Abstract
Exposure to polychlorinated biphenyls (PCBs) during pre-natal and early life can alter normal immune system development. Blood specimens from newborns, 6-, and 16-month-old infants were collected in the Michalovce and Svidnik/Stropkov districts, areas with, respectively, high and low environmental PCB contamination, and lymphocyte receptor expression was evaluated by multi-color flow cytometry. The results indicate that the percentage of lymphoid dendritic cells (DC) and naïve/resting T-lymphocytes were significantly increased at 6-months in Michalovce as compared to the same cell types in cord blood samples (p < 0.001), whereas natural regulatory T-lymphocytes and suppressor inducer T-lymphocytes were reduced (p < 0.001). Overall, a positive linear correlation of terminally differentiated effector memory (TEM) T-lymphocyte population with age, but a negative linear correlation for myeloid DC from birth to 6-months in both regions were found. Michalovce samples indicated significantly higher expression of memory T-lymphocytes (birth, 6th, and 16th month), TEM T-lymphocytes (birth and 6th month), and lymphoid DC (6th month) compared to the Svidnik/Stropkov regions. After adjustment for relevant covariates, such as maternal age, parity, season of birth, breastfeeding, birth weight, and gender, the myeloid DC, suppressor inducer T-lymphocytes, truly naïve helper/inducer T-lymphocytes, and TEM T-lymphocytes remained significantly different between districts in cord blood samples. The multivariate analysis models for 6- and 16-month samples showed district differences in all cellular determinants, except for lymphoid DC and macrophage-like cells. This study provides the first evidence that pre-natal and early post-natal exposure to PCBs affects the dynamics of cell surface receptor expression on lymphoid DC and DC-like cells, suggesting impaired immunologic development following pre-natal and early post-natal PCB exposure.
| Abbreviations: | ||
| CD, | = | cluster of differentiation; |
| PCB, | = | polychlorinated biphenyl; |
| SSC, | = | side scatter; |
| FSC, | = | forward scatter; |
| DC, | = | dendritic cells; |
| TEM, | = | terminally differentiated effector memory T-cells. |
Introduction
Persistent organic contaminants including polychlorinated biphenyls (PCBs) are widely spread throughout the aquatic and terrestrial ecosystems. The persistence of such chemicals in the environment, as well as their bioaccumulation in living organisms, has a potential to induce adverse health effects, including effects on the immune system. Children can be exposed to PCBs both pre-natally by transplacental transfer and post-natally from breast milk and food. PCBs are stored in the mother’s body predominantly in fat tissue and can be released during pregnancy and breast feeding. PCBs cross the placenta, and enter fetal tissues. Because the brain, nervous system, immune system, thyroid, and reproductive organs in the fetus and child are still under development, the effects of PCBs on these target systems may be more profound after exposure during pre-natal and neonatal periods, making fetuses and children more susceptible to PCBs than adults (ATSDR, Citation2000; Tryphonas, Citation2001). Immunotoxic effects of PCBs have been investigated in rodent and human systems, where they have been found to induce a broad range of adverse effects. Recent evidence suggests that selected PCBs may be potent agents inducing vascular inflammatory responses via cellular oxidative stress. Some authors have reported adverse effects of PCBs on child neurodevelopment, and immune system development (Hennig et al., Citation2002; Choi et al., Citation2003; Ulbrich and Stahlmann, Citation2004; Chevrier et al., Citation2007; Hertz-Picciotto et al., Citation2008).
Neubert et al. (Citation1998) described age-dependent changes in major maturational receptors on CD4+ and CD8+ lymphocytes in different age groups of healthy children (≤ 1, > 1–3, 3–7 years, etc.). The shift of cells expressing the ‘naïve’ marker CD45RA to cells carrying the ‘memory’-type surface receptor CD45RO was found in boys and girls during the course of development. In children, dendritic cell (DC) numbers change with age in a different pattern, likely reflecting difference of their lineages. Healthy children had a decrease of plasmacytoid DC, and initial rise and subsequent decline of myeloid DC subsets (Jyonouchi et al., Citation2009). Newborns generally have high numbers of CD4+CD25+ T-lymphocytes that decline rapidly over the first 2 or 3 years of life. This is dependent on T-lymphocyte production, because the fraction of CD4+CD25+ cells in the T-lymphocyte subset remains relatively constant throughout childhood (Sullivan et al., Citation2002).
We studied the expression of cell surface markers in whole blood of infants pre- and post-natally exposed to PCBs. The aim of this study was to explore the hypothesis that PCB exposure during pre-natal and early life stages can alter expression of memory CD4 T-lymphocytes (CD4+CD45RO+CD45RA−), naïve/resting T-lymphocytes (CD4+CD45RO−CD45RA+), DC cells (CD19−CD11c+CD11b−, CD19−CD11c+CD11b+), DC-like cells (CD83+CD19+), natural regulatory T-lymphocytes (CD4+CD25+), suppressor inducer T-lymphocytes (CD4+CD62L+), macrophage-like cells (CD19−CD11c−CD11b+), terminally differentiated effector memory (TEM) T-lymphocytes (CD4+CD62L−CD45RA+), and truly naïve helper/inducer T-lymphocytes (CD4+CD62L+CD45RA+). To the best of our knowledge, this report is the first to examine dynamics of the above-mentioned cell populations over time in relation to environmental PCB exposures using early life blood samples. The availability of blood samples from umbilical cord, and 6- and 16-month old children from two districts of Eastern Slovakia with different levels of environmental PCBs pollution provided a unique opportunity. Additionally, the potential confounding effects of environmental factors, maternal age, smoking, parity, breastfeeding, ethnicity, birth weight, and gender of infants on different cell populations were taken into account.
Materials and methods
Study area and population
Specimens were obtained from mothers delivering in the Michalovce district (population of 110,000)—a region with high levels of PCB contamination from a chemical manufacturing plant—and from the neighboring districts of Svidnik and Stropkov (population of 55,000) that each have (in comparison) lower environmental PCB levels. Kocan et al. (Citation2001) reported higher values of PCBs in ambient air (up to 1700 ng/m3), soil (from 0.4 to 53,000 mg/kg), surface water, sediment, and wildlife samples taken from the Michalovce district compared to in samples from Svidnik/Stropkov.
The study population was already described in detail elsewhere (Hertz-Picciotto et al., Citation2003; CitationHorváthová et al., in press), the second report described PCB congeners in relation to immunophenotypes of lymphocytes, i.e., CD3+, CD3+CD8+, CD3+CD4+, CD3−CD(56 + 16)+, CD19+, and HLADR+CD19+. Briefly, blood samples from newborns were collected from umbilical vein after delivery (ntotal = 362; Michalovce [n = 301], Svidnik/Stropkov [n = 61]). When children were ≈6- and ≈16-months-of-age, the families were invited back to their local hospital Department of Pediatrics for a follow-up examination. At 6 months-of-age, 349 (96%) infants (Michalovce [n = 291], Svidnik/Stropkov [n = 58]) and at 16 months-of-age, 313 (86%) children (Michalovce [n = 273], Svidnik/Stropkov [n = 50]) were still participating in the study and had their cells analyzed. The characteristics of the study cohort consisting of mother–infant pairs in both districts: maternal age (range 18–43 years), gestational age (range 33–42 weeks), sex of child (male/female ratio = 1), birth weight (range 1660–4740 g), ethnicity (82% Slovakian/other Eastern European, 18% Romani), maternal smoking (20% smokers), parity (range 0–4 pregnancies), and breastfeeding practice (> 90% of woman).
Mothers gave signed informed consent and completed questionnaires focused on socio-demographic characteristics, medical history, age, number of children, and selected environmental factors, including diet and smoking habits of persons in the household. In these studies, individuals that were excluded were comprised of: (a) mothers with more than four previous births; (b) mothers < 18 years-of-age; (c) mothers who had resided < 5 years in their district; (d) mothers with a major illness during pregnancy; and, (e) infants who had severe birth defects.
PCB concentrations in all biological samples here were determined in the National Reference Laboratory for Dioxins and Related Compounds for the Slovak Republic, at the Slovak Medical University in Bratislava. The PCB levels in maternal blood in Michalovce were ≈2-fold higher than in mothers in Svidnik/Stropkov. The mean maternal serum levels of the sum of six PCBs (∑PCB) were 7.5 and (718.5 ng/g lipid) in Michalovce and 3.6 ng/ml (373.7 ng/g lipid) in Svidnik/Stropkov (Sonneborn et al., Citation2008). Descriptive data for the PCB concentrations in maternal, cord, and infant serum was reported previously (Jusko et al., Citation2010, 2011). The median sum of cord PCBs was 343 ng/g lipid (25th and 75th percentiles: 224 and 535 ng/g lipid, respectively), and for 6-month-old infant sum PCB concentration the median concentration was 361 ng/g lipid (25th and 75th percentiles: 94 and 787 ng/g lipid, respectively).
Cell surface receptor expression
The frozen/thawed whole blood method was used for the cell surface receptors analysis. To perform multi-color immunophenotyping, 50 µl aliquots of whole blood were incubated for 20 min at room temperature with combinations of the following monoclonal antibodies: CD19-PC7, CD4-PC7, CD83-PC5, CD11c-PE, CD11b-FITC, CD45RO-ECD, CD45RA-PE, CD62L-PC5, CD25-FITC (Beckman Coulter, Marseille, France). Cell surface receptor analyses of blinded blood samples was performed with a Cytomics FC 500 flow cytometer and CXP software (Beckman Coulter). Cell population gating was based on forward scatter (FCS) and side scatter (SSC) characteristics and the CD45-FITC/CD14-PE antibody cocktail. Data on CD4+ T-lymphocyte subsets were generated via gating through the lymphocytes. An acquisition gate for DC, macrophage-like cells, and DC-like cells included all mononuclear cells. For multi-parametric analysis, percent positive values were determined from quadrants set with appropriate isotype control antibodies. The antigen cell surface density was determined as the percentage of positive events within a region. A minimum of 10,000 events/sample was acquired for analyses.
To assure validity of all data, all analyses were performed by the same Flow Cytometry Laboratory at the Slovak Medical University in Bratislava and by using the same methods, monoclonal antibodies, and flow analyses software each time. To assess reproducibility, the same samples were running in replicate on multiple occasions, by identical method, and using identical reporting criteria. Flow Cytometry Laboratory was accredited by the Slovak National Accreditation Service (SNAS) and regularly participated in an external quality control scheme for Clinical Immunology (SEKK Comp., Division EQA, Immunophenotyping). Internal QC procedures were performed daily using appropriate reference biological controls (Immuno-Troll Cells, Beckman). Fluorescent microspheres were used for cytometer alignment verification and fluorescence channel monitoring.
Statistical analysis
The statistical analysis was carried out using SPSS software. The non-parametric Mann-Whitney and Wilcoxon tests were used to compare variables between regions; p-values of <0.05 values represented statistical significance. The correlation of the lymphocyte receptor expression data between samples of cord blood and blood from 6- and 16-months-of-age children were represented by Kendall’s rank correlation coefficient. The variable ‘region’ (i.e., Michalovce or Svidnik/Stropkov) was used as a proxy marker for PCB exposure in the cohort. Multiple linear regression models (Stepwise method) were used for the evaluation of region effect on the subsets of T-lymphocyte populations, taking into consideration relevant confounders/predictors of the relationship of interest. Each variable, such as district, maternal age, smoking, parity, breastfeeding, ethnicity, birth weight, and gender of infants, was entered in sequence and assessed by the retain criteria in the model, i.e., p-values of <0.05.
Results
The dynamics and regional differences in the distribution of CD markers expression are shown in (DC, macrophage-like cells and DC-like cells) and (CD4+ T-lymphocyte subtypes). CD marker expression was analyzed in blood collected at birth, and at 6- and 16-months-of-age in the total group of children, and, subsequently, separately for Michalovce and Svidnik/Stropkov regions. Overall in both districts, TEM T-lymphocytes had a positive linear correlation with age, and suppressor inducer T-lymphocytes and natural regulatory T-lymphocytes had a negative linear correlation with age. Lymphoid DC and memory CD4 T-lymphocytes increased in all time periods in the samples from infants/children from the Michalovce region. Levels of naïve/resting T-lymphocytes were only seen to be increased at 6-months in the samples from infants/children from Michalovce, and at 16-months-of-age in the samples from infants/children from Svidnik/Stropkov. DC-like cells and truly naïve helper/inducer T-lymphocytes were found to be decreased in all three time periods in the subjects from the Michalovce region; myeloid DC were only seen to be decreased at the 6-months-of-age mark in both districts.
Figure 1. Expression of dendritic (DC), macrophage-like cells, and DC-like cell receptors in children living in both regions, i.e., Michalovce (n = 301) and Svidnik/Stropkov (n = 61). Cell surface receptors were analyzed by multi-color immunophenotyping of blood samples collected at birth, and at 6- and 16-months-of-age. CD19−CD11c+CD11b+ myeloid DC, CD19−CD11c+CD11b− lymphoid DC, CD19−CD11c−CD11b+ macrophage-like cells, and CD83+CD19+ DC-like cells were analyzed. Flow cytometric analysis was performed on a Beckman Coulter Cytomics FC 500 system. Statistical analyses were carried out using an SPSS System. Data are presented as mean of percentage. p-values were compared to birth, and p < 0.05 regarded as significant (H0: µ1 = µ2).
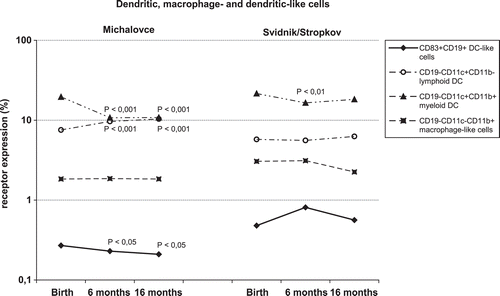
Figure 2. CD4+ T-lymphocyte subtypes in children living in the Svidnik/Stropkov (n = 61) and Michalovce (n = 301) regions. Naïve/resting T-lymphocytes (CD4+CD45RO−CD45RA+), memory T-lymphocytes (CD4+CD45RO+CDRA−), suppressor inducer T-lymphocytes (CD4+CD62L+), terminally differentiated effector memory (TEM) T-lymphocytes (CD4+CD62L−CD45RA+), truly naïve helper/inducer T-lymphocytes (CD4+CD62L+CD45RA+), and natural regulatory T-lymphocytes (CD4+CD25+) were identified. Data are presented as mean of percentage. p-values (calculated using an SPSS System) were compared to birth (cord) levels; a p < 0.05 was regarded as significant (H0: µ1 = µ2).
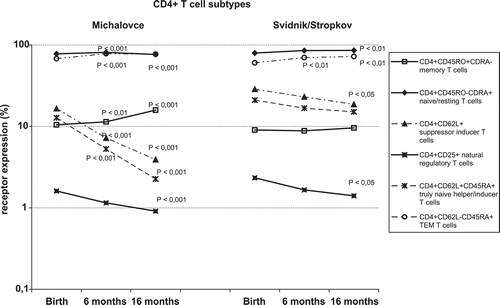
The number of CD19−CD11c+CD11b− cells—if compared to cord blood values—were significantly increased at 6- and 16-months-of-age in children in the Michalovce region, but not in those from Svidnik/Stropkov. In comparison to cord blood sample levels, CD19−CD11c+CD11b+ cell levels at 6-months-of-age were diminished 1.8-fold in Michalovce children (p < 0.001) and 1.3-fold in Svidnik/Stropkov children (p < 0.01). CD83+CD19+ expression decreased gradually from 0.27 (± 0.03)% at birth to 0.21 (± 0.02)% (p < 0.05) at 16-months-of-age in children from Michalovce. Populations of CD19−CD11c−CD11b+ did not differ significantly among timepoints. Higher expression of TEM cells at 6-months-of-age was observed in children of both regions, if compared to cord samples, and this rise continued even at 16-months-of-age in the Svidnik/Stropkov area (p < 0.01). Expression of naïve/resting T-lymphocytes increased at 6- (p < 0.001) and at 16-months-of-age (p < 0.01) in the subjects from, respectively, Michalovce and Svidnik/Stropkov. In contrast, CD4+CD62L+ cell levels decreased 4.3- and 1.8-fold between birth and 16-months-of-age among subjects from, respectively, Michalovce and Svidnik/Stropkov. Likewise, natural regulatory T-lymphocytes decreased in these subjects from both regions (p < 0.001, Michalovce; p < 0.05, Svidnik/Stropkov). Levels of CD4+CD62L+CD45RA+ expression were diminished at 6- and 16-months-of-age in children from Michalovce; at the same time, CD4+CD45RO+CD45RA− levels significantly increased (p < 0.001). Samples from children of Michalovce had significantly higher expression of memory T-lymphocytes (at birth, 6-, and 16-months-of-age; p < 0.010, p < 0.001, p < 0.001, respectively), TEM T-lymphocytes (at birth and at 6 months; p < 0.05, p < 0.01, respectively), and lymphoid DC (6-months-of-age; p < 0.01) when compared to levels in counterparts from Svidnik/Stropkov ().
Table 1. The major differences between districts (Michalovce and Svidnik/Stropkov) and cell surface receptor changes in children.
After adjustment for covariates, levels of CD4+CD62L+, CD4+CD62L+CD45RA+, and CD4+CD62L−CD45RA+, as well as CD19−CD11c+CD11b+ myeloid DC expression remained significantly different between districts in all time periods (i.e., birth, 6-, and 16-months-of-age). Furthermore, there was a significant effect of PCBs on all CD markers studied at 6- and 16-months-of-age, except for CD19−CD11c+CD11b− lymphoid DC and CD19−CD11c−CD11b+ macrophage-like cells (). Parity showed a significant effect on naïve/resting T-lymphocytes, memory T-lymphocytes, myeloid DC, TEM T-lymphocytes, macrophage-like cells, and natural regulatory T-lymphocytes in the cord blood. After exclusion of Romani ethnicity cases from the model, the findings were essentially unchanged, except for a loss of significance with respect to the measured levels of TEM lymphocytes.
Table 2. Coefficients and standard errors in models predicting cell surface markers in samples of blood from children from Michalovce and Svidnik/Stropkov.
Restriction of sampling to newborns from spontaneous deliveries and excluding Cesarean section did not change the observed associations in the cord blood model. Our findings show that the effect of PCBs on expression of cell surface markers was independent of delivery type. Similarly, stratification by ethnicity at 6- and 16-months-of-age did not significantly change the results.
Discussion
Our results indicated two main facts in the samples from the Michalovce district: an increase in lymphoid DC, memory CD4 T-lymphocytes, and TEM T-lymphocytes with age, naïve/resting T-lymphocytes at 6-months, and a decrease in the total percentages of DC-like cells and truly naïve helper/inducer cells between the studied timepoints.
The lower percentages of natural regulatory T-lymphocytes after a 16-month period of maturation by neonates in the Michalovce and Svidnik/Stropkov regions might be a key change in the infants´ immune system development. Regulatory T-lymphocytes are abundant in cord blood; they appear to be especially important in development of the immune system in the early post-natal phase, when proliferation of self-reactive T-lymphocytes may be increased during a period of relative lymphopenia (Weitkamp et al., Citation2009). Regulatory T-lymphocytes (CD4+CD25+ subset) are regarded as essential components of the immune system. They act by suppressing adaptive and possibly innate immune responses, thereby maintaining or restoring balance between immunity and tolerance. The suppressive effects of regulatory T-lymphocytes are cell-contact dependent, but a role for soluble factors, particularly in vivo, has been suggested as well (de Kleer et al., Citation2004; Curotto de Lafaille et al., Citation2008). During gestation, the immune system of the fetus is actively down-regulated to avoid immunological reactions that would end in termination of the pregnancy. This adaptation is demonstrated by: high levels of regulatory T-lymphocytes; down-regulation of antigenic-specific T-lymphocyte proliferation; and removal of activated T-lymphocytes via FasL-induced apoptosis (Clinton, Citation2010).
We demonstrated here that CD4+CD62L+ supressor inducer T-lymphocyte subset counts decreased in both districts; this effect may be related to a reduced regulatory activity with age. A possible regulatory role of CD4+CD62L+T cells for homeostasis mechanisms in experimental systems was suggested (You et al., Citation2004; Obermeier et al., Citation2005). The engagement of CD62L receptor has long been known to be important for lymphocyte homing to lymph nodes, and also contributes to chemokine-induced leukocyte migration within extravascular tissues both in acute and chronic inflammation (Chen and Bromberg, Citation2006).
The decreased numbers of CD4+CD62L+CD45RA+ truly naïve helper/inducer T-lymphocytes in 6-month-old children, and simultaneous increase in levels of CD4+CD62L−CD45RA+ TEM T-lymphocytes that lacked CD62L expression could be explained by a mechanism of naïve to memory phenotypic conversion, and CD45RA+ cells fragility depletion. Expression of CD62L is rapidly lost following T-lymphocyte receptor engagement, and CD62L− T-lymphocytes are thought to be ‘antigen experienced’ (Lecoeur and Gougeon, Citation2000; Maldonado et al., 2002; Ermann et al., Citation2005). We found an increase of memory T-lymphocytes at 6- and 16-months-of-age, and a decrease in naïve/resting T-lymphocytes at 16-months-of-age; thus, we confirm that CD4+CD45RA+ lymphocytes in newborns rapidly convert to memory CD4+CD45RO+ cells, more than in adults, thereby providing a fast, efficient, and advantageous response in vivo (Early and Reen, Citation1999).
A number of indicators of immune development differed between the two districts, suggesting a role of PCB exposures in altering the fine-tuning of this system. Our results showed a memory cell population increase in relation to PCB pollution, in agreement with Weisglas-Kuperus et al. (Citation2000), who investigated the influence of pre-natal PCB exposures on the immune system in infants. To the best of our knowledge, we are first to report the changes in lymphoid DC cells, DC-like cells, and truly naïve helper/inducer T-lymphocytes in children highly exposed to PCBs. We hypothesize that increased counts of lymphoid DC over time underwrite mainly the activation of primary immune response. Wang et al. (Citation2004) found no effect of organochlorine pesticides on DC in a murine model. Down-regulation of cell surface marker CD86 on DC upon induction by chemical compounds (organic mercury), and DC reduction by the mechanism of enhanced apoptosis, were demonstrated in previous work (Goth et al., Citation2004). Alternatively, Hulette et al. (2004), Boisleve and Pallardy (Citation2004), and Boisleve et al. (Citation2004) suggested up-regulation of CD86 and CD83 surface marker expression after chemical allergen (i.e., nickel sulfate) stimulation of DC in vitro. It is likely that a negative correlation of DC-like cells to pre-natal PCB exposure was related to failed innate immune responses. CD83+ cells possess the capability to generate numerous cytokines that may be important in the regulation of lymphocyte function and in modulating effective immune response (Zhou and Tedder, Citation1995). CD83 expression negatively regulates B-lymphocyte maturation and survival in the periphery (Luthje et al., Citation2008).
Naïve/resting CD4+ T-lymphocytes express high levels of CD45RA. Upon activation, T-lymphocytes rapidly lost CD45RA and acquired CD45RO expression. We have demonstrated the higher recruitment of memory T-lymphocytes in the peripheral blood of children exposed to high levels of PCB in comparison to in blood of children from a less-polluted area (Svidnik/Stropkov). The analysis of CD45RA and CD62L receptors showed a decrease of CD4+CD62L+CD45RA+ truly naïve helper/inducer T-lymphocytes related to pre-natal PCBs exposure.
The East Slovakian district of Michalovce is recognized as heavily polluted, cells, more than in adults, industrial PCB and other persistent organo-chlorinated pollutants (POP) (Kocan et al., Citation1994; Hovander et al., Citation2006; Jursa et al., Citation2006). Environmental exposure to organochlorines in the Michalovce district indicates association with higher rates of certain cancers and increased prevalence of thyroid disorders (Trnovec et al., Citation2004). The immune system is particularly vulnerable to POP toxicity, with observations of thymus atrophy and reduced T-lymphocyte functions (Ross and Birnbaum, Citation2001). POP are associated with changes in lymphocyte proliferation that could result in increased susceptibility to infections (Levin et al., Citation2005). Because of a high correlation that was found to exist between the measured levels of several major POP (i.e., PCB, DDE, and HCB), PCB levels were considered here to be useful as a marker for all POP components (Langer et al., Citation2009). The children living in these two East Slovakian regions are also exposed to other non-PCB pollutants that could be contributing to the observed effects. As such, additional studies will be needed to determine the effect of other non-POP pollutants exposure on the cell surface receptor expression in children born and living in the East Slovakia districts. The future evaluation of non-POP substances (e.g., heavy metals) may confirm or refute their potential confounding effects on different type of cell populations in children from a heavily PCB polluted area.
Jusko et al. (Citation2010) observed little evidence for an association between PCB exposures measured during the pre-natal and early post-natal period and post-vaccination specific antibody responses to anti-haemophilus influenzae Type b, tetanus toxoid, and diphtheria toxoid at 6-months-of-age in the same population of Michalovce and Svidnik/Stropkov districts. In addition, in utero and early post-natal PCB exposures are not associated with total serum immunoglobulin levels (IgG, IgA, IgM, or IgE) in infants at 6-months-of-age, regardless of the timing of PCB exposure (Jusko et al., Citation2011). Future research of environmental exposures will be required to detect associations in children during later childhood when immunoglobulin measures are less variable, and further determine whether relatively modest effects of PCB exposure on surface marker expression are related to total serum immunoglobulins and/or differences in the incidence of infections.
Our data suggested that maternal age, parity, breastfeeding, birth weight, gender of infant, and season of birth may alter cell surface receptor expression in children. Seasonal fluctuations in leukocyte and lymphocyte subpopulation counts have been already reported (Collinson et al., Citation2008), and our intention was to further determine these fluctuations in the extended cellular receptor family, as are CD4+ T-lymphocyte subtypes, DC, DC-like cells, and macrophage-like cells. This immunological phenotype may be consistent with enhancing requirements on the immune system during colder months; still, none of the winter coefficients were significant in our study. Conversely, increased maternal age was associated with diminution of memory T-lymphocytes and growth of TEM cells at cord blood samples. Likewise, increase in DC-like cells was found in males compared to females at 16 months-of-age. Birth weight influenced negatively the number of memory CD4+ T-lymphocytes and myeloid DC, and multi-parity was related to higher percentage of memory T-lymphocytes and myeloid DC. We also found that breastfeeding increased memory CD4+ T-lymphocyte numbers in 6-month-old infants, and also lymphoid DC at 6- and 16 months-of-age. Breast milk is rich in cytokine-producing cells, mainly macrophages and activated T-lymphocytes; most milk T-lymphocytes display the CD45RO marker of activation, at variance with the neonatal T-lymphocytes, which predominantly express a naïve CD45RA phenotype (Chirico et al., Citation2008). On the other hand, breast milk represents an important source of PCB and other lipophilic xenobiotics for the infant (Needham and Wang, Citation2002; LaKind et al., 2003). Higher concentrations of organochlorine agents were found in breast-fed, if compared to formula-fed, children (Lorber and Phillips, Citation2002; Ribas-Fito et al., Citation2005); hence, the final impact of breastfeeding on immune parameters in infants seems to be dependent on the complex network between beneficial and harmful components in the breast milk.
In conclusion, our findings indicate dynamic changes of cell surface markers expressed on lymphocytes between birth and 16 months-of-age. In children exposed to PCBs, using region as a proxy marker for PCB exposure, we have described a number of associated lymphocyte population differences for DC, DC-like cells, and a variety of CD4+ T-lymphocyte subtypes (e.g., naïve/resting T-lymphocytes, TEM T-lymphocytes, suppressor inducer T-lymphocytes, etc.). We have shown that PCB exposure in pre-natal and early post-natal life might be associated with an aberrant development of cellular immunity, which results in altered cell surface receptors expression, pointing mainly to weakened, compromised, or impaired immune responses.
Appendix
present results of the major associations between variables (e.g., maternal smoking, age, parity, season of birth, etc.) and cell surface receptor changes in children. The descriptive characteristics of DC, macrophage- and DC-like cells and CD4+ T-lymphocyte subtypes in all infants of the cohort are shown in and . The impact of age on the dynamics of cell surface receptors expression over the period from birth through infancy was determined in the total study group (Michalovce and Svidnik/Stropkov), regardless of PCB contamination ().
Table A1. The major differences between variables (i.e., parity, maternal smoking, season of birth) and cell surface receptor changes in children.
Table A2. The major differences between variables (i.e., ethnicity, gender, breastfeeding) and cell surface receptor changes in children.
Table A3. The major correlation between variables (maternal age, birth weight) and cell surface receptor changes in children.
Table A4. Total dendritic and macrophage- and dendritic-like cells in samples of blood from children in the Michalovce (n = 301) and Svidnik/Stropkov (n = 61) districts.
Table A5. Total CD4+ T-cell subtypes in samples of blood from children in the Michalovce (n = 301) and Svidnik/Stropkov (n = 61) districts.
Table A6. CD marker correlation between samples of blood from children in the total study cohort.
Acknowledgments
This work was supported by the US NIH grant no. R01-CA096525, by the grant of the Slovak Ministry of Health, no. 2005/31-SZU-09, and the 6th FP EU research projects ‘HEIMTSA’ (no. GOCE-CT-2006-036913-2) and ‘INTARESE’ (no. GOCE 018385). The authors wish to thank Dr Allen Silverstone for his excellent advice on immunophenotyping in the early parts of the project. In addition, we thank Mikulas Krnac and Zuzana Kormancikova for their technical assistance and we wish to thank our regional cooperators and all mothers with children who participated in the study.
Declaration of interest
The authors report no conflicts of interest. The authors alone are alone responsible for the content and writing of the paper.
References
- ATSDR (Agency for Toxic Substances and Disease Registry). 2000. Public Health Statement -Polychlorinated Biphenyls (PCBs). Atlanta, GA: ATSDR. Available online at: www.atsdr.cdc.gov
- Boisleve F., and Pallardy M. 2004. Mechanisms of CCR7 up-regulation by NiSO4 on human dendritic cells. Toxicol. Sci. (Suppl.) 78:47.
- Boisleve F., Aubert N., Bernard J., Pallardy M., and Roemer S. 2004. Comparison of the response of dendritic cells derived from cord blood CD34+ and from CD14+ monocytes to the contact sensitizer nickel. Toxicol. Sci. (Suppl.) 78:135.
- Chen D., and Bromberg J. S. 2006. T-regulatory cells and migration. Am. J. Transplant. 6:1518–1523.
- Chevrier J., Eskenazi B., Holland N., Bradman A., and Barr D. 2007. Effect of exposure to polychlorinated biphenyls and organochlorine pesticides on thyroid function during pregnancy. Epidemiology 18:S195–S196.
- Chirico G., Marzollo R., Cortinovis S., Fonte C., and Gasparoni A. 2008. Antiinfective properties of human milk. J. Nutr. 138:180S–1806S.
- Choi W., Eum S. Y., Lee Y. W., Hennig B., Robertson L. W., and Toborek M. 2003. PCB104-induced pro-inflammatory reactions in human vascular endothelial cells: Relationship to cancer metastasis and atherogenesis. Toxicol. Sci. 75:47–56.
- Clinton C. 2010. Development of the infant immune function and the effects of breast milk. Nat. Med. J. 2:3–6.
- Collinson A. C., Ngom P. T., Moore S. E., Morgan G., and Prentice A. M. 2008. Birth season and environmental influences on blood leukocyte and lymphocyte subpopulations in rural Gambian infants. BMC Immunol. 9:18–28.
- Conka K., Drobna B., Kocan A., and Petrik J. 2005. Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorine pesticides in human serum. J. Chromatogr. A 1084:33–38.
- Curotto de Lafaille M. A., Kutchukhidze N., Shen S., Ding Y., Uee H., and Lafaille J. J. 2008. Adaptive Foxp3 regulatory T-cell-dependent and -independent control of allergic inflammation. Immunity 29:114–126.
- De Kleer I. M., Wedderburn L. R., Taams L. S., Patel A., Varsani H., Klein M., de Jager W., Pugayung G., Giannoni F., Rijkers G., Albani S., Kuis W., and Prakken B. 2004. CD4+CD25bright regulatory T-cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J. Immunol. 172:6435–6443.
- Early E., and Reen D. J. 1999. Rapid conversion of naïve to effector T-cell function counteracts diminished primary human newborn T-cell responses. Clin. Exp. Immunol. 116:527–533.
- Ermann J., Hoffmann P., Edinger M., Dutt S., Blankenberg F. G., Higgins J. P., Negrin R. S., Fathmann C. G., and Strober S. 2005. Only the CD62L+ sub-population of CD4+CD25+ regulatory T-cells protects from lethal acute GVHD. Blood 105:2220–2226.
- Goth S. R., Chu R. A., and Pessah I. N. 2004. Dendritic cells are a sensitive target of thimerosal and ethylmercury. Toxicol. Sci. (Suppl.) 78:11.
- Hennig B., Hammock B. D., Slim R., Toborek M., Saraswathi V., and Robertson L. W. 2002. PCB-induced oxidative stress in endothelial cells: Modulation by nutrients. Int. J. Hyg. Environ. Health 205:95–102.
- Hertz-Picciotto I., Park H. Y., Dostal M., Kocan A., Trnovec T., and Sram R. 2008. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin. Pharmacol. Toxicol. 102:146–154.
- Hertz-Picciotto I., Tmovec T., Kocan A., Charles M. J., Ciznar P., Langer P., Sovcikova E., and James R. 2003. PCBs and early childhood development in Slovakia: Study design and background. Fresen. Environ. Bull. 12:208–214.
- Horváthová M., Jahnová E., Palkovičová A., Trnovec T., and Hertz-Picciotto I. Dynamics of main lymphocyte subsets in children living in the area polluted by polychlorinated biphenyls. J. Immunotoxicol. (in press).
- Hovander L., Linderholm L., Athanasiadou M., Athanassiadis I., Bignert A., Fängström B., Kocan A., Petrik J., Trnovec T., and Bergman A. 2006. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in Eastern Slovakia. Environ. Sci. Technol. 40:3696–3703.
- Hullete B., Ryan C., Gildea L., Kimber I., Dearman R., and Gerberick F. 2004. Relationship between CD86 expression, cytotoxicity and exposure of dendritic cells to chemical allergen. Toxicol. Sci. (Suppl.) 78:52.
- Jursa S., Chovancova J., Petrik J., and Loksa J. 2006. Dioxin-like and non-dioxin-like PCBs in human serum of Slovak population. Chemosphere 64:686–691.
- Jusko T. A., De Roos A. J., Schwartz S. M., Lawrence B. P., Palkovicova L., Nemessanyi T., Drobna B., Fabisikova A., Kocan A., Sonneborn D., Jahnova E., Kavanagh T. J., Trnovec T., and Hertz-Picciotto I. 2010. A cohort study of developmental polychlorinated biphenyl (PCB) exposure in relation to post-vaccination antibody response at 6-months of age. Environ. Res. 110:388–395.
- Jusko T. A., De Roos A. J., Schwartz S. M., Lawrence B. P., Palkovicova L., Nemessanyi T., Drobna B., Fabisikova A., Kocan A., Sonneborn D., Jahnova E., Kavanagh T. J., Trnovec T., and Hertz-Picciotto I. 2011. Maternal and early postnatal polychlorinated biphenyl exposure in relation to total serum immunoglobulin concentrations in 6-month-old infants. J. Immunotoxicol. 8:95–100.
- Jyonouchi H., Chu C., Geng L., Yin Z., and Fitzegerald-Bocarsly P. 2009. Age-dependent changes in peripheral blood dendritic cell subsets in normal children and children with antibody deficiency syndrome. J. Allergy Clin. Immunol. 123:S221.
- Kocan A., Petrik J., Drobna B., and Chovancova J. 1994. Levels of PCBs and some organo-chlorine pesticides in the human population of selected areas of the Slovak Republic. I. Blood. Chemosphere 29:2315–2325.
- Kocan A., Petrik J., Jursa S., Chovancova J., and Drobna B. 2001. Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere 43:595–600.
- LaKing J. S., Wilkins A. A., and Berlin C. M. 2004. Environmental chemicals in human milk: A review of levels, infant exposures and health, and guidance for future research. Toxicol. Appl. Pharmacol. 198:184–208.
- Langer P., Kočan A., Tajtáková M., Sušienková K., Rádiková Ž,Koška J., Kšinantová L., Imrich R., Hučková M., Drobná B., Gašperíková D., Trnovec T., and Klimeš I. 2009. Multiple adverse thyroid and metabolic health signs in the population from the area heavily polluted by organochlorine cocktail (PCB, DDE, HCB, dioxin). Thyroid Res. 2:3–9.
- Lecoeur, H., and Gougeon, M. L. 2000. HAART induces a strong apoptosis inhibition in CD62L-CD4+ or CD8+ T-cells, which may favor the re-expression of CD62L antigen and contribute to the emergence of phenotypically naive T-cells. Int. Conf. AIDS 13 (Abst. TuPeA3122).
- Levin M., De Guise S., and Ross P. S. 2005. Association between lymphocyte proliferation and polychlorinated biphenyls in free-ranging harbor seal (Phoca vitulina) pups from British Columbia, Canada. Environ. Toxicol. Chem. 24:1247–1252.
- Lorber M., and Phillips L. 2002. Infant exposure to dioxin-like compounds in breast milk. Environ. Health Perspect. 110:A325–A332.
- Luthje K., Kretchmer B., Fleischer B., and Breloer M. 2008. Cd83 regulates splenic B-cell maturation and peripheral B-cell homeostasis. Int. Immunol. 20:949–960.
- Maldonado A., Mueller Y. M., Thomas P., Bojczuk P., O’Connors C., and Katsikis P. D. 2003. Decreased effector memory CD45RA+CD62L−CD8+ T-cells and increased central memory CD45RA−CD62L+CD8+ T-cells in peripheral blood of rheumatoid arthritis patients. Arthritis Res. Ther. 5:R91–R96.
- Needham L. L., and Wang R. Y. 2002. Analytic considerations for measuring environmental chemicals in breast milk. Environ. Health Perspect. 110:A317–A324.
- Neubert R., Delgado I., Abraham K., Schuster Ch.,,and Helge H. 1998. Evaluation of the age-dependent development of lymphocyte surface receptors in children. Life Sci. 62:1099–1110.
- Obermeier F., Strauch U. G., Dunger N., Grunwald N., Rath H. C., and Herfarth H. 2005. In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+CD62L+ T-cells that prevent intestinal inflammation in the SCID transfer model of colitis. Gut 54:1428–1436.
- Ribas-Fito N., Grimalt J. O., Marco E., Sala M., Mazon C., and Sunyer J. 2005. Breastfeeding and concentrations of HCB and p,p´-DDE at the age of 1 year. Environ. Res. 95:8–13.
- Ross P. S., and Birnbaum L. S. 2001. III. Case studies. A. Persistent organic pollutants (POPs) in humans and wildlife. pp. 1–26. Available online at: http://whqlibdoc.who.int/hq/2001/a76785_persistent.pdf
- Sonneborn D., Park H. Y, Babinska K., Palkovicova L., Trnovec T., Kocan A., Nguyen D. V, and Hertz-Picciotto I. 2008. Serum PCB concentrations in relation to locally-produced food items in Eastern Slovakia. J. Exposure Sci. Environ. Epidemiol. 18:581–587.
- Sullivan K. E., McDonald-McGinn D., and Zackai E. H. 2002. CD4+CD25+ T-cell production in healthy humans and in patients with thymic hypoplasia. Clin. Diagn. Lab. Immunol. 9:1129–1131.
- Trnovec T., Bencko V., Langer P., van den Berg M., Kocan A., Bergman A., and Hustak M. 2004. Study design, objectives, hypotheses, main findings, health consequences for the population exposed, rationale of future research. Organohal. Comp. 66:3573–3580.
- Tryphonas H. 2001. Approaches to detecting immunotoxic effects of environmental contaminants in humans. Environ. Health Perspect. 109 (Suppl 6):877–884.
- Ulbrich B., and Stahlmann R. 2004. Developmental toxicity of polychlorinated biphenyls (PCBs): A systematic review of experimental data. Arch. Toxicol. 78:483–487.
- Wang F., Roberts S. M., and Sobel E. S. 2004. Different effects of estrogen and an organochlorine pesticide chlordecone on murine immunocyte populations. NIEHS. Available online at: www.niehs.nih.gov/news/events
- Weisglas-Kuperus N., Patandin S., Berbers G. A., Sas T. C., Mulder P. G., Sauer P. J., and Hooijkaas H. 2000. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch pre-school children. Environ. Health. Perspect. 108:1203–1207.
- Weitkamp J. H., Rudzinski E., Koyama T., Correa H., Matta P., Alberty B., and Polk D. B. 2009. Ontogeny of FoxP3+ regulatory T-cells in the postnatal human small intestinal and large intestinal lamina propria. Pediatr. Dev. Pathol. 12:443–449.
- You S., Slehoffer G., Barriot S., Bach J. F., and Chatenoud L. 2004. Unique role of CD4+CD62L+ regulatory T-cells in the control of autoimmune diabetes in T-cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA 101:14580–14585.
- Zhou L. J., and Tedder T. F. 1995. A distinct pattern of cytokine gene expression by human CD83+ blood dendritic cells. Blood 86:3295–3301.