Abstract
In addition to the effector T-cells subsets, T-cells can also differentiate into cells that play a suppressive or regulatory role in adaptive immune responses. The cell types currently identified as regulatory T-cells (Tregs) include natural or thymic-derived Tregs, T-cells which express Foxp3+CD25+CD4+ and can suppress immune responses to autoreactive T-cells, as well as inducible Tregs, that are generated from naïve T-cells in the periphery after interaction with antigens presented by dendritic cells. Inducible Tregs include TH3 cells, Tr1 cells, and Foxp3+-inducible Tregs. Tregs have been shown to be critical in the maintenance of immune responses and T-cell homeostasis. These cells play an important role in suppressing responses to self-antigens and in controlling inappropriate responses to non-self-antigens, such as commensal bacteria or food in the gut. For example, depletion of CD4+CD25+ Tregs from mice resulted in the development of multi-organ autoimmune diseases. CD4+CD25+ Tregs and/or IL-10-producing Tr1 cells are capable of suppressing or attenuating TH2 responses to allergens. Moreover, adoptive transfer of CD4+CD25+ Tregs from healthy to diseased animals resulted in the prevention or cure of certain autoimmune diseases, and was able to induce transplantation tolerance. Clinical improvement seen after allergen immunotherapy for allergic diseases such as rhinitis and asthma is associated with the induction of IL-10- and TGFβ-producing Tr1 cells as well as FoxP3-expressing IL-10 T-cells, with resulting suppression of the TH2 cytokine milieu. Activation, expansion, or suppression of CD4+CD25+ Tregs in vivo by xenobiotics, including drugs, may therefore represent a relevant mechanism underlying immunotoxicity, including immunosuppression, allergic asthma, and autoimmune diseases.
Introduction
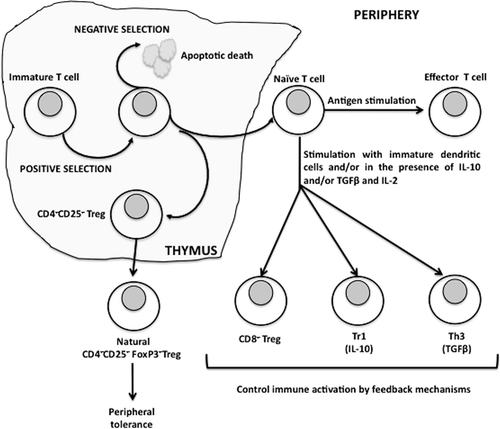
The existence of suppressor T-cells and their role in the maintenance of immune homeostasis was first proposed in the early 1970s (Gershon and Kondo, Citation1971). The re-discovery of suppressor T-cells as regulatory T-cells (Tregs) took place in the 1990s, and it is now fully accepted that distinct CD4+ T-cell subtypes, discriminated on the basis of phenotype or cytokine secretion, play a critical role in maintenance of self-tolerance (Bloom et al., Citation1992; Sakaguchi and Powrie, Citation2007). Tregs are a specialized subpopulation of T-cells critical in the modulation of immune responses and in the maintenance of T-cell homeostasis and self-tolerance (). There are several types of Tregs, including natural, T-helper (TH)-3 and T-regulatory (Tr)-1 subsets (Bach, Citation2003) as well as CD8+ Tregs (Filaci, Citation2007; Funatake et al., Citation2008; Dinesh, Citation2010).
Natural Tregs are generated in the thymus as a part of thymic selection as well as in the periphery in response to antigen exposure. Natural Tregs account for ≈5–10% of the total CD4+ T-cells in the periphery; they express CD4, high levels of CD25 and are also positive for the transcriptional repression factor fork-head box P3 (FoxP3). They are essential for the prevention of autoimmunity and keeping the immune response under control (Sakaguchi et al., Citation2006) as their absence results in multi-organ autoimmune disease in both mice and humans. Adaptive or induced Tregs are regulatory cells that develop in the periphery, and include Tr1 and TH3 cells that are CD4+CD25−FoxP3−. These Tregs secrete interleukin (IL)-10 and transforming growth factor (TGF)-β, and regulate immune responses. It has been suggested that in contrast to naturally occurring CD4+CD25+ Tregs, these cells represent altered states of differentiation rather than a unique cell lineage (Jonuleit and Schmitt, Citation2003). Tr1 cells are defined by their ability to produce large amounts of IL-10 and low to moderate levels of TGFβ, whereas TH3 cells produce preferentially TGFβ. In vitro stimulation of CD4+CD25−FoxP3− cells with TGFβ induces them to express FoxP3 and secrete TGFβ. They contribute to the development of antigen-specific immunosuppressive responses (Roncarolo and Levings, Citation2000). Immunoregulatory CD8+CD28− Tregs are present and functional in many human tumors, being able to inhibit both T-cell proliferation and cytotoxicity (Filaci, Citation2007). Data are accumulating showing the potential role of CD8+ Tregs in other diseases, including chronic infection and autoimmunity (Dinesh, Citation2010).
The constitutive expression of CD25 has been considered to be a characteristic feature in the identification of Tregs; however, activated T-cells also up-regulate CD25. CD4+CD25− T-cells that express FoxP3 have been demonstrated during immune responses associated with bacterial infection (Fontenot et al., Citation2005). The expression of FoxP3 is therefore the most definitive marker to identify Tregs. CD25 and FoxP3 are also expressed by CD8+ Tregs. Down-regulation of CD127, a subunit of the IL7R, is another mechanism to identify Tregs as it is generally accepted that the CD25high/CD127low/- phenotype is specific to these cells (Liu et al., Citation2006).
The balance between activated effector cells and Tregs is important for efficient immune responses. Just as important as mounting an immune response, organisms have to control the magnitude and duration of the response. Suppression of Treg function and/or number may lead to unchecked immune responses resulting in autoimmune diseases, allergies, transplant rejection, maternal intolerance of fetal alloantigens, septic shock and chronic inflammation (Kondelkova et al., 2010). Enhancement of regulatory T-cell functions is a therapeutic goal for the treatment of many autoimmune diseases, and therapeutics such as rapamycin and its derivatives, and corticosteroids have been shown to expand Tregs (Smith and Kumar Citation2008; Powell and Delgoffe, Citation2010; Stary et al., Citation2011). Murine Tregs are more resistant than effector T-cells to the apoptotic effects of dexamethasone in vitro and treatment of mice with dexamethasone results in an increased proportion of Tregs in immune tissues (Chen et al., Citation2004). A Treg suppression of inflammatory responses can be both a desired and undesired effect depending on the disease. There is growing evidence that the suppressive effects of Tregs may contribute to tumor growth (Colombo and Piconese, Citation2007), and to poor responses to infectious agents and vaccines (Rouse et al., Citation2006). Therefore, the ability of xenobiotics to inhibit or activate/expand Tregs may represent a relevant mechanism underlying chemical-induced immunotoxicity (Fort and Narayanan, Citation2010).
As part of the 49th Annual Meeting of the Society of Toxicology in 2010, a symposium entitled ‘Alterations in Regulatory T-Cells: Novel Pathways to Immunotoxicology’ was convened to explore the role that Tregs may play in chemical-induced immunotoxicity. The Symposium started with an ‘Introduction to the role of Tregs in immunity’ by Mohamed Oukka, and was followed by presentations on ‘Role of immunoregulatory cells in chemical and protein allergy’ by Raymond Pieters, ‘Induction of AHR-dependent Tregs: A novel pathway for TCDD immunotoxicity’ by Nancy Kerkvliet, and ‘Safety assessment of immunomodulatory biologics post-TGN1412: The promise and challenges of regulatory T-cell modulation’ by Rafael Ponce. The purpose of this review article is to highlight the role of regulatory T-cells in allergy and in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced immunosuppression; a separate paper will deal with the pharmacological modulation of regulatory T-cells.
Role of immunoregulatory cells at sites of exposure
The identification of Tregs capable of suppressing responses mediated by TH1 and TH2 cells has prompted a paradigm shift in our understanding of the regulation of immune responses. Regulatory T-cells, including Tr1 and TH3, are crucial for the induction of tolerance to food antigens, and in the maintenance of tolerance at mucosal surfaces (Battaglia et al., Citation2006; Lan et al., Citation2007). The skin, the respiratory and the gastrointestinal tract are continuously exposed to a large number of antigens, representing a relentless challenge to the immune system. An appropriate immune response must be able to differentiate between harmful and innocuous antigens. To protect the host, mechanisms must be in place to prevent deleterious reactions, such as immune responses to self-antigens, immune response to food antigens in the intestine, or exaggerated immune responses to benign environmental antigens. One key adaptation to the mucosal microenvironment is the generation within, or recruitment to the mucosal surface of Tregs (Allez and Mayer, Citation2004). Disturbance of normal immunoregulatory processes at mucosal surfaces, i.e., the breaking of tolerance, may occur, resulting in sensitization to chemicals, certain pharmaceuticals and food or airborne proteins.
Key components of the mucosal microenvironment include the luminal surface, epithelial cells, the lamina propria, and mucosal-associated lymphoid tissues (MALT), which can be found within the mucosal layers. The mucosal epithelium forms a tight protective barrier facilitated by integral membrane proteins and adhesion molecules, while the thick mucous secreted by specialized epithelial cells such as goblet cells provides another important barrier. Beneath the epithelial barrier, the lamina propria plays a critical role in host defense with its network of capillaries and lymphatics.
Communication is constantly established between the external environment and the host immune system. Mucosal epithelial cells actively participate in mucosal immunity through production of anti-microbial peptides, chemokines, and cytokines, and are equipped with pattern recognition receptors (PRRs) including Toll-like receptors and nucleotide-binding oligomerization domain (NOD) receptors: such cells also have antigen-presenting capability (Kunisawa et al., Citation2007).
Functional and customized Treg cells
Naïve CD4+ T-cells can differentiate into many different subsets of TH effector cells, notably TH1, TH2, TH9, and TH17 cells. The functional specialization of these various CD4+ T-cell subsets is due to the differential expression of ‘master’ transcription factors, namely retinoic acid receptor-related orphan receptor-γt (RORγt), T-bet, and GATA-binding protein 3 (GATA3), which turn on distinct programs of gene expression that control T-cell function and migration (Littman and Rudensky, Citation2010). However, each of these responses is pro-inflammatory and, in some circumstances, can damage tissues and lead to autoimmunity. Furthermore, TH1, TH2, and TH17 cells express various chemokine receptors that help guide these cells into appropriate tissues in which these cells are needed to fight various pathogens.
Recently, several groups demonstrated that Treg cells are not all created equal; various natural-occurring Treg subsets exist and express transcriptional factors and chemokine receptors commonly found in either TH1 or TH17 cells (Chaudhry et al., Citation2009; Koch et al., Citation2009; Zheng et al., Citation2009). These Tregs are rather specialized in preferentially suppressing TH1 or TH17 cells. T-bet is the master transcription factor controlling the differentiation, migration, and function of interferon (IFN)-γ-producing TH1 cells (Szabo et al., Citation2000). Interestingly, T-bet is also expressed by a subset of Treg cells, and is required for Treg cell homeostasis and function during polarized TH1-type immune response (Koch et al., Citation2009). Finally, deletion of signal transducer and activation of transcription-3 (STAT3) in Treg cells results in the development of a spontaneous fatal intestinal inflammation that is characterized by excessive IL-17 production, but normal levels of TH1-associated inflammatory cytokines; this indicates that there is selective dysregulation of TH17-type responses in the absence of STAT3-expressing Treg cells (Chaudhry et al., Citation2009). The mechanisms by which T-bet and STAT3 control Treg cell activity during TH1, TH2, and TH17 cell-mediated responses are still unclear, but are likely to involve a combination of influences on Treg cell migration, function and homeostasis. Additionally, loss of these transcription factors may affect the functional properties of Treg cells.
From these data, a model emerges in which selective expression or activation of transcriptional regulators associated with TH1, TH2, and TH17 cells drives the phenotypical and functional specialization of Treg cells, and the expression of subset of specific genes needed to restrain these different types of CD4+ T-cell responses.
Role of immunoregulatory cells in chemical and protein allergy
As early as 1970, studies in guinea pigs demonstrated that dermal sensitization to 2,4-dintrochlorobenzene (DNCB) is subject to tolerance induction by oral pre-exposure to DNCB (Lowney, Citation1971). In rodents, tolerance induction could be prevented by cyclophosphamide, which has been shown to inhibit Tregs (Ikezawa et al., Citation2005). Subsequently, in the 1980s and 1990s, the mechanisms regulating sensitization became clearer through studies using multiple rat strains to examine autoimmune phenomena, particularly following exposure to mercuric chloride (Pelletier et al., Citation1988, Citation1990; Mathieson et al., Citation1991; Kosuda et al., Citation1994). Brown Norway rats recovering from mercury induced autoimmunity became resistant to disease induction upon re-exposure to the same compound. A similar phenomenon was observed in rats treated with and then re-exposed to D-penicillamine (Masson et al., Citation2004; Seguin et al., Citation2004) and nevirapine (Shenton et al., Citation2003). In mice, similar immunoregulatory processes were found to occur in response to procainamide (Layland et al., Citation2004), nickel (Roelofs-Haarhuis et al., Citation2004), and contact sensitizers such as DNCB (Ju et al., Citation2003). Adoptive transfer and/or depletion of specific T-cell populations in these rodent models of allergy and autoimmunity reinforced the concept that active regulation by CD4+ and CD8+ T-cells is one of the key mechanisms for the maintenance of self-tolerance and protection from sensitization and self-reactivity (Field et al., Citation2003; Roelofs-Haarhuis et al., Citation2004; Seguin et al., Citation2004; Shenton et al., 2005).
Additional regulatory mechanisms for compounds that induce hypersensitivity include NK-T cells, dendritic cells (DC), and Kupffer cells. When activated, these cells have been shown to induce and/or expand Treg (Ju et al., Citation2003; Roelofs-Haarhuis et al., Citation2004). Tolerance, and induction of regulatory mechanisms to protein allergens, is dependent on route of exposure, dose, genotype, gender, and age (neonatal tolerance; Field et al., Citation2000, Citation2003). Oral exposure can induce anergic T-cells with regulatory activity (Artik et al., Citation2001) and the presence of commensal organisms, CD8+ intraepithelial lymphocytes, and DC in the gut foster a tolerogenic environment (Weiner et al., Citation2011). It has been suggested that low dose oral exposure to protein allergens favors the induction of Tregs, whereas higher doses favor the induction of anergy or deletion of reactive T-cells (Weiner et al., Citation2011). Low dose oral tolerance has been shown to prevent mercury induced autoimmune disease (Szeto et al., Citation1999; Pelletier et al., Citation1990; Mathieson et al., Citation1991) with both compound-specific and non-specific T-cell regulation described (Layland et al., Citation2004). For example, CD8+ suppressor cells from mercuric chloride-exposed rats appear to act through similar regulatory cytokines as has been described for CD4+ Tregs, i.e., TGFβ and IL-10, as well as via IFNγ- and receptor-mediated regulation (e.g., CTLA-4).
Orally-induced tolerance is of particular relevance in case of food allergy, where there is a lack of tolerance and proper immunoregulation to specific proteins (Mowat et al., Citation2003). Several mouse studies with food allergens indicate that CD25+ Tregs are at least of some importance in preventing responses to peanut (van Wijk et al., Citation2007) and milk proteins (Schouten et al., Citation2010). However, other regulatory processes may also be involved, including IFNγ-producing cells, other regulatory T-cells (Rezende, Citation2011), gδ T-cells (Bol-Schoenmakers et al., Citation2011), and regulatory mucosal DC (Smit et al., Citation2011).
Induction of AhR-dependent Tregs: A novel pathway for TCDD immunotoxicity
TCDD is an aryl hydrocarbon receptor (AhR) ligand that induces immunosuppression in experimental animals, including thymic involution, decreased antibody production and cytotoxic T-lymphocyte development, and increased susceptibility to a variety of infectious diseases (Kerkvliet et al., Citation2002). It has been shown that the immunosuppressive effect of TCDD in mice correlated with CD25+CD4+ regulatory T-cell generation (Funatake et al., Citation2005; Kerkvliet et al., Citation2009).
Like natural Tregs, TCDD-derived CD4+ T-cells did not produce IL-2 and their suppressive function was contact dependent (Marshall et al., Citation2008). Depending on the experimental model, TCDD-derived Tregs tended to secrete significant amounts of IL-10 or TGFβ in response to both polyclonal and allogeneic stimuli (Marshall et al., Citation2008; Quintana et al., Citation2008). Interestingly, in one study, exposure of murine immune cells to 6-formylindolo(3,2-b) carbazole (another AhR ligand) in combination with TGFβ resulted in the formation of pro-inflammatory IL-17-secreting T-cells, that could exacerbate inflammation in vivo (Quintana et al., Citation2008).
It remains to be determined whether the generation of IL-10- or TGFβ-producing CD4+ T-cells is a major component of TCDD-induced immunosuppression in experimental animals. Proposed mechanisms by which AhR signaling might promote Treg differentiation include reduction in CD62L expression in T-cells (Funatake et al., Citation2004, Citation2005), regulation of FoxP3 expression (Quintana et al., Citation2008; Hauben et al., Citation2008; Kimura et al., Citation2008), and/or modulation of DC antigen presentation. Antigen presentation by DC plays a key role in converting naïve T-cells into adaptive Tregs. TCDD-induced activation of the AhR causes an increase in CTLA-4 expression in T-cells that may induce tolerogenic DC (Funatake et al., Citation2005). In the absence of the appropriate cytokines, DC can induce clonal deletion, anergy or tolerogenic regulatory T-cells (Vorderstrasse and Kerkvliet, Citation2001; Thorstenson and Khoruts, Citation2001; Yamazaki et al., Citation2006). In addition, signaling through the AhR may up-regulate TGFβ signaling, promoting the expansion and function of Tregs (Huber et al., Citation2004).
The AhR has also been reported to play a role in the development of TH17 cells (Quintana et al., Citation2008; Veldhoen et al., Citation2008a). TH17 cells are characterized by their secretion of pro-inflammatory cytokines IL-17 and IL-22. The ligand-activated AhR regulates expression of these cytokines in tissue culture. A role for the AhR in the regulation of TH17 cells is supported further by the observation that the absolute number of TH17 cells is reduced in AhR null mice upon induction of Experimental Autoimmune Encephalomyelitis (Veldhoen et al., Citation2008b). Because TH17 cells promote the immune response and Tregs are known to decrease immune reactivity, a model has emerged suggesting that the Treg/TH17 balance distinguishes an effective immune response and self-antigen tolerance from chronic infection or autoimmunity. Preliminary evidence from multiple laboratories has suggested that the AhR modifies the Treg/TH17 cell balance through modifying the cytokine milieu. The mechanism at work may be related to the fact that TGFβ induces Treg differentiation, while the presence of IL-6 leads to TGFβ-dependent TH17 cell production. Elucidating the specific transcriptional targets of the AhR that result in the induction of Tregs through the used of potent and specific AhR ligands such as TCDD that may provide the most direct route to understand signaling steps by which the AhR influences the immune system.
Concluding remarks
The concept of regulatory or suppressor cells having a role in chemical-induced immune system toxicology has been somewhat understudied. However, it is now recognized that Tregs play a critical role in maintaining the careful balancing act that allows the immune system to respond appropriately in the face of infection or disease, resolve when the challenge has diminished, and fail to respond to self-antigens. As shown by the speakers in this symposium, various immunoregulatory T-cell subsets may be induced by environmental chemicals and protein allergens. Chemical-induced alterations in Tregs number or function may be of pivotal importance in the clinical outcomes following chemical exposure and further research is needed to elucidate the role of Tregs in chemical-induced modulation of immune function and how this may impact human health and the burden of disease.
Declaration of interest
The Authors declare of not having any financial, personal, or association with any of individuals or organizations that could have inappropriately influence the submitted work. The Authors alone are responsible for the content and writing of the paper. This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH); however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
References
- Allez, M. and Mayer, L. 2004. Regulatory T cells: Peace keepers in the gut. Inflamm. Bowel Dis. 10:666–676.
- Artik, S., Haarhuis, K., Wu, X., Begerow, J. and Gleichmann, E. 2001. Tolerance to nickel: Oral nickel administration induces a high frequency of anergic T-cells with persistent suppressor activity. J. Immunol. 167:6794–6803.
- Bach, J. F. and François Bach, J. 2003. Regulatory T-cells under scrutiny. Nat. Rev. Immunol. 3:189–198.
- Battaglia, M., Gregori, S., Bacchetta, R. and Roncarolo, M. G. 2006. Tr1 cells: From discovery to their clinical application. Semin. Immunol. 18:120–127.
- Bloom, B. R., Salgame, P. and Diamond, B. 1992. Revisiting and revising suppressor T-cells. Immunol. Today 13:131–136.
- Bol-Schoenmakers, M., Marcondes Rezende, M., Bleumink, R., Boon, L., Man, S., Hassing, I., Fiechter, D., Pieters, R. H. and Smit, J. J. 2011. Regulation by intestinal gδ T-cells during establishment of food allergic sensitization in mice. 66:331–340.
- Chaudhry, A., Rudra, D., Treuting, P., Samstein, R. M., Liang, Y., Kas, A. and Rudensky, A. Y. 2009. CD4+ regulatory T-cells control Th17 responses in a Stat3-dependent manner. 326:986–991.
- Chen, X., Murakami, T., Oppenheim, J. J. and Howard, O. M. 2004. Differential response of murine CD4+CD25+ and CD4+CD25- T-cells to dexamethasone-induced cell death. Eur. J. Immunol. 34:859–869.
- Colombo, M. P. and Piconese, S. 2007. Regulatory-T-cell inhibition versus depletion: The right choice in cancer immunotherapy. Nat. Rev. 7:880–887.
- Dinesh, R. K., Skaggs, B. J., La Cava, A., Hahn, B. H. and Singh, R. P. 2010. CD8+ Tregs in lupus, autoimmunity, and beyond. Autoimmun. Rev. 9:560–568.
- Field, A. C., Caccavelli, L., Bloch, M. F. and Bellon, B. 2003. Regulatory CD8+ T-cells control neonatal tolerance to a Th2-mediated autoimmunity. J. Immunol. 170:2508–2515.
- Field, A. C., Caccavelli, L., Fillion, J., Kuhn, J., Mandet, C., Druet, P. and Bellon, B. 2000. Neonatal induction of tolerance to T(h)2-mediated autoimmunity in rats. Int. Immunol. 12:1467–1477.
- Filaci, G., Fenoglio, D., Fravega, M., Ansaldo, G., Borgonovo, G., Traverso, P., Villaggio, B., Ferrera, A., Kunkl, A., Rizzi, M., Ferrera, F., Balestra, P., Ghio, M., Contini, P., Setti, M., Olive, D., Azzarone, B., Carmignani, G., Ravetti, J. L., Torre, G. and Indiveri, F. 2007. CD8+ CD28- T-regulatory lymphocytes inhibiting T-cell proliferative and cytotoxic functions infiltrate human cancers. J. Immunol. 179:4323–4334.
- Fontenot, J. D., Rasmussen, J. P., Williams, L. M., Dooley, J. L., Farr, A. G. and Rudensky, A. Y. 2005. Regulatory T-cell lineage specification by the forkhead transcription factor FoxP3. 22:329–341.
- Fort, M. M. and Narayanan, P. K. 2010. Manipulation of regulatory T-cell function by immunomodulators: A boon or a curse? Toxicol. Sci. 117:253–262.
- Funatake, C. J., Dearstyne, E. A., Steppan, L. B., Shepherd, D. M., Spanjaard, E. S., Marshak-Rothstein, A. and Kerkvliet, N. I. 2004. Early consequences of 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on the activation and survival of antigen-specific T-cells. Toxicol. Sci. 82:129–142.
- Funatake, C. J., Marshall, N. B. and Kerkvliet, N. I. 2008. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation of alloreactive CD8+ T-cells toward a regulatory T-cell phenotype by a mechanism that is dependent on aryl hydrocarbon receptor in CD4+ T-cells. J. Immunotoxicol. 5:81–91.
- Funatake, C. J., Marshall, N. B., Steppan, L. B., Mourich, D. V. and Kerkvliet, N. I. 2005. Cutting edge: Activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T-cells J. Immunol. 175:4184–4188.
- Gershon, R. K. and Kondo, K. 1971. Infectious immunological tolerance. Immunology. 21:903–914.
- Hauben, E., Gregori, S., Draghici, E., Migliavacca, B., Olivieri, S., Woisetschläger, M. and Roncarolo, M. G. 2008. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T-cells. Blood 112:1214–1222.
- Huber, S., Schramm, C., Lehr, H. A., Mann, A., Schmitt, S., Becker, C., Protschka, M., Galle, P. R., Neurath, M. F. and Blessing, M. 2004. Cutting edge: TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T-cells. J. Immunol. 173:6526–6531.
- Ikezawa, Y., Nakazawa, M., Tamura, C., Takahashi, K., Minami, M. and Ikezawa, Z. 2005. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T-cells, which suppress induction of contact hypersensitivity. J. Dermatol. Sci. 39:105–112.
- Jonuleit, H. and Schmitt, E. 2003. The regulatory T-cell family: Distinct subsets and their interrelations. J. Immunol. 171:6323–6327.
- Ju, C., McCoy, J. P., Chung, C. J., Graf, M. L. and Pohl, L. R. 2003. Tolerogenic role of Kupffer cells in allergic reactions. Chem. Res. Toxicol. 16:1514–1519.
- Kerkvliet, N. I. 2002. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int. Immunopharmacol. 2:277–291.
- Kerkvliet, N. I., Steppan, L. B., Vorachek, W., Oda, S., Farrer, D., Wong, C. P., Pham, D. and Mourich, D. V. 2009. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases FoxP3+ T-cells in pancreatic lymph nodes. Immunotherapy. 1:539–547.
- Kimura, A., Naka, T., Nohara, K., Fujii-Kuriyama, Y. and Kishimoto, T. 2008. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of TH17 cells. Proc. Natl. Acad. Sci. U.S.A. 105:9721–9726.
- Koch, M. A., Tucker-Heard, G., Perdue, N. R., Killebrew, J. R., Urdahl, K. B. and Campbell, D. J. 2009. The transcription factor T-bet controls regulatory T-cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10:595–602.
- Kondelková, K., Vokurková, D., Krejsek, J., Borská, L., Fiala, Z. and Ctirad, A. 2010. Regulatory T-cells (Treg) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove). 53:73–77.
- Kosuda, L. L., Hosseinzadeh, H., Greiner, D. L. and Bigazzi, P. E. 1994. Role of RT6+ T-lymphocytes in mercury-induced renal autoimmunity: Experimental manipulations of “susceptible” and “resistant” rats. J. Toxicol. Environ. Health. 42:303–321.
- Kunisawa, J., Takahashi, I. and Kiyono, H. 2007. Intraepithelial lymphocytes: Their shared and divergent immunological behaviors in the small and large intestine. Immunol. Rev. 215:136–153.
- Lan, R. Y., Mackay, I. R. and Gershwin, M. E. 2007. Regulatory T-cells in the prevention of mucosal inflammatory diseases: Patrolling the border. J. Autoimmun. 29:272–280.
- Layland, L. E., Wulferink, M., Dierkes, S. and Gleichmann, E. 2004. Drug-induced autoantibody formation in mice: Triggering by primed CD4+CD25- T-cells, prevention by primed CD4+CD25+ T-cells. Eur. J. Immunol. 34:36–46.
- Littman, D. R. and Rudensky, A. Y. 2010. TH17 and regulatory T-cells in mediating and restraining inflammation. Cell 140:845–858.
- Liu, W., Putnam, A. L., Xu-Yu, Z., Szot, G. L., Lee, M. R., Zhu, S., Gottlieb, P. A., Kapranov, P., Gingeras, T. R., Fazekas de St Groth, B., Clayberger, C., Soper, D. M., Ziegler, S. F. and Bluestone, J. A. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J. Exp. Med. 203:1701–1711.
- Lowney, E. D. 1971. Tolerance of dinitrochlorobenzene, a contact sensitizer, in man. J. Allergy Clin. Immunol. 48:28–35.
- Marshall, N. B., Vorachek, W. R., Steppan, L. B., Mourich, D. V. and Kerkvliet, N. I. 2008. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T-cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Immunol. 181:2382–2391.
- Masson, M. J. and Uetrecht, J. P. 2004. Tolerance induced by low dose D-penicillamine in the Brown Norway rat model of drug-induced autoimmunity is immune-mediated. Chem. Res. Toxicol. 17:82–94.
- Mathieson, P. W., Stapleton, K. J., Oliveira, D. B. and Lockwood, C. M. 1991. Immunoregulation of mercuric chloride-induced autoimmunity in Brown Norway rats: A role for CD8+ T-cells revealed by in vivo depletion studies. Eur. J. Immunol. 21:2105–2109.
- Mowat, A. M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3:331–341.
- Quintana, F. J., Basso, A. S., Iglesias, A. H., Korn, T., Farez, M. F., Bettelli, E., Caccamo, M., Oukka, M. and Weiner, H. L. 2008. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 453:65–71.
- Pelletier, L., Pasquier, R., Rossert, J., Vial, M. C., Mandet, C. and Druet, P. 1988. Autoreactive T-cells in mercury-induced autoimmunity. Ability to induce the autoimmune disease. J. Immunol. 140:750–754.
- Pelletier, L., Rossert, J., Pasquier, R., Vial, M. C. and Druet, P. 1990. Role of CD8+ T-cells in mercury-induced autoimmunity or immunosuppression in the rat. Scand. J. Immunol. 31:65–74.
- Powell, J. D. and Delgoffe, G. M. 2010. The mammalian target of rapamycin: Linking T-cell differentiation, function, and metabolism. Immunity 33:301–311.
- Rezende, M. M., Hassing, I., Bol-Schoenmakers, M., Bleumink, R., Boon, L., van Bilsen, J. and Pieters, R. 2011. CD4+CD25+ T-regulatory cells do not transfer oral tolerance to peanut allergens in a mouse model of peanut allergy. Clin. Exp. Allergy.
- Roelofs-Haarhuis, K., Wu, X. and Gleichmann, E. 2004. Oral tolerance to nickel requires CD4+ invariant NKT-cells for the infectious spread of tolerance and the induction of specific regulatory T-cells. J. Immunol. 173:1043–1050.
- Roncarolo, M. G. and Levings, M. K. 2000. The role of different subsets of T-regulatory cells in controlling autoimmunity. Curr. Opin. Immunol. 12:676–683.
- Rouse, B. T., Sarangi, P. P. and Suvas, S. 2006. Regulatory T-cells in virus infections. Immunol. Rev. 212:272–286.
- Sakaguchi, S., Ono, M., Setoguchi, R., Yagi, H., Hori, S., Fehervari, Z., Shimizu, J., Takahashi, T. and Nomura, T. 2006. FoxP3+ CD25+ CD4+ natural regulatory T-cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212:8–27.
- Sakaguchi, S. and Powrie, F. 2007. Emerging challenges in regulatory T-cell function and biology. Science 317:627–629.
- Schouten, B., van Esch, B. C., Hofman, G. A., Boon, L., Knippels, L. M., Willemsen, L. E. and Garssen, J. 2010. Oligosaccharide-induced whey-specific CD25+ regulatory T-cells are involved in the suppression of cow milk allergy in mice. J. Nutr. 140:835–841.
- Séguin, B., Masson, M. J. and Uetrecht, J. 2004. D-Penicillamine-induced autoimmunity in the Brown Norway rat: Role for both T- and non-T splenocytes in adoptive transfer of tolerance. Chem. Res. Toxicol. 17:1299–1302.
- Shenton, J. M., Teranishi, M., Abu-Asab, M. S., Yager, J. A. and Uetrecht, J. P. 2003. Characterization of a potential animal model of an idiosyncratic drug reaction: Nevirapine-induced skin rash in the rat. Chem. Res. Toxicol. 16:1078–1089.
- Smit, J. J., Bol-Schoenmakers, M., Hassing, I., Fiechter, D., Boon, L., Bleumink, R. and Pieters, R. H. 2011. The role of intestinal dendritic cells subsets in the establishment of food allergy. Clin. Exp. Allergy 41:890–898.
- Smith, T. R. and Kumar, V. 2008. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 29:337–342.
- Stary, G., Klein, I., Bauer, W., Koszik, F., Reininger, B., Kohlhofer, S., Gruber, K., Skvara, H., Jung, T. and Stingl, G. 2011. Glucocorticosteroids modify Langerhans cells to produce TGF ß and expand regulatory T-cells. J. Immunol. 186:103–112.
- Stevens, E. A., Mezrich, J. D. and Bradfield, C. A. 2009. The aryl hydrocarbon receptor: A perspective on potential roles in the immune system. Immunology 127:299–311.
- Szabo, S. J., Kim, S. T., Costa, G. L., Zhang, X., Fathman, C. G. and Glimcher, L. H. 2000. A novel transcription factor, T-bet, directs TH1 lineage commitment. Cell 100:655–669.
- Szeto, C., Gillespie, K. M. and Mathieson, P. W. 1999. Low-dose mercuric chloride induces resistance in Brown Norway rats to further mercuric chloride by up-regulation of interferon-γ. Scand. J. Immunol. 50:195–201.
- Thorstenson, K. M. and Khoruts, A. 2001. Generation of anergic and potentially immunoregulatory CD25+CD4+ T-cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167:188–195.
- van Wijk, F., Wehrens, E. J., Nierkens, S., Boon, L., Kasran, A., Pieters, R. and Knippels, L. M. 2007. CD4+CD25+ T-cells regulate the intensity of hypersensitivity responses to peanut, but are not decisive in the induction of oral sensitization. Clin. Exp. Allergy 37:572–581.
- Veldhoen, M., Hirota, K., Christensen, J., O’Garra, A. and Stockinger, B. 2009a. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of TH17 T-cells. J. Exp. Med. 206:43–49.
- Veldhoen, M., Hirota, K., Westendorf, A. M., Buer, J., Dumoutier, L., Renauld, J. C. and Stockinger, B. 2008b. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453:106–109.
- Vorderstrasse, B. A. and Kerkvliet, N. I. 2001. 2,3,7,8-Tetrachlorodibenzo-p-dioxin affects the number and function of murine splenic dendritic cells and their expression of accessory molecules. Toxicol. Appl. Pharmacol. 171:117–125.
- Weiner, H. L., da Cunha, A. P., Quintana, F. and Wu, H. 2011. Oral tolerance. Immunol. Rev. 241:241–259.
- Yamazaki, S., Inaba, K., Tarbell, K. V. and Steinman, R. M. 2006. Dendritic cells expand antigen-specific FoxP3+CD25+CD4+ regulatory T-cells including suppressors of alloreactivity. Immunol. Rev. 212:314–329.
- Zheng, Y., Chaudhry, A., Kas, A., deRoos, P., Kim, J. M., Chu, T. T., Corcoran, L., Treuting, P., Klein, U. and Rudensky, A. Y. 2009. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458:351–356.