPA (United States Environmental Protection Agency). 1998. Health Effects Test Guidelines OPPTS 870.7800 Immunotoxicity.
- White, K. L., Peachee, V. L., Armstrong, S. R., and Twerdok, L. E. 2004. Inhalation toxicity of gasoline and fuel oxygenates: Immunotoxicity. The Toxiclogist 78:148. ( Abstract #716).
- White, K. L., Jr., Musgrove, D. L., and Brown, R. D. 2010. The Sheep Erythrocyte T-Dependent Antibody Response (TDAR). In: Immunotoxicity Testing Methods and Protocols. (Dietert, R. R., Ed.), New York: Humana Press, pp. 173–184.
