Abstract
Bresol–a poly-herbal formulation, has been reported to be effective against bronchial asthma and allergic rhinitis in children. In vivo studies have supported the anti-histaminic and anti-anaphylactic action of bresol. However, the mechanism of action of bresol in modulation of inflammation has not been studied at the cellular and molecular level. The present study was aimed to elucidate the mechanism(s) of action of bresol at the cellular and molecular levels, using human monocyte leukemia cells. The effects of bresol on phosphodiesterase 4B (PDE4B) gene expression were analyzed using human monocytic U937 leukemia cells. The ability of bresol to stimulate cAMP formation in these cells, as well as its effects on mediators of inflammation like tumor necrosis factor-α (TNFα), nitric oxide (NO), and cycloxygenase-2 (COX-2) in lipopolysaccharide (LPS)-stimulated U937 cells, were also studied. The results here indicated that bresol exhibited potential anti-inflammatory properties by inhibiting LPS-induced PDE4B gene expression in the cells. Bresol also dose dependently activated cAMP formation, and inhibited TNFα, NO, as well as COX-2 formation in the LPS-stimulated cells. Based upon the results, we concluded that the reported anti-inflammatory activity of bresol might be attributed to its abilities to inhibit PDE4B and thus elevate cAMP levels in human monocytes. The anti-inflammatory effects of bresol might also be a result of the capacity of bresol to modulate the formation of TNFα, NO, and COX-2 in monocytes.
Introduction
Allergic rhinitis (AR) and asthma are disorders of the airway characterized by inflammation of the respiratory mucosa and involve similar inflammatory cells and mediators. AR and asthma affects ≈15–40% of individuals and have a considerable impact on the quality of life (Guerra et al., Citation2002). AR affects the upper respiratory tract especially in children whereas asthma affects the lower respiratory tract. Often, asthma and AR are comorbid conditions with AR being a major risk factor for the occurrence of asthma (Jeffery and Haahtela, Citation2006).
At present, first-line drug therapy for asthma and AR includes topical intranasal steroids and oral antihistamines. However, there is a long standing discussion on the potential side effects of these medications. Long-term treatment with the available topical steroids might lead to atrophy of the rhinal mucosa and, especially in children, to systemic side effects, and some anti-histaminic drugs might cause drowsiness or cardiac side effects (Schmidt et al., Citation2001). Despite the fact that the new generations of topical steroids and oral antihistamines seem to have considerably more moderate side effect profiles, the need remains for alternative drug therapies that are both safe and efficacious in AR and asthma.
The PDE4 family of cyclic AMP specific phosphodiesterase inhibitors are a group of potential therapeutic agents, which are currently under investigation for use in allergic asthma and rhinitis (Giembycz, 2000). These drugs exert anti-inflammatory activity by blocking the degradation of cyclic adenosine monophosphate in lymphocytes, eosinophils, neutrophils, and monocytes, and thus leading to an attenuated release of histamines, leukotrienes and several cytokines. These effects have been well established in vitro and in animal models of allergic asthma (Giembycz, 2000).
Bresol, a poly-herbal formulation, is claimed to be useful in bronchial asthma and AR in children (Chatterjee et al., Citation2004; Pandey et al., Citation2010). The anti-histaminic and anaphylactic activity of bresol is also reported (Gopumadhavan et al., Citation2005). The composition of bresol includes eleven medicinal plant extracts which include Curcuma longa, Ocimum sanctum, Adhatoda vasica, Trikatu, Triphala, Embelia ribes, Cyperus rotundus, Cinnamomum zeylanicum, Elettaria cardamomum, Cinnamomum tamala, and Mesua ferrea. The medicinal properties of each ingredient of this composition and its usage in the treatment of allergy and bronchial disorders have been described by Gopumadhavan et al. (Citation2005). However, the cellular signaling mechanism of the formulation needs to be investigated to understand the effectiveness of bresol in the treatment of AR and asthma.
In light of this information, the present study was aimed to elucidate the mechanism of action of bresol at cellular and molecular level. The effect of bresol on phosphodiesterase 4B gene expression was analyzed in vitro using human monocyte leukemia cells U937. The ability of bresol to stimulate cAMP activity in U937 cells was investigated. The effect of bresol in modulating the levels of other mediators of inflammation like TNFα, NO, and COX-2 in these cells was also studied.
Materials and methods
Materials
RPMI 1640 media, Dulbecco’s modified essential media (DMEM), fetal bovine serum (FBS), actinomycin D, dexamethasone, Rolipram, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), TRI reagent, custom-synthesized oligonucleotides, N-1-naphtyl-ethylene diamine dihydrochloride, sulfanilimide, sodium nitrite, dimethyl sulfoxide (DMSO), and lipopolyasccharide (LPS) were obtained from Sigma Chemical Co. (St. Louis, MO). Bresol syrup was obtained from the distribution unit of Himalaya Drug Company (Bangalore, India). The ingredients of the syrup have been described previously (Chatterjee et al., Citation2004). Penicillin and streptomycin were obtained from Himedia (Mumbai, India). All enzymes and other chemicals required for polymerase chain reactions (PCR) were from MBI Fermentas (Glen Burnie, MD). cAMP and COX inhibitor screening kits were purchased from Cayman (Ann Arbor, MI). All other chemicals used in the experiments were of molecular biology grade.
Cell culture
U937 cells were obtained from the National Centre for Cell Sciences (Pune, India). The cells were cultured at 37°C in a humidified 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% FBS, 100 U penicillin/ml, 10 µg streptomycin/ml, and harvested with trypsin-EDTA. Cells were seeded on 96-well plates and 40 mm petri dishes (Tarsons, Kolkata, India), and grown to confluence by incubating for 24 h at 37°C. These confluent cells were used for further experiments.
Cytotoxicity assay
The toxicity of bresol syrup was analysed by incubating the U937 cells for 24 h at 37°C with different concentrations of syrup. Bresol syrup was completely solubilized in RPMI medium, and then directly added to the cells at concentrations ranging from 0.5% to 10.0%. Thereafter, the cells were incubated for 24 h at 37°C. An MTT assay was performed (Varma et al., Citation2011a) to assess the basal cytotoxicity of the syrup. The resultant formazan dye was extracted with DMSO and spectrophotometric readings taken at 540 nm using a Synergy HT spectrophotometer (Bio-Tek, Winooski, VT, USA). From the values obtained, the percentage toxicity (relative to survival of control cells) was calculated. Only non-toxic concentrations of the drug were used for further experiments.
PDE4B gene expression
U937 cells at a density of 5 × 105/ml (cells/ml) were seeded into 40 mm petri dishes and grown to confluence overnight at 37°C. The cells were then treated with different concentrations of bresol in RPMI medium containing 2% FBS and 0.1 µg LPS/ml; rolipram, a standard PDE4B inhibitor, was added at 30 µM to parallel sets of LPS-treated cells. All dishes were then incubated at 37°C for 24 h before their cells were harvested and their RNA extracted using TRI reagent. Recovered total RNA was subjected to DNase I treatment prior to reverse transcription to rid the samples of any contaminating DNA. RNA quality and quantity was then evaluated using denaturing agarose gel electrophoresis and spectrophotometric analyses.
First-strand complementary DNA (cDNA) was generated from 3 µg total RNA using random hexamer primers and 500 U reverse transcriptase enzyme. The PCR amplification was carried out using human PDE4B-specific gene. GAPDH was used as the internal control. The primers for PDE4B and GAPDH were designed based on the previous reports (Mehats et al., 1999); the sequences of primers used for amplifications are listed in . The amplification profile consisted of initial denaturation at 94°C for 2 min followed by denaturation at 94°C for 1 min, annealing at 52°C for 30 sec, and extension at 72°C for 1 min. This was followed by a final extension at 72°C for 10 min. The PCR products were resolved by electrophoresis over a 1.5% agarose gel and visualized by ethidium bromide staining and illumination with a UV light. The molecular weight (MW) of the amplified band was calculated using standard MW marker that had been resolved in parallel lanes with the PCR-amplified samples. Quantification of PCR products was performed by densitometric analysis using Image Quant software (Microsoft Corp., Santa Rosa, CA, USA).
Table 1. List of primers used.
cAMP assay
The cAMP concentrations in cell lysates were quantified with an ELISA kit in accordance with the manufacturer’s instructions. All assays were done in triplicate. In brief, U937 cells (5 × 105 cells) were seeded into 40 mm petri plates, the cells were then treated with the different concentrations of bresol, then incubated for 24 h at 37°C. Cell controls (vehicle only) and a positive control (cells treated with 30 µM rolipram) were also maintained.
TNFα assay and gene amplification
Both a TNFα assay and PCR amplification for bresol-treated U937 cells were carried out as described previously (Mitra et al., Citation2008). U937 cells were co-treated with bresol at different concentrations and with 1 µg LPS/ml for 4 h. In brief, the cells (5 × 105 cells/ml) were seeded into 40 mm petri dishes (1 ml of cells) and treated for 4 h with bresol at 4%, 2%, and 1% concentrations along with LPS at 1 µg/ml, with LPS alone at 1 µg/ml, or with dexamethasone (100 µM) + LPS at 1 µg/ml; control dishes received only medium. The plate contents were then collected and centrifuged; the cell-free conditioned medium was then diluted 1:2 and 1:4 with fresh RPMI medium prior to assessment of cytotoxicity against L929 cells (assessed with MTT assay). These same samples of conditioned medium were also used for direct quantification of TNFα in a commercially available enzyme linked immunosorbent assay (ELISA) kit (Krishgen Biosystems, Mumbai, India) according to manufacturer’s instructions.
Note to Readers: Dexamethasone has been used by different Investigators in varying concentrations (i.e., from 10–10 all the way up to 10−4 M) to study its anti-inflammatory activity in different cell lines. The concentration of 100 µM, as reported in the present study, has been standardized in our laboratory (see Varma et al., Citation2011a,Citationb) and it has also been utilized/reported by other workers (see Sun et al., Citation2000; Ethuin et al., Citation2001; Moody et al., Citation2001; Tong et al., Citation2003; Ergun et al., Citation2004; Yadav et al., Citation2004; Olsen et al., Citation2011).
RNA isolation from drug-treated/untreated U937 cells was performed as described for PDE4B expression. PCR amplification was carried out using random hexamer primers for first-strand synthesis and human-specific TNFα primers for second-strand synthesis; the annealing temperature was 60°C (). All other PCR conditions, electrophoretic steps, and densitometric analyses were carried out as described for the PDE4B gene amplification experiment (see above).
Nitric oxide assay and iNOS gene amplification
U937 cells were treated with 1 µg LPS/ml and bresol (at different concentrations), and then incubated for 24 h before the well supernatants were recovered and used for determination of nitrite levels using the Griess method (Varma et al., Citation2011a). Each experiment was repeated three times in duplicate.
Gene amplification for iNOS was carried out using the RNA isolated from drug-treated cells according to Varma et al. (Citation2011a). Human iNOS primers were employed in the PCR reaction and the annealing temperature was set to 60°C (). All other PCR conditions, electrophoretic steps, and densitometric analyses were carried out as for the PDE4B gene amplification experiment (see above).
Cycloxygenase-2 assay
Bresol-mediated inhibition of COX-2 enzyme activity was determined using a commercially available COX-2 inhibitor screening assay kit. The assay was carried out as per the manufacturer’s instructions.
Statistical Analysis
All results were expressed as mean ± standard error (SEM). Statistical analysis of the data was determined by a one-way analysis of variance (ANOVA) followed by a Dunnett’s t-test using GraphPad Prism-4.0 software (La Jolla, CA).
Results
Cytotoxicity assay
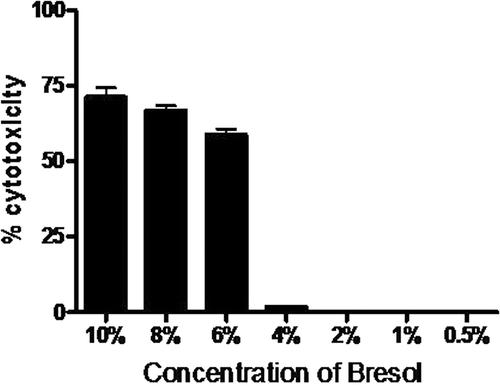
Bresol at concentrations ranging from 10-6% was found to be toxic to U937 cells as observed by the MTT assay. Cytotoxicity was not observed in concentrations below 4%, (). Three non-toxic concentrations (4%, 2% and 1%) of Bresol were used for further experiments.
Figure 1. Cytotoxicity of bresol on U937 monocytic cells. U937 cells were incubated for 24 h with different concentrations of bresol and the cell viability was then determined using an MTT assay. Data are expressed as mean (± SE) cytotoxicity (i.e., percentage reduction in viable cell numbers vs. viability level in control cultures). N = 3 samples/bresol concentration.
PDE4B gene expression
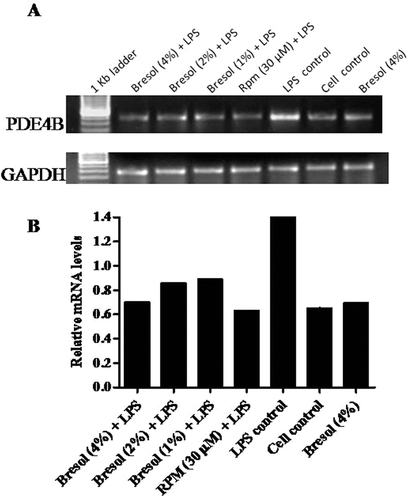
Bacterial lipopolysaccharide (LPS) has been widely recognized as an activator of proinflammatory cytokines in human monocytes. RT-PCR was carried out using PDE4B-specific primers to amplify the cDNA specific to PDE4B, and the PCR products were analyzed for the gene expression pattern of the amplicons. In the present study, 0.1 µg LPS/ml increased PDE4B gene expression in the U937 cells. Bresol at all the test concentrations successfully inhibited the LPS-induced PDE4B expression ( and ). Rolipram, a known PDE4 inhibitor, was used as a standard inhibitor in this study; at 30 µM, rolipram down-regulated LPS-induced PDE4B expression. The study also examined whether bresol at 4% (without LPS activation) had any effect on PDE4B gene expression. The results showed that bresol itself did not affect PDE4B expression levels (i.e., level of gene expression was comparable to that of unstimulated controls).
Figure 2. Effect of bresol on phosphodiesterase 4B (PDE4B) gene expression in U937 cells. (A) Cells were treated with 0.1 µg LPS/ml alone, bresol (4%) alone, 0.1 µg LPS/ml + bresol (at indicated concentrations), rolipram (30 µM) + 0.1 µg LPS/ml, or vehicle only for 24 h. RNA was then isolated from the cultures and RT-PCR carried out using oligo-dT primers and PDE4B-specific primers as described in the Methods. The image shown is a representative from among three replicates. Expression of the housekeeping GAPDH gene is included here to demonstrate loading equality. (B) Densitometric analysis of gene transcripts. The relative level of PDE4B gene expression is normalized to GAPDH. Values shown depict arbitrary units.
cAMP assay
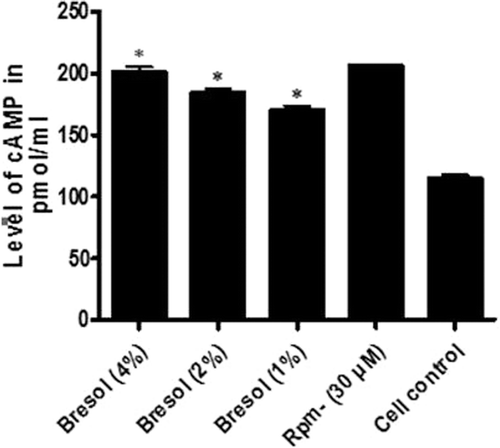
The ability of bresol to stimulate cAMP was assessed in U937 cells. The results indicated that bresol dose dependently stimulated cAMP production (; ). Specifically, 4% bresol caused an 80.57% elevation in cAMP levels as compared to those in the control cells. Bresol at doses of 2% and 1% caused 62.44% and 51.93% elevations in cAMP, respectively, as compared with the levels in the controls. Rolipram (30 µM) was used as the standard cAMP elevator; cAMP formation induced by rolipram was 83.72% a value comparable to that induced by bresol.
Table 2. Antiinflammatory activity of bresol in U937 cells.
Figure 3. Effect of bresol on cyclic AMP levels in U937 cells. U937 cells were incubated with bresol at varying concentrations, 30 µM rolipram, or vehicle for 24 h. cAMP levels in the cells were then measured using an ELISA kit. Results are represented as percentage change relative to control cell production values. Data presented are the mean (±SE) from three samples assayed in duplicate. *p < 0.01 as compared to cell control value depicted.
TNFα assay and gene amplification
Previous studies have reported that 1 µg LPS/ml could cause an increase in TNFα formation by monocytes and macrophages (Varma et al., Citation2011a). The levels of TNFα released by cells into culture supernatants were assayed using cytotoxicity against L929 cells (whose viability was calculated using an MTT assay). In this assay, L929 viability was inversely proportional to the level of TNFα released into the culture medium by the cells in response to drug treatment. Bresol at all test concentrations successfully inhibited TNFα formation by LPS-stimulated U937 cells. The percentages of inhibition of LPS-induced TNFα release caused by bresol were 65.28%, 50.09%, and 33.05% at 4%, 2%, and 1% dosages, respectively (. DXM –a glucocorticoid–was used as a reference standard in this experiment; DXM (at 100 µM) caused a 90.55% inhibition of TNFα production. Based on results of pilot studies, the concentrations of bresol and DXM in the conditioned medium were usually too low to affect viability of L929 cells outright (data not shown). As such, the viability results reported here reflected only the cytotoxic activities of TNFα that was released by the treated U937 cells.
Figure 4. Bresol-induced TNFα inhibition in human monocytes. U937 cells were incubated with 1 µg LPS/ml alone, bresol (4%) alone, 1 µg LPS/ml + bresol (at indicated concentrations), DXM (100 µM) + 0.1 µg LPS/ml, or vehicle only in media containing 2% FBS for 4 h at 37°C. Conditioned media was then recovered and analyzed for TNFα content via L929 bioassay and quantitative estimation using a human TNFα ELISA kit. RNA from the cells was extracted for RT-PCR. (A) TNFα results. Data are presented as % inhibition (mean ± SE) of LPS-induced TNFα formation over the control (LPS-treated) cells. *p < 0.01 as compared to LPS-only-treated cells. (B) Soluble TNFα secretion from U937 cells. Data are are presented as pg TNFα/ml in culture supernatant (mean ± SE) from three samples assayed in duplicate. *p < 0.01 as compared to LPS-only-treated cells. (C) RT-PCR profile of TNFα. The image shown is a representative from among three replicates. Expression of the housekeeping GAPDH gene is included here to demonstrate loading equality. (D) Densitometric analysis of gene transcripts. The relative level of TNFα gene expression is normalized to GAPDH. The values shown depict arbitrary units.
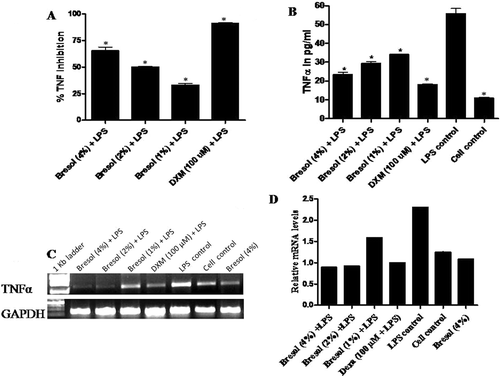
Quantitative estimation of TNFα in the cell culture supernatant (i.e., specifically, the 1:4 dilutions of the conditioned medium) was carried out using an ELISA kit. In the samples that had been diluted 1:4, untreated U937 cells demonstrated basal release of TNFα at a level of 10.88 (± 0.37) pg/ml. Treatment of U937 cells with 1 µg LPS/ml increased TNFα levels to 55.75 (±2.75) pg/ml (. Bresol at all test concentrations (4%, 2%, and 1%) significantly lowered LPS-stimulated TNFα levels to 23.38 (±1.12), 29.38 (±0.87), and 34.00 (±0.25) pg/ml, respectively (; ). These changes represent decrements from the non-bresol treated LPS-stimulated cell values of 58.06, 47.30, and 39.01%, respectively, for the 4%, 2%, and 1% test concentrations.
While these values would seem relatively low compared to what might be expected for U937 cells treated with 1 µg LPS/ml (see Abdolkhaleg and Shokooh [Citation2009] for comparison), after accounting for differences relative to those studies in cell loads (i.e., 5 × 105 vs. 2 × 106/population analyzed) and incubation timeframes (4 h vs. 24 h), these values are –after accounting for the 1:4 dilution of the parent supernatant–actually in line with expectations. That implies, overall, the decrements noted in the ELISA values approximate those noted in the cytotoxicity against L929 cells assay. As such, we can conclude that any presence of bresol in the culture supernatant did not adversely impact upon the function of TNFα per se and that the decreases in cytotoxicity activity were direct reflections of bresol-induced inhibitions of TNFα formation de novo.
In line with the observed effects on TNFα formation, TNFα-specific gene expression was substantially high in LPS-treated cells. As expected in light of the above-noted effects on TNFα release, bresol at all test concentrations down-regulated expression of the TNFα gene; gene expression levels were comparable to those in non-LPS-stimulated controls ( and ). In the cells treated with bresol alone, the gene expression level was comparable to that in unstimulated controls; this indicated that bresol itself could not trigger TNFα formation in U937 cells.
Nitric oxide assay and iNOS gene amplification
While the U937 cells produced NO in response to 1 µg LPS/ml, the presence of bresol clearly inhibited NO formation in a concentration-dependent manner (; ). Concentrations of 4%, 2%, and 1% bresol inhibited NO production by 43.09%, 34.73%, and 28.84%, respectively, relative to the LPS-induced activity in control cells. In cells treated with bresol alone, the drug had a negligible effect on NO formation.
Figure 5. Effect of bresol on nitrite production from monocytes. U937 cells were incubated with 1 µg LPS/ml alone, bresol (4%) alone, 1 µg LPS/ml + bresol (at indicated concentrations), DXM (100 µM) + 0.1 µg LPS/ml, or vehicle only for 24 h at 37°C. Conditioned media was then collected for measures of nitrite (via Griess reaction) and the cells were used for extraction of RNA and subsequent RT-PCR. (A) Nitrite in media; µM nitrite calculated by extrapolation from a standard curve generated in parallel with known concentration of sodium nitrite solutions. Data are presented as mean (± SE) of three experiments each done in duplicate. *p < 0.05 as compared to LPS only treated cells (control). (B) Inducible nitric oxide synthase (iNOS) mRNA gene expression. The image shown is a representative from among three replicates. Expression of the housekeeping GAPDH gene is included here to demonstrate loading equality. (C) Densitometric analysis of gene transcripts. The relative level of iNOS gene expression is normalized to GAPDH. The values shown depict arbitrary units.
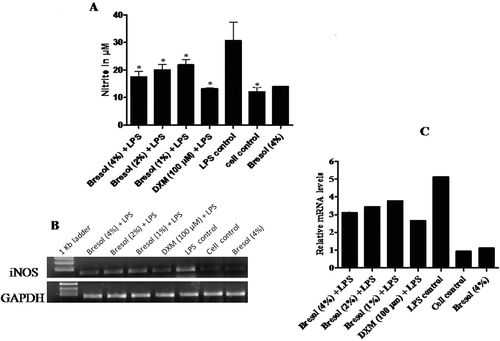
The expression of iNOS gene after treatment with bresol in cells stimulated with LPS was also studied. iNOS gene expression induced by LPS was reduced considerably by bresol at all test concentrations ( and ). DXM-treated cells also showed substantial reductions in LPS-induced iNOS gene expression. The results here suggest that the inhibition of LPS-induced NO production caused by bresol is most likely at the transcriptional level in the U937 cells.
Cycloxygenase-2 assay
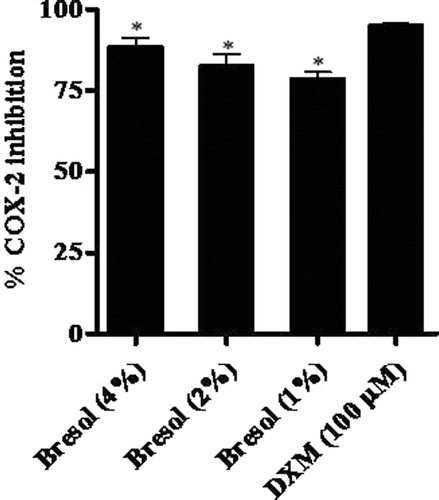
Bresol was effective in blocking COX-2 enzyme activity as determined using the COX-2 inhibition screening kit. Bresol at 4%, 2%, and 1% concentration resulted in levels of inhibition of COX-2 activity of 88.38%, 82.52%, and 78.45%, respectively, relative to the activity in control cells (; ). DXM caused a 94.88% inhibition of inducible COX-2 activity.
Figure 6. Inhibition of cycloxygenase-2 (COX-2) activity due to bresol. COX-2 activity was determined using a direct in vitro assay kit. Results are presented as as % inhibition of COX-2 activity (mean ± SE) as calculated against standard COX-2 enzyme activity (as determined using manufacturer’s instructions). *p < 0.01 as compared to DXM.
Discussion
The need for the development of new therapeutic agents for controlling asthma and AR is gaining importance as the current drugs used in the treatment of rhinitis and allergy pose severe adverse effects and also has low efficacy and compliance issues. It is now widely accepted that PDE4 inhibitors are highly effective in the treatment of AR and asthma. Inhibition of PDE4 leads to increased intracellular cyclic adenosine levels that, in turn, suppress histamine release from mast cells and the release of several cytokines and chemokines that are involved in allergic inflammation (Schmidt et al., Citation2001).
The efficacy of bresol in the management of bronchial asthma and AR has been reported (Chatterjee et al., Citation2004; Pandey et al., Citation2010). However, the mechanism of action of bresol in controlling the disease conditions has not been explored using in vitro models. The present study investigated the efficacy of bresol in inhibiting PDE4B expression in human monocyte cells. The effect of bresol in altering cAMP levels was investigated. The role of bresol in mediating cytokines like TNFα, COX-2, and NO was also studied in U937 cells.
U937 cells were chosen for study of the PDE4 gene expression studies because an earlier study had shown that this cell line expresses a range of PDE4, cAMP-specific phosphodiesterase isozymes (MacKenzie and Houslay, Citation2000). LPS has been reported to increase the PDE4 transcripts in U937 cells (Ma et al., Citation1999). Therefore, in the present study LPS-activated U937 cells were chosen and the efficacy of bresol was studied. LPS at 0.1 µg/ml activated the PDE4B gene expression and bresol abrogated the gene expression at all the test concentrations. Rolipram serves as a paradigm of a PDE4 selective inhibitor (MacKenzie and Houslay, Citation2000); in the present study, rolipram was used as standard PDE4B inhibitor. In the present study, rolipram (30 µM) was found to significantly inhibit PDE4B gene expression.
The anti-inflammatory actions of PDE4 inhibitors are well-documented both in vitro and in vivo. PDE4 inhibitors have been reported to produce marked anti-inflammatory actions in several animal models of allergic asthma (Torphy and Undem, Citation1991; Howell et al., Citation1995). Selective inhibition of PDE4 also suppresses several eosinophil functions such as superoxide anion generation, adhesion and migration (Santamaria et al., Citation1997). The results from this study demonstrate that bresol exhibits potent anti-inflammatory action by inhibiting PDE4B gene expression in monocyte cells.
Cyclic adenosine-3-5-monophosphate (cAMP) plays a central role in regulating immune responses. Increased intracellular cAMP has an inhibitory effect on release of histamine and other mediators from basophile leukocytes or mast cells as well as lysosomal enzymes from polymorphs (Ring, Citation1978). Patients suffering from asthma and AR show poor intracellular cAMP levels in peripheral leukocytes. Treatment of AR and asthma includes administration of drugs which include β2-adrenoreceptor agonists, xanthine drugs, anti-inflammatory agents (like glucocorticoids), and histamine H-1 receptor antagonists (Ring, Citation1978). The mechanism by which each of these drug acts is different. β2-adrenoreceptor agonists act mainly by dilating the bronchi by a direct action on the β2-adrenoreceptors on the smooth muscle. Xanthine drugs are adenosine antagonists which lead to increase in cyclic AMP and phosphodiesterase inhibition (Fredholm and Persson, Citation1982). It could be concluded from the present study that bresol inhibits PDE4, thereby leading to increases in cAMP levels in U937 cells.
The effect of bresol on the formation/release of inflammatory mediators like TNFα–a cytokine involved in the pathogenesis of asthma and other inflammatory conditions (Bissonnette et al., Citation1995)–was also studied here in the LPS-stimulated U937 cells. In the case of TNFα (and NO estimation), LPS was used at 1 µg/ml –rather than at the 0.1 µg LPS /ml used in the PDE4B gene expression studies –because previous reports (Sariban et al., Citation1988; Bruggen et al., 1999) as well as studies from our laboratory have clearly shown that this concentration results in significant triggering of TNFα and NO expression in U937 cells. Along with the measures of the production/release of this cytokine, gene expression levels of this protein were also assessed to ascertain if any effects from the bresol were potentially mediated at the transcriptional level. In this study, the effects of bresol in inhibiting TNFα levels approached those of dexamethasone (DXM) –a glucocorticosteroid that inhibits TNFα formation in LPS-activated monocytes and macrophages (Das et al., Citation1997). Gene expression studies employing TNFα-specific primers also showed that bresol significantly down-regulated LPS-induced TNFα gene expression in the U937 cells. Such outcomes emphasized the potency of bresol as an anti-inflammatory agent.
NO, endogenously produced in mammalian airways by nitric oxide synthase (NOS), is known to regulate many aspects of human asthma, including modulation of airway and vascular smooth muscle tone and inflammation (Batra et al., Citation2007). Asthmatics demonstrate an increased expression of inducible NOS (iNOS) in their airway epithelial cells and an increased level of NO in exhaled air (Batra et al., Citation2007). In the present study, U937 cells were stimulated with LPS to produce NO and the effect of bresol in inhibiting NO synthesis was studied. Bresol dose dependently inhibited LPS induced NO formation. The level of NO in cells treated with bresol alone was similar to that in the cell control; this showed that bresol alone does not interfere with NO formation in U937 cells. To confirm whether the inhibitory effect on NO production was targeted at the transcription level, iNOS gene expression was analyzed. There was a significant increase in iNOS mRNA in LPS-treated cells; however, this effect was abrogated by bresol at all test concentrations. The level of inhibition was comparable to that induced by DXM, a standard iNOS inhibitor in the present study. In the cells treated with bresol alone, the level of iNOS gene expression was similar to that in the controls.
Recent studies have linked COX-2 induction to the asthmatic inflammatory response (Szczeklik et al., Citation2001). The safety of COX-2 inhibitors in aspirin induced asthma has been reported (Szczeklik et al., Citation2001). In the present study, COX-2 inhibitory properties of bresol were studied using ELISA and the results showed that bresol successfully inhibited COX-2 production at all test concentrations. The level of inhibition was comparable to dexamethasone, the standard COX-2 inhibitor used in the present study.
These results clearly showed that bresol is highly effective in inhibiting LPS-induced PDE4B gene expression; this, in turn, resulted in increases in cAMP levels in the U937 cells. The results also confirmed the immunomodulatory role of bresol in inhibiting the formation of the proinflammatory cytokine TNFα, as well as COX-2 and NO synthesis, in LPS-activated monocytes. Conventional drugs used for treating asthma, particularly steroids, can impair immune function and lead to more serious health problems. Herbal based medicines for the treatment of asthma and rhinitis are widely accepted mainly because of its low side effects. A number of studies were found that support the use of herbal medicines in asthma and allergy (Beilory and Lupoli, Citation1999). Various derivatives from specific medicinal plants were identified as the antiasthma components and some mechanisms of action were explored. The results showed positive effects of these herbs on bronchodilation, pulmonary function tests, and antagonism of asthma mediators (such as histamine and platelet activating factor [PAF]), corticosteroid levels, and clearance of mucus. Improved symptoms were also seen in patients with AR specifically on histamine-induced reactions (Bielory and Lupoli, Citation1999).
In summary, our results demonstrate that in human monocytes-U937 cells, bresol exhibits strong anti-inflammatory properties by inhibiting PDE4B expression and thus elevating cAMP levels. Bresol has shown potent anti-inflammatory properties by inhibiting formation of TNFα, NO, and COX-2 in LPS-stimulated monocyte cells. In vivo studies and clinical trials have suggested the anti-allergic properties of bresol. The results obtained from the present in vitro studies provide strong evidence to the anti-inflammatory properties of this drug.
Acknowledgements
We thank the management, The Himalaya Drug Company for providing all the necessary facilities for carrying out this work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
References
- Abdolkhaleg, D. and Shokooh, D. 2009. Secreted tumor necrosis factor-alpha by human myeloid cells: A valuable parameter for evaluation of endotoxin contamination in vitro. Immunopharmacol Immunotoxicol 31:405–413.
- Batra, J., Chatterjee, R. and Ghosh, B. 2007. Inducible nitric oxide synthase (iNOS): Role in asthma pathogenesis. Indian J Biochem Biophys 44:303–309.
- Bielory, L. and Lupoli, K. 1999. Herbal interventions in asthma and allergy. J Asthma 36:1–65.
- Bissonnette, E. Y., Enciso, J. A. and Befus, A. D. 1995. Inhibition of tumour necrosis factor-α (TNF-α) release from mast cells by the anti-inflammatory drugs, sodium cromoglycate and nedocromil sodium. Clin Exp Immunol 102:78–84.
- van der Bruggen, T., Nijenhuis, S., van Raaij, E., Verhoef, J. and van Asbeck, B. S. 1999. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect Immun 67:3824–3829.
- Chatterjee, S., Pal, S., Panna, S., and Kolhapure, S. A. 2004. Evaluation of efficacy and safety of bresol (HK-07) syrup in pediatric allergic rhinitis. Ind. J. Clin. Practice 15:25–36.
- Das, A. M., Flower, R. J., Hellewell, P. G., Teixeira, M. M. and Perretti, M. 1997. A novel murine model of allergic inflammation to study the effect of dexamethasone on eosinophil recruitment. Br J Pharmacol 121:97–104.
- Ergun, M. A., Konac, E., Erbas, D. and Ekmekci, A. 2004. Apoptosis and nitric oxide release induced by thalidomide, gossypol and dexamethasone in cultured human chronic myelogenous leukemic K-562 cells. Cell Biol Int 28:237–242.
- Ethuin, F., Delarche, C., Benslama, S., Gougerot-Pocidalo, M. A., Jacob, L. and Chollet-Martin, S. 2001. Interleukin-12 increases interleukin-8 production and release by human polymorphonuclear neutrophils. J Leukoc Biol 70:439–446.
- Fredholm, B. B. and Persson, C. G. 1982. Xanthine derivatives as adenosine receptor antagonists. Eur J Pharmacol 81:673–676.
- Gopumadhavan, S., Rafiq, M., Venketaranganna, M. V., and Mitra, S. K. 2005. Anti histaminic and antianaphylactic activity of Bresol (HK-07), a herbal formulation. Ind. J. Pharmacol. 37:300–303.
- Guerra, S., Sherrill, D. L., Martinez, F. D. and Barbee, R. A. 2002. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol 109:419–425.
- Howell, R. E., Jenkins, L. P., Fielding, L. E. and Grimes, D. 1995. Inhibition of antigen-induced pulmonary eosinophilia and neutrophilia by selective inhibitors of phosphodiesterase types 3 or 4 in Brown Norway rats. Pulm Pharmacol 8:83–89.
- Jeffery, P. K. and Haahtela, T. 2006. Allergic rhinitis and asthma: Inflammation in a one-airway condition. BMC Pulm Med 6 Suppl 1:S5.
- Ma, D., Wu, P., Egan, R. W., Billah, M. M. and Wang, P. 1999. Phosphodiesterase 4B gene transcription is activated by lipopolysaccharide and inhibited by interleukin-10 in human monocytes. Mol Pharmacol 55:50–57.
- MacKenzie, S. J. and Houslay, M. D. 2000. Action of rolipram on specific PDE4 cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase in U937 monocytic cells. Biochem J 347:571–578.
- Méhats, C., Tanguy, G., Dallot, E., Robert, B., Rebourcet, R., Ferré, F. and Leroy, M. J. 1999. Selective up-regulation of phosphodiesterase-4 cyclic adenosine 3′,5′-monophosphate (cAMP)-specific phosphodiesterase variants by elevated cAMP content in human myometrial cells in culture. Endocrinology 140:3228–3237.
- Mitra, S. K., Varma, S. R., Godavarthi, A. and Nandakumar, K. S. 2008. Liv.52 regulates ethanol induced PPARγ and TNFα expression in HepG2 cells. Mol Cell Biochem 315:9–15.
- Moody, M. D., Van Arsdell, S. W., Murphy, K. P., Orencole, S. F. and Burns, C. 2001. Array-based ELISAs for high-throughput analysis of human cytokines. BioTechniques 31:186–90, 192.
- Olsen, P. C., Ferreira, T. P., Serra, M. F., Farias-Filho, F. A., Fonseca, B. P., Viola, J. P., Cordeiro, R. S., Silva, P. M., Costa, J. C. and Martins, M. A. 2011. Lidocaine-derivative JMF2-1 prevents ovalbumin-induced airway inflammation by regulating the function and survival of T-cells. Clin Exp Allergy 41:250–259.
- Pandey, M. K., Singh, S. K., and Patki, P. S. 2010. Efficacy and safety of bresol tablets in the management of bronchial asthma. Ind. J. Clin. Practice 20:707–713.
- Ring, J. 1978. [Cyclic adenosine 3-5 monophosphate (c-AMP) and allergy]. Hautarzt 29:625–631.
- Santamaria, L. F., Palacios, J. M. and Beleta, J. 1997. Inhibition of eotaxin-mediated human eosinophil activation and migration by the selective cyclic nucleotide phosphodiesterase type 4 inhibitor rolipram. Br J Pharmacol 121:1150–1154.
- Sariban, E., Imamura, K., Luebbers, R. and Kufe, D. 1988. Transcriptional and posttranscriptional regulation of tumor necrosis factor gene expression in human monocytes. J Clin Invest 81:1506–1510.
- Schmidt, B. M., Kusma, M., Feuring, M., Timmer, W. E., Neuhäuser, M., Bethke, T., Stuck, B. A., Hörmann, K. and Wehling, M. 2001. The phosphodiesterase 4 inhibitor roflumilast is effective in the treatment of allergic rhinitis. J Allergy Clin Immunol 108:530–536.
- Sun, L., Chang, J., Kirchhoff, S. R. and Knowlton, A. A. 2000. Activation of HSF and selective increase in heat-shock proteins by acute dexamethasone treatment. Am J Physiol Heart Circ Physiol 278:H1091–H1097.
- Szczeklik, A., Nizankowska, E., Bochenek, G., Nagraba, K., Mejza, F. and Swierczynska, M. 2001. Safety of a specific COX-2 inhibitor in aspirin-induced asthma. Clin Exp Allergy 31:219–225.
- Tong, Z., Dai, H., Chen, B., Abdoh, Z., Guzman, J. and Costabel, U. 2003. Inhibition of cytokine release from alveolar macrophages in pulmonary sarcoidosis by pentoxifylline: Comparison with dexamethasone. Chest 124:1526–1532.
- Torphy, T. J. and Undem, B. J. 1991. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax 46:512–523.
- Varma, R. S., Ashok, G., Vidyashankar, S., Patki, P. and Nandakumar, K. S. 2011a. Anti-inflammatory properties of Septilin in lipopolysaccharide-activated monocytes and macrophage. Immunopharmacol Immunotoxicol 33:55–63.
- Varma, R. S., Ashok, G., Vidyashankar, S., Patki, P. and Nandakumar, K. S. 2011b. Ethanol extract of Justicia gendarussa inhibits lipopolysaccharide-stimulated nitric oxide and matrix metalloproteinase-9 expression in murine macrophage. Pharm Biol 49:648–652.
- Yadav, A. K., Paul, B. N., Naik, S., Saxena, A. K. and Patel, D. K. 2004. Human hemoglobin shares bioactivities ascribed to human tumor necrosis factor-α. Immunopharmacol Immunotoxicol 26:559–572.